SUMMARY
Reproductive cessation is perhaps the earliest aging phenotypes humans experience. Similarly, C. elegans’ reproduction ceases in mid-adulthood. Although somatic aging has been studied in both worms and humans, mechanisms regulating reproductive aging are not yet understood. Here we show that TGF-β Sma/Mab and Insulin/IGF-1 signaling regulate C. elegans reproductive aging by modulating multiple aspects of the reproductive process, including embryo integrity, oocyte fertilizability, chromosome segregation fidelity, DNA damage resistance, and oocyte and germline morphology. TGF-β activity regulates reproductive span and germline/oocyte quality non-cell-autonomously, and is temporally and transcriptionally separable from its regulation of growth. Chromosome segregation, cell cycle, and DNA damage response genes are upregulated in TGF-β mutant oocytes, decline in aged mammalian oocytes, and are critical for oocyte quality maintenance. Our data suggest that C. elegans and humans share many aspects of reproductive aging, including the correlation between reproductive aging and declining oocyte quality, and mechanisms determining oocyte quality.
INTRODUCTION
Many biological functions associated with quality of life decline with age, but female reproductive aging is one of the earliest declines humans experience. Although progressive loss of ovarian follicles leads to menopause between the ages of 45 and 55, the risk of infertility, birth defects, and miscarriage increase a decade earlier, likely due to age-related declines in oocyte quality (te Velde and Pearson, 2002). While aged mammalian oocytes exhibit increased errors in fertilization, chromosome segregation, and cleavage divisions (Goud et al., 1999; te Velde and Pearson, 2002), little is known about mechanisms that regulate oocyte quality maintenance with age.
C. elegans is a useful model for aging studies due to its short lifespan and the conservation of longevity pathways from C. elegans to humans (Kenyon, 2005; Suh et al., 2008). Recently, C. elegans has also been developed as a model of reproductive aging (Andux and Ellis, 2008; Hughes et al., 2007; Luo et al., 2009). These studies established that (1) C. elegans reproductive aging is independent of sperm contribution; (2) simply reducing ovulation rate or progeny production do not extend reproductive span; and (3) reproductive aging is usage-independent, i.e., independent of the magnitude and timing of oocyte use. That is, in worms as in humans, simply delaying the reproductive schedule does not delay reproductive aging.
One argument against using C. elegans as a model of human reproductive aging is that oocytes are continually produced in worms, while humans’ total oocyte supply is produced at birth. However, both human and C. elegans females reproduce for about one-third to one-half of their lives, and thus undergo significant reproductive aging on proportional time scales, implying that genetic mechanisms may link reproduction to longevity in both organisms (Cant and Johnstone, 2008; Luo et al., 2009). Furthermore, both human and C. elegans oocytes are cell-cycle arrested at meiotic prophase I, release from arrest is coordinated with oocyte maturation in both, and the mechanisms underlying oocyte maturation are highly conserved between the two organisms (Greenstein, 2005; Mehlmann, 2005). Most importantly, human reproductive aging occurs a decade prior to the exhaustion of the oocyte supply, suggesting that oocyte quality, rather than quantity, is the limiting factor for successful reproduction with age. Thus, the critical question that we address in this study is whether worms’ reproduction is similarly limited by oocyte quality, and if so, by what mechanisms.
Several long-lived C. elegans mutants, including the insulin/IGF-1 receptor mutant daf-2, delay reproductive aging (Huang et al., 2004; Hughes et al., 2007; Luo et al., 2009). daf-2 mutants extend life span, delay distal germline integrity decline, and extend reproductive span through the activity of the FOXO transcription factor DAF-16 (Garigan et al., 2002; Hughes et al., 2007; Kenyon et al., 1993; Luo et al., 2009), but daf-2’s role in oocyte quality maintenance and the mechanisms by which daf-2 mutants extend reproductive span are unknown.
We recently found that mutants of the TGF-β Sma/Mab pathway also significantly extend reproductive span (Luo et al., 2009), while mutants in the TGF-β Dauer pathway extend life span (Shaw et al., 2007) without greatly extending reproductive span (Luo et al., 2009). The TGF-β Sma/Mab pathway, which is highly conserved from worms to humans, consists of extracellular ligands (DBL-1), type I (SMA-6) and type II receptors (DAF-4), R-Smads (SMA-2 and SMA-3), a co-Smad (SMA-4), and a transcription co-factor (SMA-9) (Massagué, 2000; Savage-Dunn, 2005). Notably, Sma/Mab regulation of reproductive span is genetically independent of Insulin/IGF-1 signaling (IIS) and Dietary Restriction (Luo et al., 2009).
Here we show that C. elegans oocytes, like human oocytes, degrade functionally and morphologically with age, and that reduction of TGF-β Sma/Mab signaling and IIS delays reproductive aging by maintaining oocyte and germline quality. Although the TGF-β Sma/Mab pathway acts autonomously in the hypodermis to regulate body size (Wang et al., 2002), surprisingly, we find that both insulin and TGF-β Sma/Mab signaling regulate oocyte and distal germline quality maintenance non-autonomously. TGF-β regulates reproductive aging separately from the developmental regulation of growth, both temporally and transcriptionally. We find that TGF-β oocyte transcriptional targets that are required for C. elegans embryonic and germline integrity maintenance also change with age in mammalian oocytes. The conserved nature of these signaling pathways suggests that the mechanisms underlying the maintenance of C. elegans reproductive capacity with age may also influence reproductive capacity decline in higher organisms.
RESULTS
TGF-β Sma/Mab and Insulin/IGF-1 signaling regulate embryo viability and oocyte quality maintenance
Wild-type C. elegans reproduction declines with age, but reduced Insulin/IGF-1 signaling (IIS) delays reproductive cessation (Huang et al., 2004; Hughes et al., 2007). We recently found that reduced TGF-β Sma/Mab signaling also significantly extends reproductive span in a manner that is independent of insulin signaling, caloric restriction, sperm contribution, and ovulation rate (Luo et al., 2009). To identify the molecular mechanisms underlying normal reproductive aging and its delay in insulin and TGF-β signaling mutants, we systematically investigated each component of the reproductive system, from fertilized embryos through the distal germline (Figure 1A).
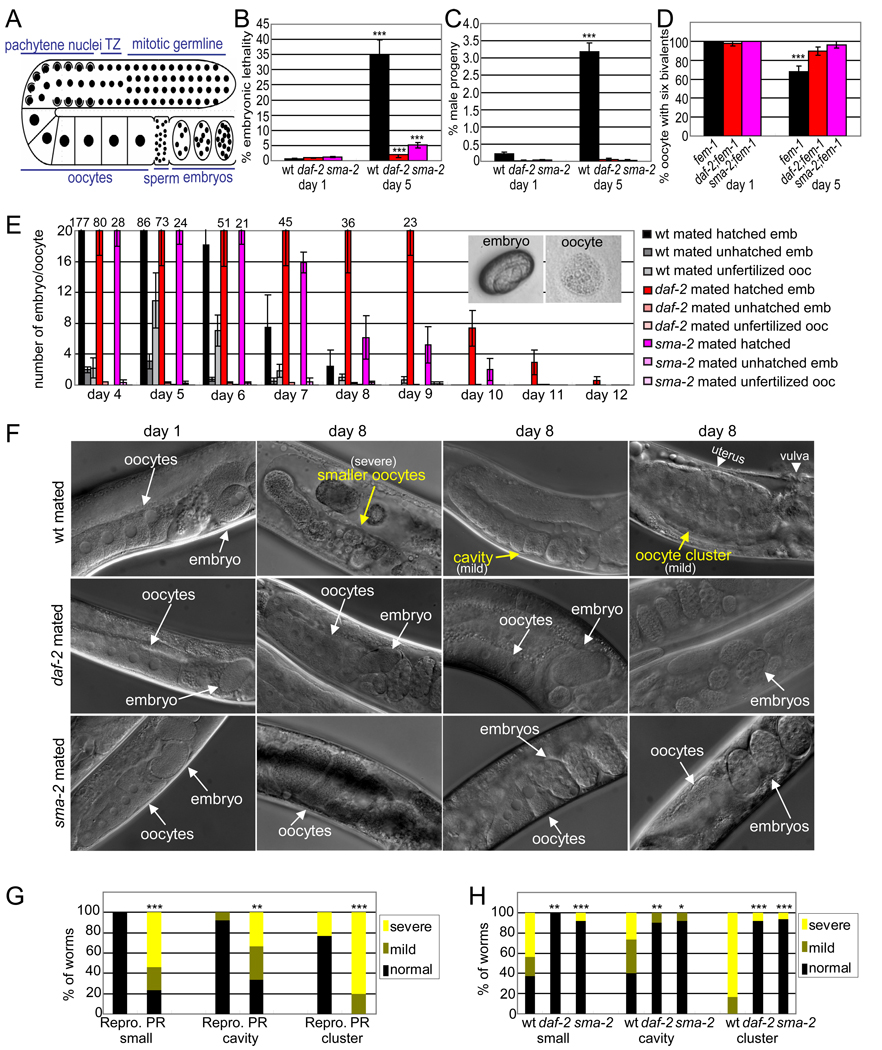
Figure 1. TGF-β Sma/Mab and insulin/IGF-1 signaling regulate embryo viability, oocyte fertilizability, and oocyte morphology.
