Abstract
Because nuclear factor-κB (NF-κB) is a ubiquitously expressed proinflammatory transcription factor that regulates the expression of over 500 genes involved in cellular transformation, survival, proliferation, invasion, angiogenesis, metastasis, and inflammation, the NF-κB signaling pathway has become a potential target for pharmacological intervention. A wide variety of agents can activate NF-κB through canonical and noncanonical pathways. Canonical pathway involves various steps including the phosphorylation, ubiquitnation, and degradation of the inhibitor of NF-κB (IκBα), which leads to the nuclear translocation of the p50- p65 subunits of NF-κB followed by p65 phosphorylation, acetylation and methylation, DNA binding, and gene transcription. Thus, agents that can inhibit protein kinases, protein phosphatases, proteasomes, ubiquitnation, acetylation, methylation, and DNA binding steps have been identified as NF-κB inhibitors. Here, we review the small molecules that suppress NF-κB activation and thus may have therapeutic potential.
Keywords: Inflammation, NF-κB, small molecule inhibitors, therapeutics
1. Introduction
The nuclear factor-κB (NF-κB) signaling pathway plays a major role in the development, maintenance, and progression of most chronic diseases. NF-κB controls the expression of genes involved in a number of physiological responses, including immune inflammatory responses, acute-phase inflammatory responses, oxidative stress responses, cell adhesion, differentiation, and apoptosis [1]. Recent studies have suggested that NF-κB dysregulation is associated with many diseases including AIDS, atherosclerosis, asthma, arthritis, diabetes, inflammatory bowel disease, stroke, muscle wasting and viral infections. Mounting evidence indicates that NF-kB acts as a link between inflammation and cancer progression [2–10], making NF-κB essential to and a potential drug target in hematological malignancies and solid tumors [11, 12]. NF-κB was first identified in 1986 by Sen and Baltimore [5] in the nucleus bound to an enhancer element of the immunoglobulin kappa light chain gene in B cells [5, 13]. It is now known to be ubiquitous in nature present in all the cell types and is evolutionary conserved. It belongs to the family of Rel proteins that includes c-Rel, RelA (p65), RelB, NF-κB1 (p50 and its precursor p105), and NF-κB2 (p52 and its precursor p100) all of which can form hetero- or homodimers [14–16].
NF-κB activation is tightly regulated mainly through its localization. In resting cells, NF-κB proteins are kept in the cytoplasm in association with inhibitory IkB proteins including IκBα, IκBβ, and IκBε [15] among which IκBα is the most abundant. NF-κB signaling occurs through the canonical (classical) pathway initiated by NF-κB1 (p50/p105) and a noncanonical (alternative) pathway initiated by NF-κB2 (p52/p100) (Fig 1). Before the active NF-κB is translocated into the nucleus, NF-κB1 and NF-κB2 are cleaved to the active p50 and p52 subunits, respectively. While the classical pathway depends on IKK complex consisting of IKKα, IKKβ, IKKγ and the inhibitory subunit IκBs, the alternative pathway depends on IKKα homodimers and NF-κB inducing kinase (NIK) [17–19]. During classical activation, the IKK complex specifically phosphorylates IκBs on two conserved N-terminal serine residues which target them for E2- and E3-ligase-mediated polyubiquitination and subsequent 26S proteasomal mediated degradation. This process releases and activates NF-κB which now translocates to the nucleus. The activation of alternative pathway, which is commonly associated with RelB results in regulated processing of the p100 precursor protein to p52 and subsequent translocation of p52-RelB heterodimers to the nucleus[18]. Although NF-κB activation occurs mainly through canonical and non-canonical pathways, during the past decade a number of pathways for NF-κB activation has been elucidated (Fig 1).
Figure 1.
Schematic representation of major NF-κB activation pathways. In the classical pathway, binding of TNFα to the receptor triggers the sequential recruitment of the adaptors TRADD, TRAF2 and RIP to the membrane. TRAF2 then recruits the IKK complex composed of IKKα, IKKβ and IKKγ (NEMO) through mediation of kinases like TAK1, MEKK1, MEKK3. Activation of the IKK complex leads to the phosphorylation and ubiquitination of IκBα at specific residues followed by its degradation via the proteasome pathway. The p105 subunit of NF-κB then undergoes GSK3β and Tpl2 mediated phosphorylation at S903 and S907 and subsequent degradation. The heterodimer p50–p65 is then released and migrates to the nucleus where it undergoes a series of posttranslational modifications including phosphorylation, acetylation and methylation and binds to specific κB sites and activates NF-κB target genes [49, 205, 206]. The alternative pathway is IKKγ independent and is triggered by binding of the CD40, RANK, LTβR, BAFF ligands to their receptor, leading to recruitment of TRAF proteins and the sequential activation of NIK and IKKα. Activation of IKKα then induces the processing of the inhibitory protein p100. p100 proteolysis releases p52 which then translocates to the nucleus and triggers transcription of NF-κB target genes [207]. NF-κB activation in response to UV-C does not depend on IKK activation and relies on sequential recruitment of p38MAPK and CKII. Activated CKII phosphorylates IκBα at C-terminus (S283-T299). The phosphorylated IκBα undergoes ubiquitination and degradation leading to release of active NF-κB in to the nucleus [208, 209]. EGF induced NF-κB activation proceeds without serine phosphorylation and ubiquitination of IκBα and is IKK independent. It relies on phosphorylation of IκBα at Tyr42 through mediation of tyrosine kinases that triggers its proteasome mediated degradation and subsequent release of active NF-κB to the nucleus [210]. NF-κB activation in response to bacterial endotoxin LPS involves Toll like receptor and is mediated through recruitment of MyD88, TRAF6 and ECSIT. Recruitment of these adaptors leads to sequential activation of IRAK1/2 and IKK and eventual release of active NF-κB [211]. NF-κB activation by pervanadate and H2O2 induces phosphorylation of IκBα at Tyr42 by protein tyrosine kinase like Syk. The Tyr phosphorylation does not lead to IκBα degradation but makes the binding weak thereby dissociating the IκBα and releasing active NF-κB to the nucleus [212, 213]. Antigen receptor viz., T-cell receptor and B-cell receptor mediated signaling to NF-κB activation depends on recruitment of a trimolecular protein complex CARMA1-BCL10-MALT1. In this pathway PKCθ (in T cells) and PKCβ (in B cells) alongwith other kinases act upstream to the trimolecular complex to promote IKKγ polyubiquitination and consequent IKK activation. Activation of IKK through this pathway involves mediation of TRAF2, TRAF6, TAK1 and TAB1 [214, 215]. A novel pathway of NF-κB activation originating from the nucleus is associated with DNA damage. Double-stranded DNA breaks in response to genotoxic agents initiate signals that trigger SUMOylation of nuclear-localized IKKγ, preventing its nuclear export. Concomitantly, these breaks activate ATM which phosphorylates SUMO-modified IKKγ, promoting the removal of SUMO and enhancing IKKγ ubiquitination. Ubiquitinated IKKγ then translocates to the cytoplasm, where it activates IKK in cooperation with ATM and the ELKS protein, leading to IκBα phosphorylation and degradation, p65 nuclear translocation and induction of NF-κB dependent target genes [216–219]. NF-κB can also be regulated by phosphatases. WIP1, a Ser/Thr phosphatase was recently shown to negatively regulate NF-κB activation by dephosphorylating p65 at Ser536 [80].
Abbreviations: AgR, antigen receptor; ATM, ataxia-telangiectasia mutant; BAFF, B-cell activating factor; BCL, B-cell lymphoma; BCR, B cell receptor; CARMA, CARD-containing MAGUK protein; CD40L, CD40 ligand; CK, casein kinase; DSBS, Double-stranded DNA breaks; ECSIT, evolutionary conserved signaling intermediates on Toll pathways; EGF, epidermal growth factor; EGFR, EGF receptor; ELKS, glutamate, leucine, lysine, serine-rich protein; GSK, glycogen synthase kinase; Hsp90, heat shock protein 90; IκB, inhibitor of NF-κB; IKK, IκB kinase; IRAK, IL-1R-associated kinase; LTβ, lymphotoxin β; LPS, lipopolysaccharide; MALT, mucosa-associated lymphoid tissue; MAPK, mitogen activated protein kinase; MAPK/Erk kinase kinase; MyD88, myeloid differentiation factor; NF-κB, nuclear factor-κB; NIK, NF-κB-inducing kinase; NEMO, NF-κB essential modulator; PDK, Phosphoinositide-dependent kinase; PI3K, phosphatidylinositol 3-kinase; PKC, protein kinase C; PLC, phospholipase C; RANKL, receptor activator of NF-κB ligand; RIP, receptor-interacting protein; Syk, Spleen tyrosine kinase; TAB, TAK1-binding protein; TAK, transforming growth factor-β-activated kinase; TCR, T cell receptor; TLR, Toll-like receptor; TNF, tumour necrosis factor; TNFR1, TNF receptor 1; Tpl2, tumour progression locus-2; TRADD, TNF-receptor-associated death domain protein; TRAF, TNF-receptor-associated factor.
Once in the nucleus, activated NF-κB undergoes a series of posttranslational modifications, including phosphorylation, acetylation, and methylation. These modifications regulate both the strength and duration of NF-κB activity. RelA/p65 is directly phosphorylated by cAMP- dependent protein kinase (PKA) at Ser276, casein kinase II (CKII) at Ser529, and IKK at Ser536 [20, 21]. RelA dephosphorylation by protein phosphatase 2A (PP2A) has been reported to decrease NF-κB activity [22]. RelA is subject to inducible acetylation by p300/CBP, and acetylated RelA interacts weakly, if at all, with IκBα [23, 24], but maintains its nuclear localization and NF-κB transcriptional response. RelA is also subject to methylation by lysine methyltransferase Set9 (also called Set7 or KMT7) at Lys314/315 [25].
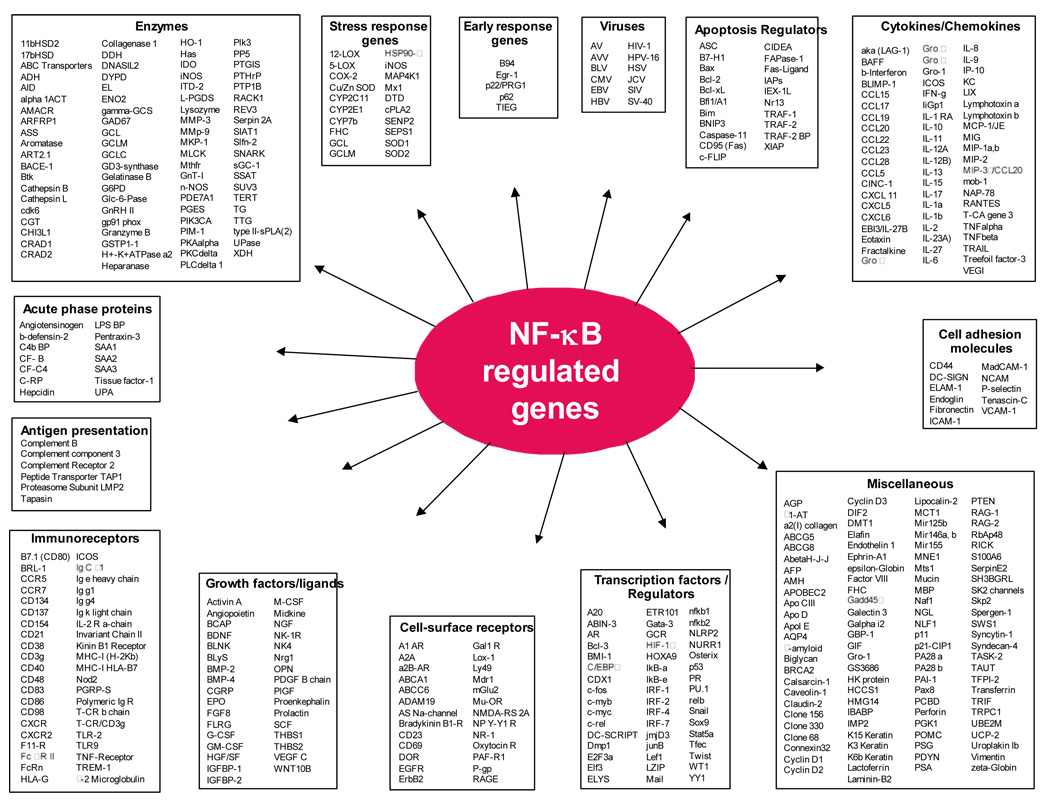
Activated NF-κB binds to specific DNA sequences in target genes, which are designated as κB elements, and regulates the transcription of over 500 genes involved in immunoregulation, growth regulation, inflammation, carcinogenesis, and apoptosis (Fig 2). NF-κB is frequently constitutively activated in patients with chronic inflammatory conditions such as cancer and pulmonary, cardiovascular, autoimmune, skin, and neurodegenerative diseases [26]. NF-κB’s ability to control multiple genes involved in human diseases makes the NF-κB signaling pathway a novel target for therapy [27, 28].
Figure 2.
A list of gene products regulated by NF-κB.
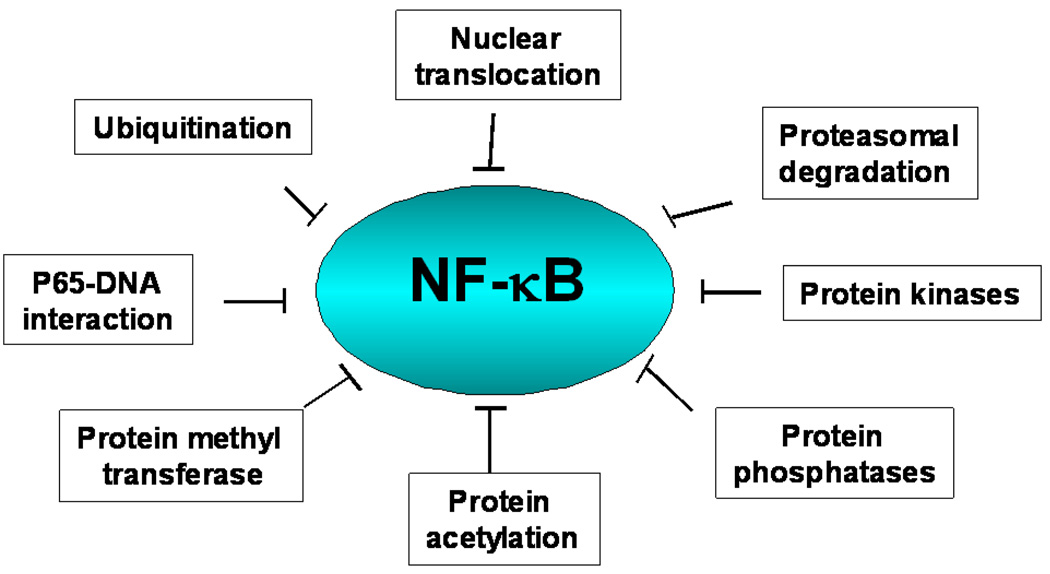
Due to the various levels of regulation, NF-κB signaling pathway can be potentially targeted at various levels including kinases, phosphatases, ubiquitination, nuclear translocation, DNA binding, protein acetyl transferases and methyl transferases (Fig 3).