(A) Schematic of the C. elegans gonad.
(B) Percentage of embryos that fail to hatch (± SEP).
(C) Percentage of male progeny (± SEP).
(D) Percentage of oocytes with 6 DAPI-stained bodies (± SEP).
(E) Number of hatched embryos (inset, left), unhatched embryos, and unfertilized oocytes (inset, right) produced each day after mating with young wild-type(wt) males (mean ± SEM); percentages shown in Figure S1F.
(F) Oocyte morphology, with defects in yellow.
(G) Oocyte morphology markers scored in mated wt animals that are either reproductive (Repro) or post-reproductive (PR).
(H) Oocyte morphology markers scored in day 8 mated worms. p-values for wild-type versus daf-2 or sma-2 indicated.
For all figures, * indicates p-value < 0.05, ** < 0.01, *** < 0.001.
To examine fertilized embryo quality, we determined embryonic lethality rates. Compared to age-matched daf-2 and sma-2 animals, older wild-type animals produce significantly more embryos that fail to hatch, though they all produced more unhatched embryos with age (Figure 1B, 1E, and S1A) and that are susceptible to damage by bleaching, a test of eggshell integrity (Figure S1C, S1D). Thus, the reproductive span extension exhibited by daf-2 and sma-2 mutants is at least partly a manifestation of increased embryo integrity late in reproduction.
Chromosomal abnormalities, in particular aneuploidies, are a major cause of mammalian embryonic developmental defects (Magli et al., 2007; Rubio et al., 2003), and nondisjunction rates also increase with age in Drosophila (Tokunaga, 1970). Increased chromosomal abnormalities, particularly autosome loss, could contribute to C. elegans embryonic lethality. Meiotic X chromosome non-disjunction produces males (Hodgkin et al., 1979), which in combination with embryonic lethality, provides a simple measure of chromosomal loss (Saito et al., 2009). Strikingly, the fraction of male progeny produced by wild-type mothers increased 16-fold from day 1 to day 5 (Figure 1C). By contrast, the rate of male production by daf-2 and sma-2 mutants was significantly lower (Figure 1C, S1B). To directly test disjunction fidelity, we counted DAPI-stained bodies (Saito et al., 2009) in oocytes of spermless (fem-1) animals. We found that the number of oocytes with the normal number of stained bodies (six bivalents) decreased significantly with age in fem-1, but insignificantly in sma-2;fem-1 and daf-2;fem-1 animals (Figure 1D and S1E), suggesting an increased frequency of chromosomal segregation errors in wild type oocytes with age. Therefore, worms with reduced insulin/IGF-1 and TGF-β Sma/Mab signaling better maintain oocyte chromosome segregation fidelity with age.
Oocyte quality decline is also a cause of human age-related infertility (Goud et al., 1999). To test fertilizability, we mated hermaphrodites with young adult (Day 1 or 2) wild-type males and counted the number of fertilized embryos and unfertilized oocytes produced each day (Figure 1E, S1F), excluding mothers that stopped producing cross-progeny before reproductive cessation. (While fertilized embryos are ovoid with a distinct eggshell, unfertilized oocytes are fuzzy and round (Figure 1E)). Aging wild-type animals produced a significant number of unfertilized oocytes with age, while daf-2 and sma-2 mutants produced almost exclusively successfully fertilized embryos (Figures 1E, S1F). Although daf-2 and sma-2 mutants produce fewer total progeny, such usage-dependent mechanisms as total progeny number, early progeny production, and ovulation rate have been previously eliminated as contributing factors in reproductive aging (Andux and Ellis, 2008; Hughes et al., 2007; Luo et al., 2009). To ensure that sperm is not limiting in our mated assays, we examined oocytes of mated worms for ribonucleoprotein (RNP) foci, which form in sperm-depleted oocytes (Jud et al., 2007); the Day 8 non-reproductive mated worms do not form RNP foci (Figure S1G). This suggests that sufficient sperm are available throughout the reproductive period in our mating experiments, and the unfertilized oocytes that the wild-type worms produce in old age are likely due to lower oocyte quality. Mutations in both the TGF-β Sma/Mab and IIS pathways delay such decline, rendering the oocytes fertilizable longer.
TGF-β Sma/Mab and IIS regulate oocyte morphology maintenance
To determine whether IIS and TGF-β Sma/Mab signaling regulate oocyte morphology maintenance, we examined wild-type and mutant oocytes with age. On Day 1 of adulthood, wild-type oocytes are large and closely packed with their neighboring oocytes (Figure 1F). sma-2 mutants have fewer oocytes aligned in the gonad due to their short length, but the morphology of the oocytes in both the daf-2 and sma-2 mutants is similar to wild type in early adulthood.
On Day 8, when mated wild-type animals have nearly ceased reproduction, their oocytes have visibly degraded: some become much smaller, as previously reported (Andux and Ellis, 2008); some lose contact with their neighbors, resulting in cavities; and others fuse into large clusters packed in the uterus (Figure 1F, Figure S1H-K). The defects were independent of levamisole paralysis treatment used for microscopy (Figure S1L, S1M, S2A, S2B). To test whether these defects are morphological predictors of reproductive capacity, we compared reproductive and post-reproductive wild-type animals; oocytes from the post-reproductive animals were significantly more degraded in terms of oocyte size, cavities, and cluster formation (Figure 1G). By contrast, oocytes in aged daf-2 and sma-2 animals were still young-looking, with significantly fewer morphological defects than age-matched wild type oocytes (Figure 1F, 1H, S1N-S). Thus, reduced TGF-β Sma/Mab and IIS activity both improve oocyte morphology maintenance. Together, our data suggest that oocyte quality, as defined by chromosome segregation fidelity, fertilizability, and morphology, declines with age in C. elegans, and that reduced TGF-β Sma/Mab and IIS signaling delay this decline.
Distal germline morphology and proliferation is maintained in TGF-β Sma/Mab and IIS mutants
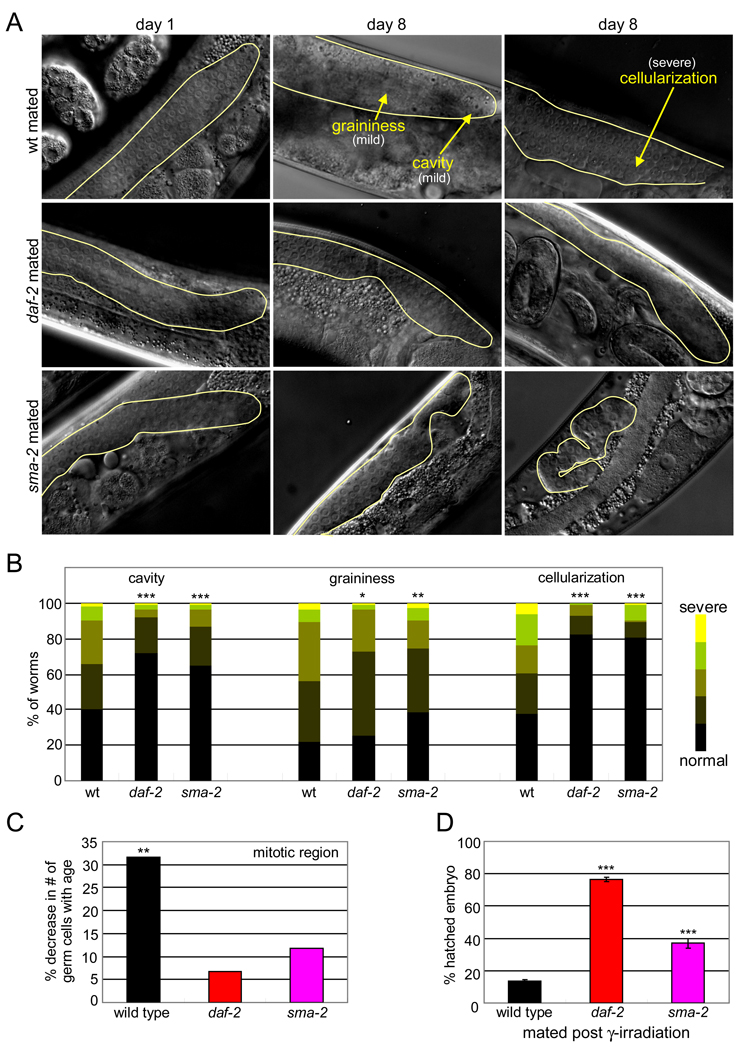
The distal germline undergoes significant morphological decline with age, but IIS mutations significantly slow this deterioration (Garigan et al., 2002)(Figure 2A, 2B, S2C-F). We scored the appearance of cavities, graininess, and cellularization, the major morphological markers of germline aging (Garigan et al., 2002), and found that sma-2 mutations also significantly slow germline deterioration (Figure 2A, 2B, S2G, S2H). While these may be independent effects of the pathways, oocyte and distal germline morphology characteristics in the same population of wild-type worms are correlated (Figure S2J).
Figure 2. TGF-β Sma/Mab and insulin/IGF-1 signaling regulate DNA damage response and distal germline integrity.
(A) Distal germline morphology, with defects in yellow.
(B) Distal germline morphology scores of day 8 mated worms; p-values compare wt versus daf-2 or sma-2. *p < 0.05, ** < 0.01, *** < 0.001.