Figure 3.
Potential targets for inhibiting the NF-κB activation pathway.
2. Inhibitors of the NF-κB activation pathway
Given NF-κB’s relevance in human diseases and the fact that many drugs interfere with NF-κB signaling, the NF-κB signaling pathway provides a highly attractive target for the therapeutic development. More than 700 inhibitors of the NF-κB activation pathway, including antioxidants, peptides, small RNA/DNA, microbial and viral proteins, small molecules, and engineered dominant-negative or constitutively active polypeptides have been described (Table 1). Several of these molecules act as general inhibitors of NF-κB activation, while other molecules target specific steps; some molecules possibly target multiple steps in the NF-κB pathway (Fig 3).
Table 1.
A list of small molecules as inhibitors of NF-kB pathway
| A. Upstream NF-kB | Kahweol | AIDCA derivative | Catalposide | E-73 | RelA peptides (P1 & P6) |
| Natural product | Kava derivatives2 | TDZD | Cyclolinteinone | Ecabet sodium | Viral Protein |
| 15d-PGJ(2) | Licorce extracts | TPCA-1 | Dihydroarteanniun | Gabexate mesilate | 3C protease (EMC virus) |
| Calagualine | Manumycin A | Pyridine derivatives | Docosahexaenoic acid | Glimepiride | Canine Distemper Virus |
| Conophylline | Monochloramine | ACHP | Emodin | Hypochlorite | MNF (myxoma virus) |
| Evodiamine | N-acetylcysteine | Acrolein | Ephedrae herba (Mao) extract | Losartin | Protein |
| Geldanamycin | Nitric oxide | AGRO100* | Equol | LY294002 | C5a |
| Perrilyl alcohol | Nitrosylcobalamin | Amino-pyrimidine | Erbstatin | Pervanadate | DQ 65-79 |
| PSK | Oleandrin | AS602868 | Estrogen | Phenylarsine oxide | Fox1j |
| Rocaglamides | Omega 3 fatty acids | Aspirin | Ethacrynic acid | Phenytoin | GILZ |
| Viral protein | ox-LDL | Azidothymidine | Fosfomycin | Ro106-9920** | HSCO |
| Adenovirus E1A | Panduratin A | BAY-11-7082 | Fungal gliotoxin | Sabaeksan | HSP-72 |
| NS5A (Hep-C virus) | PEITC | BAY-11-7083 | Gamisanghyulyunbueum*** | U0126 (MEK inhibitor) | Interleukin-10 |
| Protein | Petrosaspongiolide M | Benzoimidazole derivative | Genipin | Others | Interleukin -11 |
| Erbin overexpression | Phytic acid | Benzyl isothiocyanate | Genistein | Vagus nerve stimulation | Interleukin -13 |
| Golli BG21 | Piceatannol | BMS-345541 | Glabridin | Low level laser therapy | MTS-SR-IκBα |
| KSR | Pinosylvin | Carboplatin | Glucosamine sulfate | Zinc | Onconase |
| MAST205 | Plagius flosculosus extract | CDDO-Me | Glutamine | D. IκB upregulators/ | RASSF1A gene |
| NPM-ALK oncoprotein | Plumbagin | CHS 828* | Gumiganghwaltang*** | NF-κB translocation | ROR-alpha |
| Hep-C virus protease | Pomegranate extract | Compound 5 | Isomallotochromanol | Natural product | Surfactant protein A |
| PEDF | Prostaglandin A1 | Compound A | Isomallotochromene | PGG | TAT-SR- IκBα |
| Rituximab | Quercetin | Cyclopentenones | Kochia scoparla fruit extract | 15-deoxyspergualin | ZAS3 protein |
| TNAP | Rengyolone | CYL-19s | L-ascorbic acid | 2',8"-biapigenin | ZUD protein |
| Synthetic | Rosmarinic acid | CYL-26z | Leflunomide metabolite | 5F (from Pteri syeminpinnata) | β-amyloid protein |
| Betaine | Rottlerin | Diaylpyridine derivative | Melatonin | Agastache rugosa leaf extract | Synthetic |
| Desloratadine | Saikosaponin-d | DPE | Midazolam | Alginic acid | BMD |
| LY29 and LY30 | Salvia miltiorrhoza extract | Epoxyquinone | Momordin I | Antrodia camphorata extract | Carbaryl |
| MOL 294 ** | Sanguinarine | Gabexate mesilate | Morinda officinalis extract | Apigenin | CGS 25462 |
| Pefabloc | SAm extract | Gleevec | Mosla diantherra extract | Astragaloside IV | DHMEQ |
| Rhein | Staurosporine | Hydroquinone | Opuntia ficus indica extract | AT514 (serratamolide) | Diltiazem |
| SMI and FP | Sesquiterpene lactones | Ibuprofen | Platycodin saponins | Atorvastatin | Dioxin |
| B. IKK activity and IkB | Scoparone | IQCAD | Polymyxin B | Blue honey suckle extract | Dipyridamole |
| phosphorylation | Silibinin | Indolecarboxamide | Poncirus trifoliata fruit extract | Buthus martensi extract | Disulfiram |
| Natural product | Silymarin | Isobutyl nitrite | Probiotics | Cantharidin | Enalapril |
| [6]-gingerol | Sulforaphane | Jesterone dimer | Prostaglandin | Chiisanoside | mEET |
| 1'-Acetoxychavicol acetate | Sulindac | 15-deoxyspergualine analog | Resiniferatoxin | Clarithromycin | Fluvastatin |
| 20(S)-Protopanaxatriol | Tetrandine | Methotrexate | Stinging nettle extracts | Cornus officinalis extract | Indole-3-carbinol |
| 4-Hydroxynonenal | Theaflavin | MLB120** | Thiopental | Eriocalyxin B | JSH-23 |
| Acetyl-boswellic acids | Thienopyridine | Monochloramine | Tipifarnib | Gangliosides | KL-1156 |
| Anandamide | Tilianin | MX781 (Retinoid antagonist) | Titanium | Glucocorticoids | Leflunomide |
| Anethole | Ursolic acid | 4-HPR | TNP-470 | HP extracts | Levamisole |
| Apigenin | Vesnarinone | Nafamostat mesilate | Trichomomas vaginalis | Hirsutenone | MEB |
| Artemisia vestita1 | Wedelolactone | NSAIDs | TG-rich lipoproteins | Human breast milk | Moxifloxacin |
| Baoganning | Withanolides | PS-1145 (MLN1145) | Ursodeoxycholic acid | JM34 | Omapatrilat |
| Betulinic acid | Xanthoangelol D | PQD | Xanthium strumarium extract | KIOM-79 | R-etodolac |
| Black raspberry extracts | Zerumbone | Pyridooxazinone derivative | β- PEITC | Leptomycin B | Rolipram |
| Buddlejasaponin IV | β-carboline | SC-514** | 8-MSO | Neomycin | SC236 (COX-2 inhibitor) |
| Cacospongionolide B | γ-mangostin | Scytonemin | β-lapachone | Nucling | Triflusal |
| Calagualine | γ-Tocotrienol | Sodium salicylate | Peptide | Oregonin | Volatile anesthetics |
| Cardamomin | Peptide | Statins (several) | Penetratin | OXPAPC | E. NF-kB DNA-binding |
| Cardamonin | IKKβ peptide | Sulfasalazine | VIP | Paeoniflorin | Inorganic Complex |
| Casparol | NEMO CC2-LZ peptide | Sulfasalazine analogs | Protein | Phallacidin | Metals (chromium, cadmium, gold, |
| Cobrotoxin | Protein | Survanta | Activated protein C | Piperine | lead, mercury, zinc, arsenic) |
| Cycloepoxydon | Anti-thrombin III | Thalidomide | HSP-70 | Pitavastatin | Natural product |
| Decursin | Chorionic gonadotropin | THI 52 | Interleukin-13 | Platycodi radix extract | Actinodaphine |
| Dehydroascorbic acid | FHIT | YC-1** | Intravenous Ig | Probiotics | Anthocyanins |
| Dexanabinol | HB-EGF | Others | Murr1 gene product | Rapamycin | Arnica montana extract |
| Digitoxin | Hepatocyte growth factor | Lead | Neurofibromatosis-2 protein | Rhubarb aqueous extract | Artemisinin |
| Diosgenin | Interferon-α | Mild hypothermia | PACAP | Salvia miltiorrhoza extract | Baicalein |
| Diterpenes | Interleukin-10 | Saline (low Na+) | SAIF | SH extract | Bambara groundnut |
| Docosahexaenoic acid | PAN1 | C. IkB degradation | ST2 (IL-1-like receptor) | Selenomethionine | β-lapachone |
| Falcarindol | PTEN | Natural product | α-MSH | Shenfu | Biliverdin |
| Flavopiridol | SOCS1 | 5'-methylthioadenosine | γ-glutamylcysteine synthetase | Sophorae radix extract | Brazilian |
| Furonaphthoquinone | Viral Protein | Artemisia iwayomogi extract | Bacterial/Viral Protein | Sopoongsan | Calcitriol |
| Garcinone B | Adenovirus | Alachlor | K1L (Vaccinia virus protein) | Sorbus commixta extract | Campthothecin |
| Glossogyne tenuifolia extract | Core protein (Hep-C virus) | Amentoflavone | Nef (HIV-1) | Sphondin | Sutherlandia frutescens |
| Glycine chloramine | Cytomegalovirus | Antrodia camphorata# | Vpu protein (HIV-1) | T. polyglycosides | Capsiate |
| Guggulsterone | E7 (Papillomavirus) | Artemisia capillaries extract | YopJ | Younggaechulgam-tang*** | Catalposide |
| Herbimycin A | MC159 | Aucubin | Synthetic | α-pinene | Cat's claw bark |
| Honokiol | MC160 | Baicalein | 1-Bromopropane | Peptide | Cheongyeolsaseuptang*** |
| Hypoestoxide | NS5B (Hep-C virus) | Blackberry extract | Acetaminophen | NCPP | Chitosan |
| Indirubin-3'-oxime | vIRF3 (KSHV) | Buchang-tang*** | Diamide | PN50 | Chicory root |
| Isorhapontigenin | Synthetic | Capsaicin | Dobutamine | CSPDP | |
| Clarithromycin | AIM2 overexpression | Raxofelast | F. Proteasome/protease | Mangifera indica bark | |
| Cloricromene | Angiopoietin-1 | Ribavirin | Natural product | extract | |
| C-K and Rh(2) | Antithrombin | Rifamides | Cyclosporin A | Mangiferin | |
| Cortex cinnamomi | AvrA protein | Ritonavir | Lactacystine | Melatonin | |
| extract | (Salmonella) | Rosiglitazone | β-lactone | Mn-SOD | |
| Cryptotanshinone | β-catenin | Roxithromycin | Peptide | Mulberry anthocyanins | |
| Cytochalasin D | Bromelain | DAAS | ALLnL | Myricetin | |
| Black rice extract | CaMKK | Serotonin derivative | LLM | N-acetyl-L-cysteine | |
| Danshenshu | CD43 overexpression | Simvastatin | Ubiquitin ligase | Nacyselyn | |
| Diterpenoids | FLN29 overexpression | SM-7368** | Z-LLL | Naringin | |
| Ent-kaurane diterpenoids | FLIP | T-614 | Z-LLnV | N-ethyl-maleimide | |
| Epinastine hydrochloride | G-120 | Sulfasalazine | Synthetic | Nitrosoglutathione | |
| Epoxyquinol A | Gax (homeobox protein) | SUN C8079 | APNE | NDGA | |
| Erythromycin | HIV-1 Resistance Factor | Triclosan plus CPC | Boronic acid peptide | Ochnaflavone | |
| Evodiamine | Interleukin 4 | Tobacoo smoke | BTEE | Orthophenanthroline | |
| Fish oil | SspH1 and IpaH9.8 | Verapamil | 3,4-dichloroisocoumarin | Phenylarsine oxide | |
| Fomes fomentarius | NDPP1 (CARD protein) | Others | Deoxyspergualin | PhIP | |
| extracts | Overexpressed ZIP1 | Heat (fever-like) | DFP | Phyllanthus urinaria | |
| Fucoidan | p8 | Hypercapnic acidosis | Disulfiram | PMC | |
| Gallic acid | p202a | Hyperosmolarity | FK506 (Tacrolimus) | PTX | |
| Ganoderma lucidum | p21 (Rec) | Hypothermia | Bortezomib | Pyrithione | |
| Garcinol | PIAS1 | Alcohol | Salinosporamide A | Pyrrolinedithiocarbamate | |
| Geranylgeraniol | Pro-opiomelanocortin | E. NF-kB transactivation | TLCK | Quercetin | |
| Ginkgolide B | PYPAF1 protein | Natural products | TPCK | Quinozolines | |
| Glycyrrhizin | Raf Kinase inhibitor | 4'-DM-6-Mptox | G. Antioxidants | Rebamipide | |
| Halofuginone | protein Rhus verniciflua | 4-phenylcoumarins | 23-hydroxyursolic acid | Red wine | |
| Hematein | fruits | AHUP | Aged garlic extract | Redox factor 1 | |
| Herbal compound 861 | SLPI | Adenosine | Anetholdithiolthione | Resveratrol | |
| Hydroxyethyl starch | Siah2 | c-AMP | Apocynin | Ginseng derivative | |
| Hydroxyethylpuerarin | SIRT1 Deacetylase | Artemisia sylvatica extract | Apple juice/extracts | Rotenone | |
| Hypericin | overexpression | Bifodobacteria | Arctigenin | Roxithromycin | |
| Kamebakaurin | Siva-1 | Blueberry & berry mix | Aretemisa p7F | S-allyl-cysteine | |
| Linoleic acid | Solana nigrum L. | BSASM | Astaxanthin | Sauchinone | |
| Lithospermi radix | Surfactant protein A | BF phenylpropanoids | Benidipine | Spironolactone | |
| Macrolide antibiotics | Tom1 overexpression | cPrG.HC | bis-eugenol | Strawberry extracts | |
| Mediterranean plant | Transdominant p50 | Seaweed extract | BG compounds | Taxifolin | |
| extracts | Uteroglobin | Fructus benincasae extract | BHA | Tempol | |
| 2-methoxyestradiol | VEGF | Glucocorticoids | CAPE | Tepoxaline | |
| 6-MITC | Synthetic | Gypenoside XLIX | Carnosol | tert-butyl hydroquinone | |
| Nicotine | ADP ribosylation inhibitor | Kwei Ling Ko3 | Carvedilol | Tetracylic A | |
| Ochna macrocalyx bark | 7-amino-4-methylcoumarin | LC root | Catechol derivatives | Vitamin B6 | |
| ext. | Amrinone | Luteolin | Celasterol | Vitamin C | |
| Oridonin | Atrovastat | Manassantins A,B | Cepharanthine | Vitamin D | |