(C) Percent decrease in mitotic germ cell number with age (raw values in Figure S2I).
(D) daf-2 and sma-2 lay significantly more hatched embryos than wild type after γ-irradiation (% ± SEP). Animals were mated with young wt males after irradiation.
The distal germline contains proliferating germline stem cells (GSCs) and their mitotic descendents. The number of DAPI-stained germ cell nuclei in this zone declines significantly with age in wild-type animals (Figure 2C, S2I), but declines less in daf-2 and sma-2 animals (Figure 2C, S2I), possibly due to better maintenance of proliferative ability. Together, our data suggest that both IIS and TGF-β Sma/Mab signaling may regulate the maintenance of distal germline proliferation and germline quality, as well as oocyte quality.
Physiological and DNA damage-induced apoptosis are not major contributors to TGF-β and IIS regulation of reproductive aging
Prior to cellularization into oocytes, germ cell nuclei undergo programmed cell death (Gumienny et al., 1999). This “physiological germ cell apoptosis” has been proposed to be an important factor in maintaining oocyte quality via resource re-allocation (Andux and Ellis, 2008). We found that sma-2 and daf-2 mutants have higher levels of physiological apoptosis than wild type, but wild-type levels decreased insignificantly with age (Figure S2K).
C. elegans’ germline also undergoes apoptosis as a response to DNA damage from ionizing radiation (Gartner et al., 2000). We examined animals after γ-irradiation, and found that DNA damage-induced apoptosis declined significantly with age in wild-type animals, but the rates in older TGF-β and IIS mutants were not significantly different from wild type (Figure S2L). While neither insulin/IGF-1 nor TGF-β signaling appears to regulate this process, the significant decrease in irradiation-induced apoptosis with age likely contributes to reproductive aging in general.
DNA damage response contributes to reproductive maintenance by TGF-β and IIS
A different aspect of the DNA damage response is improved both by reduced IIS and TGF-β signaling: the number of viable progeny produced after ionizing radiation treatment increased significantly in daf-2 and sma-2 mutants compared to wild type (Figure 2D). The proportion of arrested larvae is also slightly increased in the mutants (Figure S2M), suggesting that even damaged animals are more developmentally competent than the wild-type progeny. Thus, while the rate of DNA-damage induced apoptosis is not increased, sma-2 and daf-2 germ cells may better repair damaged DNA or be better protected against genotoxic stress, which in turn may be partially responsible for slowed reproductive aging.
TGF-β and IIS signaling regulate reproductive aging non-autonomously
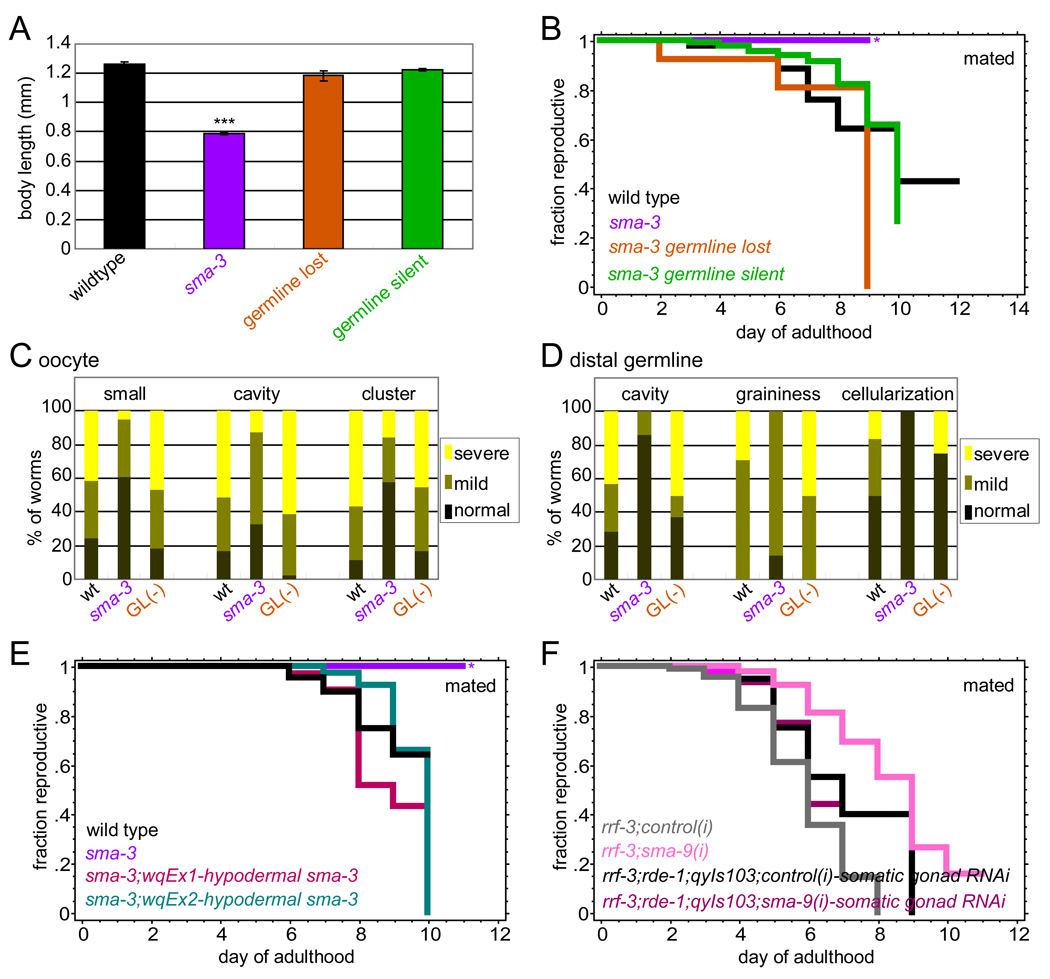
TGF-β Sma/Mab signals cell-autonomously in the hypodermis to regulate body growth (Wang et al., 2002). To test the cell autonomy of TGF-β Sma/Mab signaling in the regulation of reproductive aging, we performed mosaic analyses. Hypodermal expression of the TGF-β Sma/Mab signal transducer SMA-3, which forms a transcriptional complex with SMA-2, is necessary and sufficient for normal body length (Wang et al., 2002). Like sma-2 mutants, sma-3 mutants extend reproductive span (Luo et al., 2009), and maintain oocyte and germline morphology longer with age (Figure 3C, 3D). If reproductive aging is dependent on cell-autonomous TGF-β Sma/Mab signaling in the germline, loss of the sma-3 transgene in the germline alone should recapitulate sma-3 reproductive span extension. Alternatively, if reproductive aging is dependent on somatic (non-autonomous) TGF-β signaling, somatic sma-3 expression should be sufficient to suppress sma-3’s long reproductive span. We screened a synchronized population of sma-3(wk30);qcEx26[sma-3 gDNA;sur-5::gfp] transgenic animals (Wang et al., 2002), selecting worms expressing GFP in most somatic tissues, including hypodermis, but without germline fluorescence (Figure S3A-C) (“germline silent” animals). Because the sma-3 transcript could still be present but undetectable, we also selected somatically fluorescent animals that produced no fluorescent progeny, indicating that they had completely lost the transgenic array in the germline (“germline lost”). As previously reported, somatic sma-3 activity rescued body length (Figure 3A). Surprisingly, both the germline-silent and germline-lost animals had wild-type-like reproductive spans (Figure 3B), indicating that somatic sma-3 expression is sufficient to rescue reproductive span regulation. We also found that the sma-3 germline-silent mosaic animals reduced ovulation rate and progeny number, but have a normal reproductive span (Figure 3B, S3D, S3E), underscoring our previous finding that low ovulation rates and progeny numbers do not extend reproductive span (Luo et al., 2009). Additionally, the morphology of day 8 oocytes and distal germlines of somatic sma-3 animals were more similar to wild type than to sma-3 (Figure 3C, 3D). Thus, TGF-β signaling regulates reproductive aging non-autonomously, signaling from somatic tissues to the germline to maintain quality.
Figure 3. TGF-β Sma/Mab signaling regulates reproductive aging non-autonomously in hypodermis.
(A) Body length of wt, sma-3(wk30), sma-3(wk30);qcEx26[sma-3 gDNA; sur-5::gfp] animals that have lost or silenced transgenic sma-3 expression in the germline (mean ± SEM).
(B) Mated reproductive spans of worms in (A). *high matricide rate due to internal progeny hatching. (All reproductive span statistics are shown in Table S1.)
(C and D) Scoring of oocyte (C) and distal germline (D) morphology markers in day 8 mated wt, sma-3, and sma-3 germline-lost (GL) animals.
(E) Two independent transgenic lines (sma-3(wk30);Pvha-7::gfp::sma-3) expressing sma-3 in the hypodermis have mated reproductive spans similar to wild type (Table S1).
(F) sma-9 RNAi significantly extends mated reproductive span of rrf-3 worms, but does not extend the mated reproductive span of the somatic-gonad-only RNAi strain rrf-3;rde-1;qyIs103[Pfos-1a::rde-1].