| PC-SPES (8 herb mixture) | Benfotiamine | MI bark extract | Chlorogenic acid | Vitamin E derivatives | |
| PGG | Benzamide | Mesuol | Chlorophyllin | Wogonin | |
| Pepluanone | Bisphenol A | Nobiletin | Cocoa polyphenols | xanthohumol | |
| Phyllanthus amarus | Caprofen | Phomol | Curcumin | Yakuchinone A, B | |
| extracts | Carbocisteine | Psychosine | DHEA | α-lipoic acid | |
| Plant compound A | Celecoxib | Qingkailing# | DHEA sulfate | α-tocopherol | |
| Polyozellin | Germcitabine | Saucerneol D & E | Dehydroevodiamine | α-torphryl acetate | |
| Prenylbisabolane 3 | Cinnamaldehyde | Shuanghuanglian# | Demethyltraxillagenin | α-torphryl succinate | |
| Prostaglandin E2 | 2-methoxy CNA | Smilax bockii extract | Diethyldithiocarbamate | β-Carotene | |
| PSK | 2-hydroxy CNA | Trilinolein | Diferoxamine | ||
| Quinic acid | CDS | Uncaria tomentosum extract | Dihydroisoeugenol | ||
| Sanggenon C | CP Compound | WS extracts | Dihydrolipoic acid | ||
| Sesamin | Cyanoguanidine | Wortmannin | Dilazep | ||
| Shen-Fu# | HMP | α-zearalenol | Fenofibric acid | ||
| Silibinin | α-difluoromethylornithine | Viral Protein | DMDTC | ||
| Sinomenine | DTD | BZLF1 (EBV protein) | Dimethylsulfoxide | ||
| Sword brake fern extract | Evans Blue | SH gene products (PMV) | Disulfiram | ||
| Tanacetum larvatum | Evodiamine | Protein | Ebselen | ||
| extract | Fenoldopam | Antithrombin | Edaravone | ||
| Tansinones | FEX | NF-kappaB-repression factor | EGTA | ||
| Taurine + niacine | Fibrates | PIAS3 | EPC-K1 | ||
| TZD MCC-555 | FK778 | PTX-B | Epigallocatechin-3-gallate | ||
| Trichostatin A | Flunixin meglumine | Synthetic | Ergothioneine | ||
| Triptolide | Flurbiprofen | 17-AAG | Ethyl pyruvate | ||
| Tyrphostin AG-126 | Hydroquinone | TMFC | Ganoderma lucidum | ||
| Ursolic acid | IMD-0354 | AQC derivatives | polysaccharides | ||
| Withaferin A | JSH-21 | 9-aminoacridine | Garcinol | ||
| Xanthohumol | KT-90 | derivatives | γ-glutamylcysteine synthetase | ||
| Xylitol | Lovastatin | Chromene derivatives | Ginkgo biloba extract | ||
| Yan-gan-wan# | Mercaptopyrazine | D609 | Glutathione | ||
| Yin-Chen-Hao# | Mevinolin, | Dimethylfumarate | Hematein | ||
| Yucca schidigera extract | Monoethylfumarate | EMDPC | Hydroquinone | ||
| Peptide | Moxifloxacin | Histidine | Hydroquinone | ||
| Ghrelin | Nicorandil | HIV-1 PI | IRFI 042 | ||
| Peptide YY | Nilvadipine | Mesalamine | Iron tetrakis | ||
| Rapamycin | NO-ASA | PEITC | Isovitexin | ||
| Viral Protein | Panepoxydone | Pranlukast | Kangen-karyu extract | ||
| African Swine Fever virus | Peptide nucleic acids | RO31-8220 (PKC | Ketamine | ||
| Sendai Virus-C,V proteins | Perindopril | inhibitor) | Lacidipine | ||
| E1B (Adenovirus) | PAD | SB203580 (MAPK inhibitor) | Lazaroids | ||
| ICP27 (HSV-1) | α-PBN | Tetrathiomolybdate | L-cysteine | ||
| H4/N5 (bracovirus) | Pioglitazone | Tranilast | Ligonberries | ||
| NS3/4A (Hep-C) | Pirfenidone | Troglitazone | Lupeol | ||
| Protein | PNO derivatives | Others | Magnolol | ||
| Adiponectin | Quinadril | Low gravity | Maltol |
Tibetan medicine;
Piper methysticum;
Tortoise shell-Rhizome jelly;
Anticancer drugs;
small molecules;
Oriental medicines;
Traditional Chinese Medicine; 15d-PGJ(2), 15-deoxy-prostaglandin J(2); 17-AAG, 17-allylamino-17-demethoxygeldanamycin; 20(S)-PPT, 20(S)-Protopanaxatriol; 4’-DM-6-Mptox, 4’-demethyl-6-methoxypodophyllotoxin; 6-MITC, 6-Methylsulfinyl) hexyl isothiocyanate; α-MSH, α-melanocyte-stimulating hormone; α-PBN, alpha-phenyl-N-tert-butylnitrone; ACHP, 2-amino-6-[2-(cyclopropylmethoxy)-6-hydroxyphenyl]-4-piperidin-4-yl nicotinonitrile; AGRO100, G-quadruplex oligodeoxynucleotide; AHUP, 8-acetoxy-5-hydroxyumbelliprenin; AIDCA, (Amino)imidazolyl carboxaldehyde derivative; ALLnL, N-acetyl-leucinyl-leucynil-norleucynal; APNE, N-acetyl-DL-phenylalanine-b-naphthylester; AQC, 6-aminoquinazoline; BAY-11-7082, E3((4-methylphenyl)-sulfonyl)-2-propenenitrile; BAY-11-7083, E3((4-t-butylphenyl)-sulfonyl)-2-propenenitrile; BF, Bupleurum fruticosum; BG, Bruguiera gymnorrhiza; BHA, Butylated hydroxyanisole; BMD, N(1)-Benzyl-4-methylbenzene-1,2-diamine; BMT, o,o’-bismyristoyl thiamine disulfide; BSASM, plant extract mixture; BTEE, N-benzoyl L-tyrosine-ethylester; CaMKK, Calcium/calmodulin-dependent kinase kinase; CAPE, Caffeic Acid Phenethyl Ester; CDDO-Me, C-28 methyl ester of 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid; CDS, Commerical peritoneal dialysis solution; CMP, Chinese medicinal preparations; CNA, Cinnamaldehyde; Compound 5, Uredio-thiophenecarboxamide derivative; CP Compound, 6-Hydroxy-7-methoxychroman-2-carboxylic acid phenylamide); CPC, cetylpyridinium chloride; cPrG.HC, Cycloprodigiosin hycrochloride; CSPDP, Chondrotin sulfate proteoglycan degradation product; CYL-19 s and CYL-26z, two synthetic alpha-methylene-gamma-butyrolactone derivatives; D609, phosphatidylcholine-specific phospholipase C inhibitor; DAAS, diacetoxy acetal derivative of santonin; DFP, diisopropyl fluorophosphates; DHEA, Dehydroepiandrosterone; DHMEQ, Dehydroxymethylepoxyquinomicin; DMDTC, Dimethyldithiocarbamates; DPE, 2-(3,4-dihydroxyphenyl)ethanol; DTD, 4,10-dichloropyrido[5,6:4,5]thieno[3,2-d’:3,2- d]-1, 2, 3-ditriazine; EGTA, Ethylene glycol tetraacetic acid; EMC virus, encephalomyocarditis virus; EMDPC, Ethyl 2-[(3-methyl-2,5-dioxo(3-pyrrolinyl)) pyrimidine-5-carboxylate; EPC-K1, phosphodiester compound of vitamin E; FEX, Fexofenadine hydrochloride; FHIT, Fragile histidine triad protein; FLIP, FLICE-Like Inhibitory Protein; G-120, Ulmus davidiana Nakai glycoprotein; GILZ, Glucorticoid-induced leucine zipper protein; HB-EGF, Heparin-binding epidermal growth factor-like growth factor; HMP, 7-(4’-hydroxy-3’-methoxyphenyl)-1-phenylhept-4-en-3-one; HP, Harpagophytum procumbens; HSCO, Hepatoma Subtracted-cDNA library Clone One; IQCAD, Imidazolylquinoline-carboxaldehyde derivative; JSH-23, 4-Methyl- -(3-phenyl-propyl)-benzene-1,2-diamine; KL-1156, 6-Hydroxy-7-methoxychroman-2-carboxylic acid phenylamide; KSHV, Kaposi’s sarcoma-associated herpesvirus; KSR, Kinase suppressor of ras; LC, Ligusticum chuanxiong; LLM, N-acetyl-leucinyl-leucynil-methional; LY294002, [2-(4-morpholinyl)-8-phenylchromone]; MAST205, a serine/threonine kinase; MC160, Mollusum contagiosum virus; MEB, 2-(4-morpholynl) ethyl butyrate hydrochloride; mEET, Mouse estrogen enhanced transcript; Mn-SOD,Manganese superoxide dismutase; MSO, 8-methylsulphinyloctyl; N-(4-hydroxyphenyl) retinamide [4-HPR; NDGA, Nordihydroguaiaritic acid; NCPP, NLS Cell permeable peptides; NFD-37, 2-Methyl-2-(2-methylpropenyl)-2,3-dihydronaphthoquinone [2,3-b]furan-4,9-dione; NH(2)Cl, monochloramine; NO-ASA, Nitric-oxide-donating aspirin; ox-LDL, Oxidized low density lipoprotein; OXPAPC, oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine; PACAP, Pituitary adenylate cyclase-activating polypeptide; PAD, 6(5H)-phenanthridinone; PEDF, pigment epithelium derived factor; PEITC, Phenethyl isothiocyanate; PGG, 1,2,3,4,6-penta-O-galloyl-β-D-glucose; PhIP, 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine; PI, protease inhibitor; PIAS1, protein inhibitor of activatated STAT1; Plant compound A, plant-derived phenyl aziridine precursor; PMC, (2,2,5,7,8-pentamethyl-6-hydroxychromane); PMV, Paromyxovirus; PNO, Pyridine N-oxide; PQD, Pyrazolo[4,3-c]quinoline derivative; PSK, Protein-bound polysaccharide; PTEN, phosphatase and tensin homolog; PTX, Pentoxyifylline (1-(5’-oxohexyl) 3,7-dimehylxanthine; PTX-B, pertussis toxin binding protein; Pyridine derivatives, 2-amino-3-cyano-4-aryl-6-(2- hydroxy-phenyl)pyridine derivatives; RH(2) & C-K, intestinal bacterial metabolites Rh(2) and compound K (C-K); RORalpha, Retinoic acid receptor-related orphan receptor-alpha; SH, Sargassum hemiphyllum; SAIF, Saccharomyces boulardii anti-inflammatory factor; SLPI, Secretory leucoprotease inhibitor; SMI and FP, Salmeterol and Fluticasone propionate; SOCS, suppressor of cytokine signaling proteins; SspH1 and IpaH9.8, Leucine-rich effector proteins of Salmonella & Shigella; TDZD, 1,2,4-thiadiazolidine derivative; TG, triglyceride; THI 52, 1-naphthylethyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline; TLCK, N-α-tosyl-L-lysine chloromethyl ketone; TMFC, 3,4,5-trimethoxy-4’-fluorochalcone; TNAP, TRAFs and NIK-associated protein; ; TP, Tripterygium polyglycosides; TPCA-1, 2-[(aminocarbonyl)amino]-5-acetylenyl-3-thionphenecarboxamides; TPCK, N-α-tosyl-L-phenylalanine chloromethyl ketone; TZD, Thiazolidinedione; VEGF, Vascular endothelial growth factor; VIP, Vasoactive intestinal peptide; VVP, Vaccinia virus protein; WS, Witheringia solanacea; Z-LLL, N-carbobenzoxyl-L-leucinyl-L-leucinyl-L-norleucinal; Z-LLnV (carbobenzoxyl-leucinyl-leucynil-norvalinal;
2.1. Inhibition of protein kinases
NF-κB activation requires the phosphorylation, polyubiquitination, and subsequent degradation of its inhibitory subunit, IκBα. Hence, inhibiting IκBα phosphorylation ultimately inhibits NF-κB’s transcriptional activity [29, 30]. IκBα phosphorylation is carried out by IKK, a serine/threonine protein kinase composed of three basic subunits: the kinases IKKα, IKKβ, and the regulatory subunit IKKγ (NEMO). The IKK activation is usually the first common step in the integration of many NF-κB-activating pathways; therefore, one strategy for inhibiting NF-κB activation is to block IKK activation. However, although more than 150 agents have been shown to inhibit NF-κB activation at the IKK step, few studies have investigated the mechanism by which a given agent can inhibit IKK or its activation. The few IKK inhibitors for which a mechanism of action is known can be divided into three general groups: adenosine triphosphate (ATP) analogs, which show some specificity for interacting with IKK; compounds that have allosteric effects on IKK structure; and compounds that interact with a specific cysteine residue (Cys-179) in the activation loop of IKKβ. ATP analogs include natural products such as β-carboline and synthetic compounds such as SC-839, which has an approximately 200-fold preference for IKKβ compared to IKKα [27, 31]. Compounds that have allosteric effects on IKK structure include BMS-345541, a synthetic compound that binds to an allosteric site on both IKKα and IKKβ and has an approximately 10-fold greater inhibitory effect on IKKβ than on IKKα [32]. Compounds that interact with Cys-179 IKKβ include thiol-reactive compounds such as parthenolide, arsenite, and certain epoxyquinoids [33–36]; these compounds’ interactions with Cys-179 are believed to interfere with phosphorylation- induced IKKβ activation because Cys-179 is located between Ser177 and Ser181, which are required for IKKβ activation in response to upstream signals such as tumor necrosis factor (TNF) and lipopolysaccharide (LPS) [37, 38]. Gene-based inhibitors can also block IKK activation. Specifically, mutations at the ATP-binding site or in the kinase activation loop can create dominant-negative IKKα and IKKβ, which are capable of blocking NF-κB activation [39–43]. Because of their distinct roles in the canonical and non-canonical NF-κB activation pathways, dominant-negative IKK mutants’ can show stimulus-dependent inhibition [44]. Adenoviral-mediated delivery of an IKKβ dominant-negative kinase has been shown to have therapeutic potential for airway inflammatory diseases such as asthma [45, 46]. NEMO can also serve as a target for inhibiting the IKK complex. In particular, introducing a cell-permeable 10 amino-acid peptide that corresponds to the NEMO-binding domain of IKKβ can block the binding of NEMO to IKK in response to TNF in the canonical pathway [47].