Expression of sma-3 under the vha-7 promoter, which is primarily hypodermal, rescues the small body size phenotype of sma-3 mutants (Wang et al., 2002). To determine the tissue-specificity of non-autonomous TGF-β signaling in reproductive aging regulation, we selected large Pvha-7::sma-3;sma-3(wk30) worms (Figure S3F, S3G), and found that the reproductive span extension of sma-3 mutants was also rescued by hypodermal sma-3 expression (Figure 3E, S3H). Because we were concerned that the vha-7 promoter might also express in somatic gonad tissues, we checked the effect of sma-9 RNAi in a somatic-gonad-only RNAi strain (rrf-3;rde-1;qyIs103[Pfos-1a::rde-1+Pmyo-2::yfp])(Hagedorn et al., 2009). Somatic gonad-specific knockdown of TGF-β signaling did not recapitulate the reproductive span extension we observed in whole-animal RNAi (Figure 3F). Together our results suggest that TGF-β signaling in the hypodermis acts autonomously to regulate body size, but non-autonomously to regulate oocyte and distal germline quality maintenance and subsequently, reproductive aging.
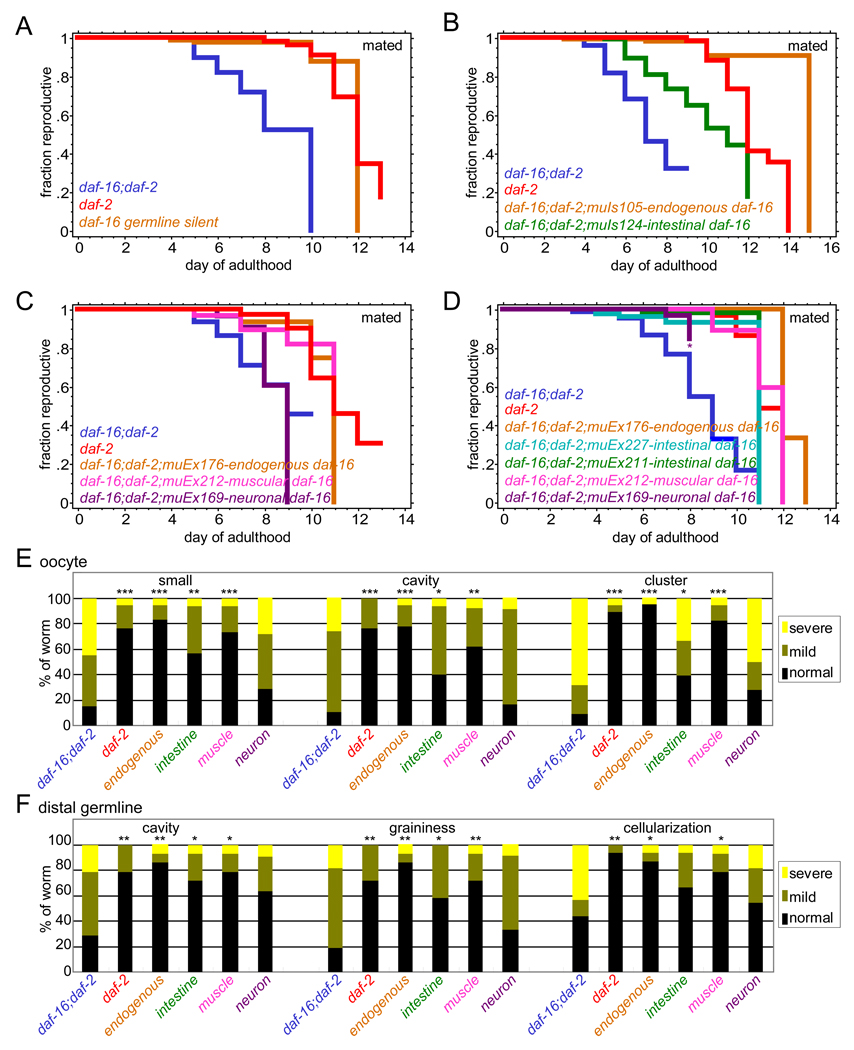
IIS acts both autonomously (Libina et al., 2003) and non-autonomously to regulate life span (Apfeld and Kenyon, 1998; Wolkow et al., 2000). We found that germline silencing of daf-16 activity still allows daf-2-mutant-like reproductive span extension (Figure 4A, S4), suggesting that IIS also acts germline-non-autonomously to regulate reproductive aging. Tissue-specific expression analysis of the DAF-16 transcription factor showed that intestinal expression DAF-16, which increases life span (Libina et al., 2003), also significantly increased reproductive span and improved oocyte and germline morphology of daf-16;daf-2 mutants (Figure 4B, 4D-F). Surprisingly, muscle-expressed DAF-16, which has no effect on life span (Libina et al., 2003) also increased reproductive span and improved germline and oocyte quality significantly (Figure 4C-F), while neuronal DAF-16 had little effect on reproductive aging (Figure 4C-F). Thus, IIS acts non-autonomously to regulate germline and oocyte aging, acting partially in different tissues from its non-autonomous regulation of longevity.
Figure 4. Insulin/IGF-1 signaling regulates reproductive aging non-autonomously in intestine and muscle.
(A) daf-16 germline silent worms (daf-16(mu86);daf-2(e1370);muIs105 [Pdaf-16::gfp::daf-16 +rol-6(su1006)], Figure S4) with only somatic daf-16 activity have a reproductive span similar to daf-2 mutants (statistics in Table S1).
(B-D) daf-16 activity in intestine (B and D) and muscle (C and D) significantly restores reproductive span extension, while neuronal daf-16 activity (C and D) does not.
(E and F) Oocyte (E) and distal germline (F) morphology scores of day 8 mated daf-16;daf-2, daf-2, endogenous-promoter-driven and tissue-specific promoter-driven daf-16 transgenic animals. *p < 0.05, ** < 0.01, *** < 0.001 for daf-16;daf-2 versus other genotypes.
TGF-β Sma/Mab signaling acts in adulthood to regulate reproductive aging
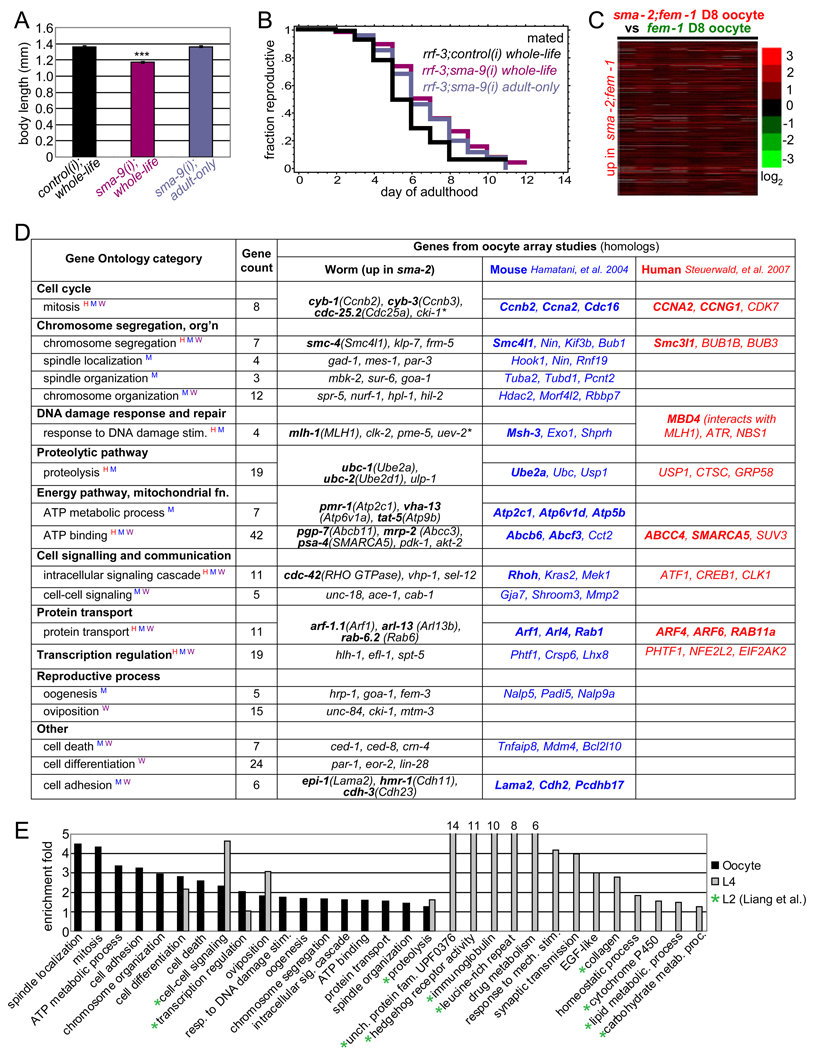
The timing of IIS’ effects on reproduction and longevity largely overlap, acting primarily in adulthood with some contribution to reproduction in late larval stages (Dillin et al., 2002)(Figure S5A, S5B). By contrast, TGF-β signaling acts in earlier larval stages to regulate body size (Liang et al., 2003; Savage-Dunn et al., 2000). To determine the timing of TGF-β regulation of reproductive span, we used RNAi to knock down Sma/Mab signaling in RNAi-sensitive rrf-3 mutants either during the animals’ whole life or only during adulthood. Whole-life sma-9(RNAi) treatment both reduced body size (Figure 5A, S5C) and increased reproductive span (Figure 5B). sma-9(RNAi) treatment only in adulthood, however, did not reduce body size (Figure 5A), but increased reproductive span to the same extent as whole-life sma-9(RNAi) treatment (p=0.46, Figure 5B). Thus, TGF-β signaling’s effects on body size are temporally separable from its effects on reproduction. Additionally, small body size is not required for extended reproductive span through TGF-β signaling. Our tissue specificity and temporal analyses suggest that the downstream effectors that control body size and reproductive aging may be distinct, despite the fact that they are both controlled by TGF-β signaling in the hypodermis.