While activation of NF-κB by many stimuli depends on the phosphorylation of IκBs at N-terminal sites by the IKK complex, the mechanism of NF-κB activation by ultraviolet (UV) radiation involves the IKK-independent phosphorylation of IκBα at a cluster of C-terminal sites that are recognized by casein kinase II (CKII). CKII activity toward IκBα depends on p38 mitogen-activated protein kinase (MAPK) activation. CKII’s role as a key survival signal that activates NF-κB and protects tumor cells from apoptosis suggests that CKII may be an attractive target for the treatment of diverse cancers. Apigenin, a plant flavonoid, and emodin, a plant anthraquinone, are competitive inhibitors of CKII that directly interact with the nucleotide-binding sites of CKII [48].
Besides phosphorylating and subsequently degrading the molecules that inhibit NF-κB, protein kinases can also target the functional domains of NF-κB proteins themselves to optimally activate NF-κB. NF-κB proteins can be phosphorylated in the cytoplasm or nucleus by such kinases as glycogen synthase kinase 3β (GSK3β) [49], TRAF-associated NF-κB activator (TANK)-binding kinase 1 (TBK1) [50, 51], PKAc [20], mitogen- and stress-activated protein kinase-1 (MSK-1) [52], MAP3K NIK[53], Tpl2, PKC-θ [54], PI3K, Akt [55–57], p38 MAPK [58], protein tyrosine kinase, PKC-δ [59], RHO-kinase 2 [60], Mitogen activated protein kinase kinase 3 (MEKK3) [61], and receptor tyrosine kinases such as epidermal growth factor receptor, human epidermal growth factor receptor 2 [62]. Antagonistic antibodies or kinase inhibitors that target these molecules may decrease NF-κB activation. Some kinase inhibitors that have the potential to inhibit NF-κB activation include SB203580 and PD0980589 (MAPK inhibitors) [58]; denbinobin (TAK1 inhibitor) [63]; tyrosine kinase inhibitors [62]; rhein, (an MEKK inhibitor) [64, 65]; TNAP, betaine (NIK inhibitors) [66–68], epoxyquinol B (a TAK1 crosslinker) [69]; M2L (an extracellular signal-regulated kinase 2 inhibitor) [70, 71]; CCK-8 (a p38 kinase kinase inhibitor) [72], KSR2 (an MEKK3 inhibitor) [73], golli BG21 (a PKC inhibitor) [74].
2.2. Inhibition of NF-κB activation by protein phosphatases
Because protein phosphorylation is a dynamic process whereby phosphatases counterbalance kinase action, phosphatases may be used to inhibit NF-κB activation. Protein phosphatase 2A is a serine/threonine phosphatase that has been reported to dephosphorylate and modulate the activity of IKKβ [75]. Cytosine arabinoside, a pyrimidine analogue used to effectively treat acute leukemia, has been reported to induce apoptosis by activating protein phosphatases 2A and 2B-A and dephosphorylating the p65 subunit of NF-κB [22, 76]. Recently, OspF, a protein phosphatase from Shigella flexneri, was found to dephosphorylate MAPK and prevent histone H3 phosphorylation at Ser10 in a gene-specific manner to block the activation of a subset of NF-κB responsive genes [77]. Our previous studies have shown that protein tyrosine phosphatase (PTP) inhibitors can suppress NF-κB activation and that phenylarsine oxide, a specific PTP inhibitor, can promote tyrosine 42 phosphorylation of IκBα [78]. While some PTPs stimulate NF-κB activation, other PTPs negatively regulate NF-κB activation. For instance, PTEN, a tumor suppressor with phosphatase activity is known to inhibit NF-κB activation [79]. Recently, Chew et al., [80] found that WIP1, a Ser/Thr PP2C family of phosphatases act as a negative regulator of NF-κB activation. Overexpression of WIP1 was associated with decreased NF-κB activation, whereas WIP1 knockdown resulted in increased NF-κB activation. The group further showed that WIP1 target Ser536 of the p65 subunit of NF-κB.
2.3. Proteasome inhibitors and IκB ubiquitination blockers
The step before NF-κB leaves the cytoplasm involves the ubiquitination of IκB by the SCF-β-TrCP ubiquitin ligase complex followed by the rapid degradation of ubiquitinated IκB by the 26S proteasome [38]. Because IκBα degradation is an important step in the NF-κB activation pathway, inhibiting the proteasomes that degrade IκBα may also serve as a tool for pharmacological intervention. Very specific and potent proteasome inhibitors have been engineered by coupling boronic acid to dipeptides [81]. The dipeptide boronate, bortezomib, the most-studied proteasome inhibitor in clinical development [82], has been shown to inhibit proliferation and induce apoptosis in head and neck [83–85], prostate [86], pancreatic [87], gastric [88], and ovarian [89] cancers. Bortezomib’s antitumor properties correlate in part with its ability to inhibit IκBα degradation [83, 90]. Other well-known proteasome inhibitors include ALLnL, LLM, Z-LLnV, and Z-LLL, lactacystine, N-cbz-Leu-Leu-leucinal (MG132), MG115, and ubiquitin ligase inhibitors [91]. In addition, we recently identified a novel proteasome inhibitor, salinosporamide A (NPI-0052), which can suppress both constitutive and inducible NF-κB activation in a nanomolar range [92].