Figure 5. TGF-β Sma/Mab signaling regulates oocyte quality and body size through distinct sets of downstream targets.
(A) sma-9 RNAi adult-only treatment reduces body size significantly (p<0.001, 14% decrease), while adult-only treatment does not (mean ± SEM).
(B) Mated reproductive spans of rrf-3 animals treated with control RNAi whole-life, with sma-9 RNAi whole-life, or with sma-9 RNAi in adulthood only (Table S1).
(C) Expression heatmap of 386 genes significantly upregulated in sma-2;fem-1 oocytes (FDR=0%).
(D) Enriched GO terms for genes in (C). Example genes from this study (worm) and genes up-regulated in young vs. old mouse (Hamatani et al., 2004) or human (Steuerwald et al., 2007) oocytes are listed, with highly homologous or important interacting genes in bold. (Expanded gene list in Table S3.) GO terms also enriched in younger human (H), mouse (W), or worm (W) oocytes are labeled with corresponding superscript letters. * indicates a gene involved in corresponding GO function but failed to be recognized by DAVID (not included in gene counts).
(E) GO terms enriched in TGF-β Sma/Mab mutant oocytes are largely distinct from those enriched in Sma/Mab L4 and L2 (Liang et al., 2007) larvae.
TGF-β oocyte quality targets are shared with mammalian oocyte aging genes
To identify the targets of TGF-β Sma/Mab signaling that regulate reproductive aging, we compared the transcription of unfertilized oocytes isolated from day 8 spermless fem-1 and sma-2;fem-1 worms (Figure S5D). Gene Ontology (GO) analysis of significantly-upregulated and downregulated TGF-β oocyte genes (Figure 5C, S5F; Table S2A, S2B) identified such categories as oogenesis, cell cycle, chromosome segregation and organization, DNA damage response, proteolysis, ATP binding, signaling, transcription regulation, protein transport, aging, GTP binding, and oxidoreductases, etc (Figure 5D, 5E, S5G, Table S3 and S4). >70% of the sma-2-regulated genes are regulated in the same direction in young relative to old (day 8) fem-1 oocytes (Figure S5E, S5F), and similar GO terms are also enriched (Figure 5D), suggesting that these genes are good markers of the “youthfulness” of oocytes.
A striking number of the genes and GO terms identified in our array analysis of sma-2;fem-1 and fem-1 oocytes that were associated with “youthful” oocytes are shared with genes downregulated in aging mouse and human oocytes (Hamatani et al., 2004; Steuerwald et al., 2007) (Figure 5D), such as mitotic cell cycle regulation, chromosome segregation, response to DNA damage, proteolysis, ATP binding, signaling, transcriptional regulation, and protein transport. The condensin SMC is upregulated in sma-2 oocytes, and declines in both mouse and human oocytes with age, suggesting that chromosome segregation is a shared key determinant of oocyte quality. Cell cycle regulators (CYB-1/3), and DNA mismatch repair proteins (e.g., MLH-1, MBD4) are also higher in sma-2 oocytes and decline with age in mammalian oocytes. Interestingly, several TGF-β signaling genes are upregulated with age in mouse oocytes (Hamatani et al., 2004), paralleling our observations on the extension of reproductive span in C. elegans TGF-β mutants.
In addition to the genes that are shared between sma-2 mutants and age-regulated in mouse and human oocytes, our analysis has uncovered new genes that are potential regulators of reproductive aging. lin-28, which is important in reproductive development regulation (Hartge, 2009) and the reprogramming of differentiated cells into induced pluripotent stem cells (Nimmo and Slack, 2009), and clk-2, a telomere length regulator that is involved in DNA damage response and cell cycle checkpoint, are also significantly upregulated in sma-2 oocytes (Table S2A). Several Class 2 longevity genes, including dod-23 and dod-24 (Murphy et al., 2003), as well as many oxidoreductases and protein metabolism genes, are significantly downregulated in sma-2 oocytes (Table S2B), suggesting additional novel mechanisms that may contribute to the regulation of oocyte aging. Finally, expression of the insulin-like peptide genes ins-22 and ins-23 is significantly upregulated in sma-2 oocytes, while ins-7 (Murphy et al., 2003) is downregulated, possibly indicating insulin signaling from the oocytes themselves.
TGF-β somatic and oocyte transcriptional targets are distinct
Sma/Mab L2 transcriptional targets regulate body size and male tail patterning (Liang et al., 2007). We compared the expression profiles of Sma/Mab mutant and wild-type early L4 whole animals, prior to oocyte development; these targets are similar to Liang, et al.’s L2 targets (Figure 5E). In contrast to sma-2 oocyte gene expression, the genes upregulated in Sma/Mab larvae (Table S2C) include the GO terms of hedgehog signaling, immunoglobulin domain proteins, leucine-rich repeat proteins, cuticle collagens, and lipid and carbohydrate metabolism genes (Figure 5E). Thus, both at the gene and GO term level, the targets of Sma/Mab signaling in body size and oocyte quality regulation are largely non-overlapping (Figure 5E).
TGF-β oocyte targets are required for reproductive quality maintenance
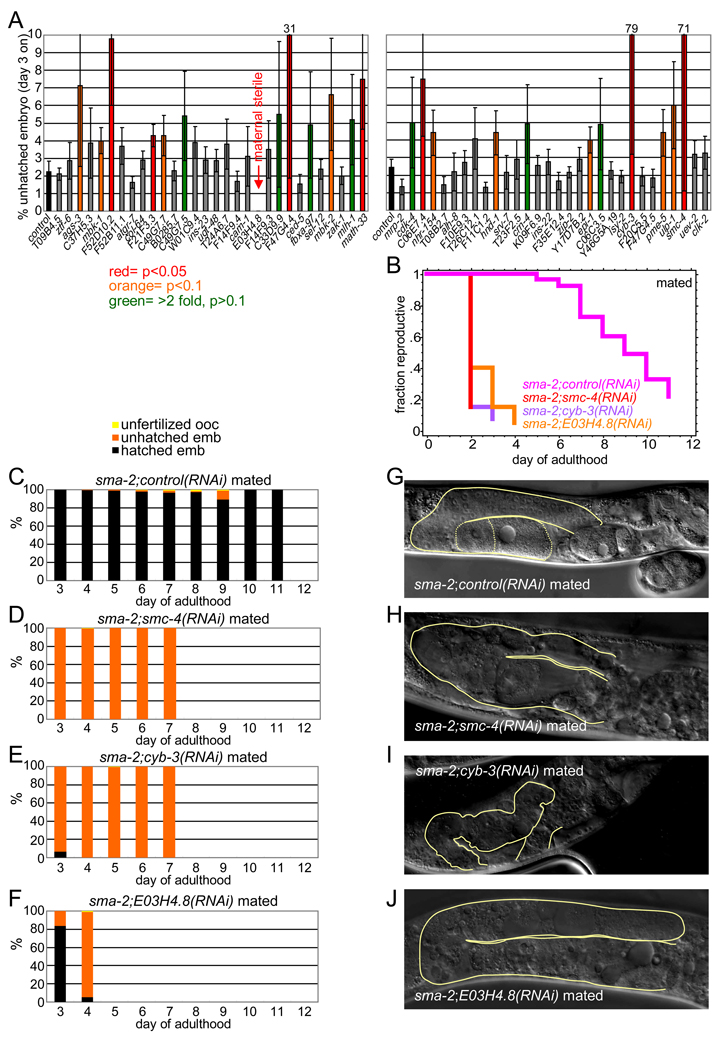
To test candidate genes for their roles in reproduction, we used RNAi knockdown to screen the top-ranking oocyte target genes for their effects on sma-2 late embryo hatching, reasoning that loss of important sma-2-upregulated genes might reduce reproductive success. Of 60 genes tested, 27 reduced sma-2 embryo hatching rates (Figures 6A, S6A). We then tested the genes with the strongest hatching effects for their contributions to reproductive span determination and embryo/oocyte quality (Figures 6B-J, S6). Three genes, smc-4 (condensin, structural maintenance of chromosomes), cyb-3 (cyclin B, sister chromatid segregation), and E03H4.8 (unknown, predicted vesicle coat complex), shortened sma-2 reproductive span substantially, from sma-2’s mean of 9 days to <3 (Figure 6B). We found that these “early effect” genes also had severe effects on sma-2 embryonic viability, producing almost exclusively unhatched embryos (Figure 6C-F, S6B). These genes are critical for oocyte quality, as knocking them down in wild type also resulted in severe effects on embryonic viability (Figure S6D-F). Knockdown of these genes also severely affected germline and oocyte morphology; oocytes were largely unidentifiable, distal germline cells were not well-defined, and the gonads themselves were misshapen (Figure 6G-J, S6C). The loss of other sma-2-oocyte regulated genes also increased the rate of unhatched embryos and/or unfertilized ooctyes with age in both sma-2 and wild type, but later or more mildly (Figure 7B-D, S7); these include math-33 (putative apoptosis gene), F47G4.4 (putative chromosome segregation gene), F52D10.2 and C06E7.4 (both unknown), and F21F3.3 (methyltransferase).
Figure 6. TGF-β Sma/Mab signaling regulates genes essential for embryonic viability in oocytes.
(A) RNAi knockdown of many sma-2-regulated oocyte targets increase sma-2 mutant’s embryonic lethality (mean ± SEM).
(B) RNAi knockdown of smc-4, cyb-3, and E03H4.8 have early and severe effects on reproductive span (Table S1).
(C-F) RNAi knockdown of smc-4, cyb-3, and E03H4.8 greatly increase the percentage of unhatched embryos (orange) in mated sma-2 mutants (compare D-F with C). Wild type treated with RNAis shown in Figure S6D-F.
(G-J) smc-4, cyb-3, and E03H4.8 RNAi-treated sma-2 animals exhibit severely degraded germlines at day 8 (compare H-J with G). Contours of gonads shown in yellow, visible oocytes outlined by dotted lines in (G).
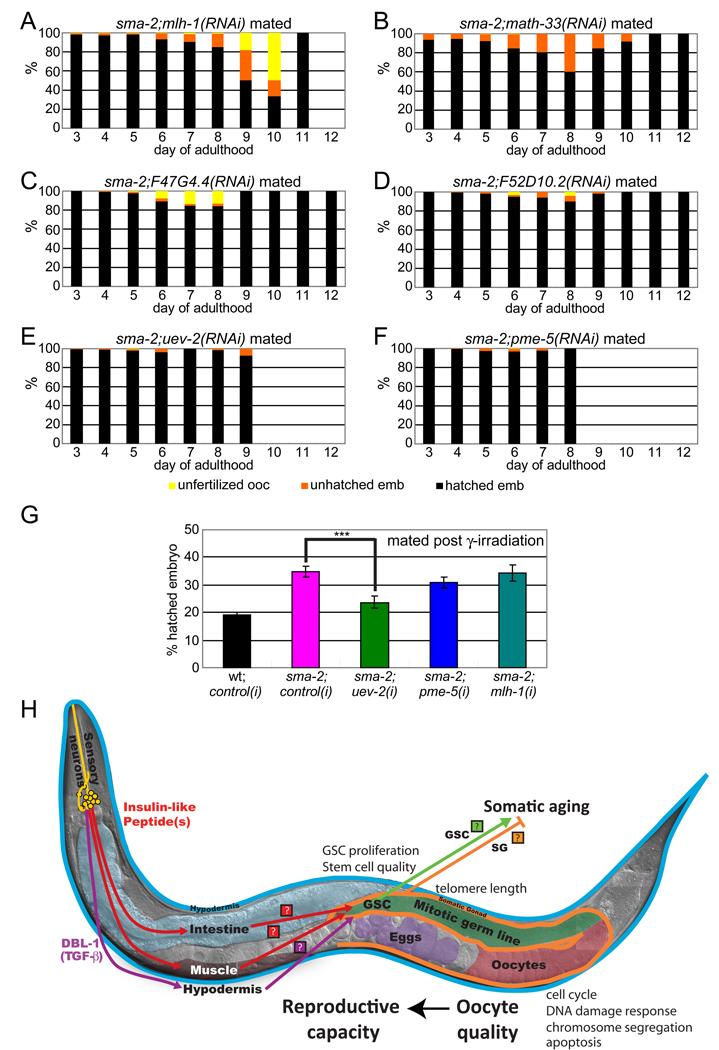
Figure 7. TGF-β Sma/Mab signaling regulates genes important for age-associated oocyte quality maintenance.
(A-F) RNAi treatments of TGF-β target genes accelerate oocyte quality decline, increasing the percentage of unhatched embryos (orange) and/or unfertilized oocytes (yellow) earlier in life (compare with Figure 6C). mlh-1, math-33, and F47G4.4 RNAis have greater effects (A-C), while F52D10.2, uev-2, and pme-5 have milder effects (D-F). Wild type treated with RNAis shown in Figure S7C-F, I-J).
(G) uev-2 RNAi treatment significantly increases sma-2’s production of unhatched embryos after γ-irradiation, while pme-5 and mlh-1 do not. Animals were mated with young wt males after irradiation.
(H) Model of reproductive aging regulation by the TGF-β Sma/Mab (pink) and insulin/IGF-1 signaling (red) pathways. Ligands (Insulin-Like Peptides, TGF-β DBL-1) are secreted neuronally and mediate signaling to the soma (hypodermis, intestine, and muscle), generating as yet unidentified secondary signals to regulate reproduction. These secondary signals block distal germline and oocyte integrity maintenance with age, resulting in germline morphology decline, slowed germ cell proliferation, and a decline in oocyte quality. Downstream effectors in oocytes include chromosome segregation, cell cycle, and DNA damage response/repair genes, etc. Declines in embryonic viability and infertility mark reproductive cessation. The germline and somatic gonad regulate somatic aging (Hsin and Kenyon, 1999), suggesting a bi-directional signaling flow in the coordination of somatic and germline aging. (Photo courtesy of Ian Chin-Sang.)
Because we had observed that progeny survival after DNA damage was increased in sma-2 mutants (Figure 2D), and a number of the DNA damage response genes upregulated in sma-2 oocytes and were required for embryo viability (Figure 5D, Table S3), we investigated these genes’ effects on sma-2’s oocyte quality and post-γ-irradiation embryonic lethality. We found that loss of mlh-1, a DNA mismatch repair homolog of human MLH1, increased the rate of unhatched embryos and unfertilized oocytes late in sma-2 reproduction (days 7–10, Figure 7A, 6A, S6A). Loss of uev-2 (stress/DNA damage response) and pme-5 (PARP/tankyrase) had milder effects on hatching (Figures 7E, 7F, 6A and S6A). However, loss of uev-2 had a significant effect on post-γ-irradiation sma-2 embryonic lethality (Figure 7G).
Together, our expression results show that sma-2 regulates a distinct set of genes in oocytes from its targets in body size determination. Furthermore, our functional analyses of sma-2 oocyte targets suggest several mechanisms that are required for successful extended reproduction, and many of these mechanisms are shared with mammalian oocytes. Chromosome segregation and cell cycle genes are more highly expressed in sma-2 than in wild-type worms, and their loss causes severe functional and morphological germline defects, suppressing reproduction. DNA damage response and other novel genes, by contrast, are required later in reproduction to maintain reproductive fidelity.
DISCUSSION
Here we have systematically examined the processes involved in reproduction, from embryonic viability through distal germline morphology, to determine which are most susceptible to aging and which are altered in mutants with extended reproductive spans. Our data establish that oocyte and distal germline quality correlate well with reproductive success, and that TGF-β Sma/Mab and Insulin/IGF-1 signaling regulate reproductive aging primarily through their control of these aspects of reproduction.
A model for TGF-β Sma/Mab and IIS regulation of Reproductive Aging
Our mosaic and hypodermal rescue data suggest that the TGF-β pathway regulates reproductive aging through an interaction between the soma and germline. We previously showed that TGF-β signaling regulates reproductive aging independently of such somatically-controlled mechanical processes as ovulation and body growth (Luo et al., 2009), and our mosaic analysis supports this uncoupling of reproductive span and ovulation (Figure S3D, S3E). Thus, the interaction between the soma and germline to regulate reproductive aging is likely to be mediated by molecular signals. These secondary signals must originate in somatic (hypodermal) tissues downstream of TGF-β signaling, and subsequently act in the germline to control quality (Figure 7H). Similarly, IIS acts in the muscle and intestine to regulate germline and oocyte maintenance. Although the specific signals have not yet been identified, insulin-like peptides are regulated by IIS and coordinate the state of the insulin pathway between tissues (Murphy et al., 2007), and a nuclear hormone receptor is required for starvation-induced adult reproductive diapause (Angelo and Van Gilst, 2009). Together with our data, the observation that signals from the germline and somatic gonad regulate longevity (Flatt et al., 2008; Ghazi et al., 2009; Hsin and Kenyon, 1999), suggests a bi-directional flow of information between somatic and reproductive tissues normally coordinates their rates of aging.
The germline and reproductive aging
TGF-β Sma/Mab and IIS mutations prevent age-related decline in the integrity of the distal germline-containing germline stem cells, and the quality of the distal germline and oocytes are correlated (Figure S2J). Interestingly, germline stem cells protected by starvation have the capacity to re-generate and re-establish reproduction, even after a long period of quiescence (Angelo and Van Gilst, 2009). Although this is the first report of C. elegans TGF-β signaling possibly regulating germline stem cell activity in C. elegans, TGF-β/BMP signaling is known to affect GSC development in other organisms, including Drosophila germline and mammalian muscles (Carlson et al., 2009; Yamashita et al., 2005; Zhao et al., 2008). The upregulation of LIN-28, a key regulator of stem cell induction, in the TGF-β mutant reproductive system is particularly intriguing.
TGF-β Sma/Mab signaling regulates reproductive aging distinctly from body growth
Although TGF-β Sma/Mab signaling regulates both body growth and reproductive aging, the downstream molecular mechanisms of these two processes are distinct. First, Sma/Mab signaling is required for body size regulation during development, before gametogenesis (Liang et al., 2003; Savage-Dunn et al., 2000), while Sma/Mab regulation of germ line aging is carried out in adulthood (Figure 5B). Second, body size and reproductive span are not correlated (Luo et al., 2009). Furthermore, despite the fact that Sma/Mab activity in the hypodermis directs both body growth and oocyte quality, the Sma/Mab pathway has distinct transcriptional targets in the body and oocytes. Interestingly, we find that these oocyte-specific targets can be separated into early and late-effect genes, with chromosome segregation and cell cycle genes having early and severe effects on reproductive tissues, and DNA damage response genes primarily regulating late effects. The late effects are particularly interesting, as they are most likely to become increasingly important as ooyctes age.
C. elegans as a model of reproductive aging
Although worms and humans have vastly different life spans and reproductive strategies, the cellular and molecular bases of reproductive span regulation are strikingly similar. As we have shown here for C. elegans, oocyte quality decline is the major reason for human reproductive capacity decline, resulting in sterility and developmental birth defects. Chromosomal abnormalities, in particular aneuploidies, are the major defect in human embryos from aging mothers (te Velde and Pearson, 2002). Worms also increase chromosome non-disjunction rates with age (Rose and Baillie, 1979; Tang et al., 2010)(Figure 1B-D), and we find that mutants with extended reproductive success significantly reduce chromosomal non-disjunction rates. Oocyte fertilizability, stress resistance, and morphology are compromised with age in humans (Blondin et al., 1997; Goud et al., 1999); we found this is also the case for C. elegans, but these declines are delayed in TGF-β and IIS mutants. Finally, our oocyte transcriptional and functional analyses show that genes upregulated in TGF-β mutants are strikingly similar to mammalian oocyte genes that decline with age, suggesting that many of the molecular mechanisms underlying reproductive cessation are shared between C. elegans and humans. Therefore, C. elegans not only regulates reproductive aging through oocyte quality control, as do humans, but also, such control is mediated through the regulation of similar oocyte quality maintenance mechanisms.
The fact that both Insulin/IGF-1 and TGF-β signaling, two pathways that are evolutionarily conserved from worms to humans, have significant roles in regulating the rate of reproductive decline and utilize similar mechanisms, suggests that these pathways are also likely to be important in the regulation of human reproductive decline. Several recent genome-wide association studies of human reproductive development and menopause identified genes that regulate development and longevity in C. elegans (Ong et al., 2009; Stolk et al., 2009). These genes include FOXO3a, the human homolog of the DAF-16/FoxO transcription factor downstream of the Insulin/IGF-1 signaling pathway, and LIN-28, which we find is regulated by TGF-β signaling in oocytes.
TGF-β signaling has also been implicated in several aspects of mammalian reproduction and reproductive aging. TGF-β members are upregulated in mouse oocytes with age (Hamatani et al., 2004) and many TGF-β superfamily ligands regulate folliculogenesis (Knight and Glister, 2006; Trombly et al., 2009). While humans have a more complex TGF-β pathway family that carries out many different functions, it is likely that TGF-β signaling may be involved in regulation of reproductive cessation. Therefore, the similarities in the regulation of reproductive aging in worms and humans may allow us to use worms as genetic and molecular models to study this important human problem, enabling the development of therapies to address maternal age-related birth defects and reproductive decline.
EXPERIMENTAL PROCEDURES
*Expanded Experimental Procedures are presented in Supplemental Information, and include C. elegans strains used and analyses of embryonic lethality, male progeny production, chromosome bivalents, oocyte fertilizability, RNP foci, mitotic germ cell number, physiological and irradiation-induced apoptosis, reproductive span, ovulation rate, body length, and temporal RNAi effects.
Oocyte morphology analysis
For each oocyte image, a score was assigned for each of the three signs of deterioration (cavities, graininess, and cellularization), based on the severity of the phenotype, with 1 = normal, 2 = mild, 3 = severe. Mann-Whitney analysis was used to determine whether there were significant differences in pairwise comparisons. Three individuals who were blind to the genotypes scored the images independently.
Distal germline morphology analysis
For each distal germline image, a score was assigned for each of the three signs of deterioration (cavities, graininess, and cellularization), based on the severity of the phenotype, with 1 = normal, and 5 = most severe, by four individuals (3 were blind to the genotypes) and averaged. Mann-Whitney (pairwise) analyses were used as described above.
Immunostaining
Staining with RME-2 antibody, a gift from Dr. Barth Grant, was performed as described previously (Grant and Hirsh, 1999).
Mosaic analysis
Developmentally synchronized sma-3(wk30) III;qcEx26 X [pCS29+sur-5::gfp] worms with somatic GFP expression were picked; green fluorescence in tissues including hypodermis, intestine, neurons, but not germline, was verified at high magnification (Figure S3A-C). Worms were screened for large body size before mating. Animals with no fluorescent progeny are “germline-lost” worms.
Hypodermal rescue strain construction
sma-3(wk30) were injected with pCS227[Pvha-7::sma-3] at 90 ng/µl (strains and plasmid kindly provided by Dr. Cathy Savage-Dunn) with Pmyo-2::mCherry (PFC590, Addgene) as a co-injection marker (5 ng/µl). Large F1s were picked to establish independent lines for follow-up analysis.
Oocyte and L4 microarrays
Hypochlorite-synchronized wild-type and sma-2 or sma-4 larvae were collected at mid-L4. Oocytes were isolated (Miller, 2006) from fem-1 and sma-2;fem-1 animals on day 8 of adulthood; RNA was extracted, cRNA was linearly amplified, Cy3/Cy5 labeled, hybridized to the Agilent 44k C. elegans microarray, and analyzed as previously described (Shaw et al., 2007). GO analysis was performed using DAVID (Dennis et al., 2003; Huang et al., 2008) on significantly differentially expressed genes (FDR=0%, SAM (Tusher et al., 2001)). The raw microarray data set is publically available through PUMAdb (http://puma.princeton.edu).
Supplementary Material
ACKNOWLEDGMENTS
We thank Cathy Savage-Dunn (CUNY) for CS122 and pCS227; David Sherwood (Duke University) for NK640; Barth Grant (Rutgers) for RT130 and α-RME-2 antibody; members of the Murphy Lab & Z. Gitai for comments on the manuscript; and March of Dimes Basil O’Connor Starting Scholar and NIH New Innovator (1DP2OD004402-01) awards for funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andux S, Ellis RE. Apoptosis maintains oocyte quality in aging Caenorhabditis elegans females. PLoS Genet. 2008;4 doi: 10.1371/journal.pgen.1000295. e1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelo G, Van Gilst MR. Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science. 2009 doi: 10.1126/science.1178343. 1178343. [DOI] [PubMed] [Google Scholar]
- Apfeld J, Kenyon C. Cell nonautonomy of C. elegans daf-2 function in the regulation of diapause and life span. 1998;95:199–210. doi: 10.1016/s0092-8674(00)81751-1. [DOI] [PubMed] [Google Scholar]
- Blondin P, Coenen K, Sirard MA. The impact of reactive oxygen species on bovine sperm fertilizing ability and oocyte maturation. J Androl. 1997;18:454–460. [PubMed] [Google Scholar]
- Cant MA, Johnstone RA. Reproductive conflict and the separation of reproductive generations in humans. Proceedings of the National Academy of Sciences. 2008;105:5332–5336. doi: 10.1073/pnas.0711911105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson ME, Conboy MJ, Hsu M, Barchas L, Jeong J, Agrawal A, Mikels AJ, Agrawal S, Schaffer DV, Conboy IM. Relative roles of TGF-beta and Wnt in the systemic regulation and aging of satellite cell responses. Aging Cell. 2009;8:676–689. doi: 10.1111/j.1474-9726.2009.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis G, Sherman B, Hosack D, Yang J, Gao W, Lane H, Lempicki R. DAVID: database for annotation, visualization, and integrated discovery. Genome Biology. 2003;4:R60. [PubMed] [Google Scholar]
- Dillin A, Crawford DK, Kenyon C. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science. 2002;298:830–834. doi: 10.1126/science.1074240. [DOI] [PubMed] [Google Scholar]
- Flatt T, Min K-J, D'Alterio C, Villa-Cuesta E, Cumbers J, Lehmann R, Jones DL, Tatar M. Drosophila germ-line modulation of insulin signaling and lifespan. Proceedings of the National Academy of Sciences. 2008;105:6368–6373. doi: 10.1073/pnas.0709128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garigan D, Hsu A-L, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Genetic analysis of tissue aging in Caenorhabditis elegans: a role for heat-shock factor and bacterial proliferation. Genetics. 2002;161:1101–1112. doi: 10.1093/genetics/161.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner A, Milstein S, Ahmed S, Hodgkin J, Hengartner MO. A conserved checkpoint pathway mediates DNA damage induced apoptosis and cell cycle arrest in C. elegans. 2000;5:435–443. doi: 10.1016/s1097-2765(00)80438-4. [DOI] [PubMed] [Google Scholar]
- Ghazi A, Henis-Korenblit S, Kenyon C. A transcription elongation factor that links signals from the reproductive system to lifespan extension in Caenorhabditis elegans. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000639. e1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goud P, Goud A, Van Oostveldt P, Van der Elst J, Dhont M. Fertilization abnormalities and pronucleus size asynchrony after intracytoplasmic sperm injection are related to oocyte postmaturity. Fertility and Sterility. 1999;72:245–252. doi: 10.1016/s0015-0282(99)00231-9. [DOI] [PubMed] [Google Scholar]
- Grant B, Hirsh D. Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol Biol Cell. 1999;10:4311–4326. doi: 10.1091/mbc.10.12.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein D. Control of oocyte meiotic maturation and fertilization. In: Kimble J, Strome S, editors. WormBook. The C. elegans Research Community; 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumienny T, Lambie E, Hartwieg E, Horvitz H, Hengartner M. Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development. 1999;126:1011–1022. doi: 10.1242/dev.126.5.1011. [DOI] [PubMed] [Google Scholar]
- Hagedorn EJ, Yashiro H, Ziel JW, Ihara S, Wang Z, Sherwood DR. Integrin acts upstream of netrin signaling to regulate formation of the anchor cell's invasive membrane in C. elegans. 2009;17:187–198. doi: 10.1016/j.devcel.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamatani T, Falco G, Carter MG, Akutsu H, Stagg CA, Sharov AA, Dudekula DB, VanBuren V, Ko MS. Age-associated alteration of gene expression patterns in mouse oocytes. Hum Mol Genet. 2004;13:2263–2278. doi: 10.1093/hmg/ddh241. [DOI] [PubMed] [Google Scholar]
- Hartge P. Genetics of reproductive lifespan. Nat Genet. 2009;41:637–638. doi: 10.1038/ng0609-637. [DOI] [PubMed] [Google Scholar]
- Hodgkin J, Horvitz HR, Brenner S. Nondisjunction mutants of the nematode Caenorhabditis Elegans. Genetics. 1979;91:67–94. doi: 10.1093/genetics/91.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399:362–366. doi: 10.1038/20694. [DOI] [PubMed] [Google Scholar]
- Huang C, Xiong C, Kornfeld K. Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8084–8089. doi: 10.1073/pnas.0400848101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protocols. 2008;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Hughes SE, Evason K, Xiong C, Kornfeld K. Genetic and pharmacological factors that influence reproductive aging in nematodes. PLoS Genet. 2007;3:e25. doi: 10.1371/journal.pgen.0030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jud M, Razelun J, Bickel J, Czerwinski M, Schisa J. Conservation of large foci formation in arrested oocytes of Caenorhabditis nematodes. Development Genes and Evolution. 2007;217:221–226. doi: 10.1007/s00427-006-0130-3. [DOI] [PubMed] [Google Scholar]
- Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132:191–206. doi: 10.1530/rep.1.01074. [DOI] [PubMed] [Google Scholar]
- Liang J, Lints R, Foehr ML, Tokarz R, Yu L, Emmons SW, Liu J, Savage-Dunn C. The Caenorhabditis elegans schnurri homolog sma-9 mediates stage- and cell type-specific responses to DBL-1 BMP-related signaling. Development. 2003;130:6453–6464. doi: 10.1242/dev.00863. [DOI] [PubMed] [Google Scholar]
- Liang J, Yu L, Yin J, Savage-Dunn C. Transcriptional repressor and activator activities of SMA-9 contribute differentially to BMP-related signaling outputs. Developmental Biology. 2007;305:714–725. doi: 10.1016/j.ydbio.2007.02.038. [DOI] [PubMed] [Google Scholar]
- Libina N, Berman JR, Kenyon C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell. 2003;115:489–502. doi: 10.1016/s0092-8674(03)00889-4. [DOI] [PubMed] [Google Scholar]
- Luo S, Shaw WM, Ashraf J, Murphy CT. TGF-beta Sma/Mab signaling mutations uncouple reproductive aging from somatic aging. Plos Genet. 2009;5 doi: 10.1371/journal.pgen.1000789. e1000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magli MC, Gianaroli L, Ferraretti AP, Lappi M, Ruberti A, Farfalli V. Embryo morphology and development are dependent on the chromosomal complement. Fertility and Sterility. 2007;87:534–541. doi: 10.1016/j.fertnstert.2006.07.1512. [DOI] [PubMed] [Google Scholar]
- Massagué J. How cells read TGF-[beta] signals. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM. Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction. 2005;130:791–799. doi: 10.1530/rep.1.00793. [DOI] [PubMed] [Google Scholar]
- Miller MA. Sperm and oocyte isolation methods for biochemical and proteomic analysis. 2006:193–201. doi: 10.1385/1-59745-151-7:193. In. [DOI] [PubMed] [Google Scholar]
- Murphy CT, Lee S-J, Kenyon C. Tissue entrainment by feedback regulation of insulin gene expression in the endoderm of Caenorhabditis elegans. Proceedings of the National Academy of Sciences. 2007;104:19046–19050. doi: 10.1073/pnas.0709613104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Nimmo R, Slack F. An elegant miRror: microRNAs in stem cells, developmental timing and cancer. Chromosoma. 2009;118:405–418. doi: 10.1007/s00412-009-0210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong KK, Elks CE, Li S, Zhao JH, Luan Ja, Andersen LB, Bingham SA, Brage S, Smith GD, Ekelund U, et al. Genetic variation in LIN28B is associated with the timing of puberty. Nat Genet. 2009;41:729–733. doi: 10.1038/ng.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AM, Baillie DL. The effect of temperature and parental age on recombination and nondisjunction in Caenorhabditis elegans. Genetics. 1979;92:409–418. doi: 10.1093/genetics/92.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio C, Simon C, Vidal F, Rodrigo L, Pehlivan T, Remohi J, Pellicer A. Chromosomal abnormalities and embryo development in recurrent miscarriage couples. Hum Reprod. 2003;18:182–188. doi: 10.1093/humrep/deg015. [DOI] [PubMed] [Google Scholar]
- Saito TT, Youds JL, Boulton SJ, Colaiacovo MP. Caenorhabditis elegans HIM-18/SLX-4 interacts with SLX-1 and XPF-1 and maintains genomic integrity in the germline by processing recombination intermediates. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000735. e1000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage-Dunn C. TGF-β signaling. In: Greenwald I, editor. WormBook. The C. elegans Research Community, WormBook; 2005. [Google Scholar]
- Savage-Dunn C, Tokarz R, Wang H, Cohen S, Giannikas C, Padgett RW. SMA-3 Smad has specific and critical functions in DBL-1/SMA-6 TGF-beta-related signaling. Developmental Biology. 2000;223:70–76. doi: 10.1006/dbio.2000.9713. [DOI] [PubMed] [Google Scholar]
- Shaw WM, Luo S, Landis J, Ashraf J, Murphy CT. The C. elegans TGFbeta Dauer pathway regulates longevity via insulin signaling. Curr Biol. 2007;17:1635–1645. doi: 10.1016/j.cub.2007.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuerwald NM, Bermudez MG, Wells D, Munne S, Cohen J. Maternal age-related differential global expression profiles observed in human oocytes. Reprod Biomed Online. 2007;14:700–708. doi: 10.1016/s1472-6483(10)60671-2. [DOI] [PubMed] [Google Scholar]
- Stolk L, Zhai G, van Meurs JBJ, Verbiest MMPJ, Visser JA, Estrada K, Rivadeneira F, Williams FM, Cherkas L, Deloukas P, et al. Loci at chromosomes 13, 19 and 20 influence age at natural menopause. Nat Genet. 2009;41:645–647. doi: 10.1038/ng.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh Y, Atzmon G, Cho M-O, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proceedings of the National Academy of Sciences. 2008;105:3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Machacek T, Mamnun YM, Penkner A, Gloggnitzer J, Wegrostek C, Konrat R, Jantsch M, Loidl J, Jantsch V. Mutations in Caenorhabditis elegans him-19 show meiotic defects that worsen with age. Mol Biol Cell. 2010 doi: 10.1091/mbc.E09-09-0811. E09-09-0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update. 2002;8:141–154. doi: 10.1093/humupd/8.2.141. [DOI] [PubMed] [Google Scholar]
- Tokunaga C. The effects of low temperature and aging on nondisjunction in Drosophila. Genetics. 1970;65:75–94. doi: 10.1093/genetics/65.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombly DJ, Woodruff TK, Mayo KE. Roles for transforming growth factor beta superfamily proteins in early folliculogenesis. Semin Reprod Med. 2009;27:014–023. doi: 10.1055/s-0028-1108006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Tokarz R, Savage-Dunn C. The expression of TGF-beta signal transducers in the hypodermis regulates body size in C. elegans. Development. 2002;129:4989–4998. doi: 10.1242/dev.129.21.4989. [DOI] [PubMed] [Google Scholar]
- Wolkow CA, Kimura KD, Lee MS, Ruvkun G. Regulation of C. elegans life-span by insulinlike signaling in the nervous system. Science. 2000;290:147–150. doi: 10.1126/science.290.5489.147. [DOI] [PubMed] [Google Scholar]
- Yamashita YM, Fuller MT, Jones DL. Signaling in stem cell niches: lessons from the Drosophila germline. J Cell Sci. 2005;118:665–672. doi: 10.1242/jcs.01680. [DOI] [PubMed] [Google Scholar]
- Zhao R, Xuan Y, Li X, Xi R. Age-related changes of germline stem cell activity, niche signaling activity and egg production in Drosophila. Aging Cell. 2008;7:344–354. doi: 10.1111/j.1474-9726.2008.00379.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.