Several serine protease inhibitors with chymotrypsin-like specificity, including DCIC, TPCK, TLCK, BTEE, APNE, are also able to block proteasome function. However, unlike other protease inhibitors that block only IκB degradation, serine protease inhibitors can block IκB phosphorylation as well as degradation. However, not all serine protease inhibitors can inhibit NF-κB activation [93–95].
Among IκB ubiquitination blockers, the YopJ protein of the bacterial pathogen Yersinia deubiquitinates and stabilizes IκBα to prevent NF-κB nuclear translocation [96]. The small molecule R0196-9920 has been reported to inhibit IκBα ubiquitination and oral inflammation in mouse models [97, 98]. Yaron et al., [98] blocked TNFα-induced IκBα degradation by microinjecting phosphopeptides that corresponded to IκBα’s signal-dependent phosphorylation site. Presumably these phosphopeptides acted as competitive inhibitors for binding to the ubiquitin ligase complex essential to IκBα degradation. Inhibiting β-TrCP (the recognition subunit of the SCF E3 ligase complex) by specific RNAi treatment or by overexpression of dominant-negative β-TrCP mutants blocked NF-κB activity and sensitized breast cancer cells to chemotherapeutic agents [99]. Recently, A20 (TNFAIP3), a cytoplasmic zinc finger protein, was shown to inhibit NF-κB activation in the TNFR and TLR pathways. The ubiquitin editing property of A20 was shown to be essential for NF-κB inhibition [100].
2.4. Blockage of NF-κB nuclear translocation
One approach for inhibiting NF-κB activation is to use small peptides that cross the cell membrane and block the nuclear translocation of the NF-κB complex [101–103]. For example, SN50, a forty-one-residue synthetic peptide that contains a hydrophobic membrane-translocating region and the nuclear localization sequence of NF-κB p50 [101], can enter cells and compete with NF-κB complexes for the machinery responsible for the nuclear translocation of NF-κB. SN50 effectively inhibits the LPS- and TNF-α-induced nuclear translocation of NF-κB in different cell lines [101, 104–107] and mitigates inflammatory responses in vivo [108, 109]. However, SN50 also blocks the nuclear translocation of a number of other transcription factors [102]. Dehydroxymethylepoxyquinomicin, a fungal epoxyquinoid that has anti-inflammatory and antitumor activity in several mouse models, has been reported to be a specific inhibitor of NF-κB nuclear translocation [110].
2.5. Blocking NF-κB activation by inhibitors of p65 acetylation
The activated p65 subunit of NF-κB undergoes acetylation in the nucleus at multiple lysine residues including K122, K123, K218, K221 and K310 [23, 111]. The opposing activities of histone acetyltransferases and histone deacetylases (HDACs) regulate p65 complex acetylation [24]. Acetylation of p65 also depends on coactivators such as p300 and CREB-binding protein (CBP) [112]. The K221 and K310 acetylation are associated with increased NF-κB target gene transcription [112] and are required for p65 activation [24], which is supported by the observations that SIRT driven deacetylation at K310 inhibits NF-κB target gene transcription [113]. Additionally, K122 and K123 acetylation reduces p65 DNA binding affinity and increases IκB interaction and nuclear export [111]. Site-specific p300-mediated p65 acetylation thus regulates the specificity of NF-κB- dependent gene expression [111, 114].
During the last 5 years, a number of compounds have been reported to inhibit NF-κB by inhibiting acetylation. Gallic acid obtained from natural products such as gallnuts, sumac, oak bark, and green tea was recently reported to possess anti-histone acetyltransferase activity, thus showing potential to downregulate NF-κB activation [115]. Daxx, a protein associated with the death domain of Fas receptor, has been reported to suppress NF-κB transcriptional activity by inhibiting p300/CBP-mediated p65 acetylation [116]. Anacardic acid derived from traditional medicinal plants can also inhibit NF-κB activation by inhibiting p65 acetylation [117].
2.6. Blocking NF-κB activation by methyltransferases
The RelA subunits of NF-κB undergo various posttranslational modifications that create specific marks to recruit different effectors to control NF-κB’s temporal and spatial activation [118]. RelA is subject to monomethylation by lysine methyltransferase Set9 (also called Set7 or KMT7) at Lys314/315 in vitro and in vivo in response to stimuli [25]. RelA methylation at these two residues negatively regulates NF-κB function by triggering the ubiquitination and proteasome-mediated degradation of promoter-associated RelA. RelA methylation also serves as a “death” signal for the destruction of DNA-bound, activated NF-κB [25]. Because RelA subunit methylation negatively impacts NF-κB function, designing a molecule that activates Set9 function could potentiate NF-κB inhibition.
2.7. Blockage of NF-κB to DNA binding
The most direct strategy for blocking NF-κB activation is to block NF-κB from binding to specific κB sites on DNA. Some sesquiterpene lactones (SLs) have been reported to inhibit NF-κB [119] by interacting with Cys-38 in the DNA-binding loop of RelA [120, 121]. Most SLs can also inhibit DNA binding through an analogous Cys residue in the DNA-binding loops of p50 and c-Rel. Recently, a computer-based structural comparison of 103 SLs predicted that a methylene-carbonyl substructure is important for SL-based inhibition of RelA at Cys-38 [122]. Some SLs, including parthenolide, have been shown to inhibit IKKβ through the reactive Cys-179 in the kinase activation loop [34, 121]. Thus, SLs, which target both IKK activity and NF-κB subunit DNA binding [36], have multistep inhibitory activity within the NF-κB signaling pathway.
Blocking specific NF-κB-DNA binding can also be accomplished with decoy oligodeoxynucleotides (ODNs). These ODNs have κB sites and competes for NF-κB dimer binding to specific genomic promoters [123–125]. These oligonucleotides have modifications to increase their stability and their affinity for NF-κB in vivo [126–128]. Decoy ODNs have been reported to have therapeutic potential in a number of animal models of inflammation and cancer; for example, directly injecting NF-κB decoy ODNs into implanted adenocarcinoma colon 26 tumors in mice inhibited cachexia without affecting tumor growth [129].
2.8. Other mechanisms of NF-κB inhibition
2.8.1. By gene transfer
One strategy to block NF-κB activation is through the transfer of genes that code for proteins shown to suppress NF-κB activation. The most direct target is IκBα. IκBα mutation at specific phosphorylation sites (Ser32 and Ser36 replaced to alanine) and ubiquitination sites (Lys21 and Lys22 mutated to arginine) results in a nondegradable form of IκBα. This results in a stable cytoplasmic pool of IκBα, thereby preventing NF-κB activation [130–132]. Injecting a nonphosphorylatable form of IκBα into bone marrow macrophages has been shown to inhibit osteoclastogenesis and block bone resorption [133]. Additionally, specific C-terminal serine-to-alanine mutations are sometimes included to reduce the constitutive turnover of IκBα [134]. These super-repressor forms of IκBα can still interact with NF-κB dimers to keep the dimers in the cytoplasm permanently [132, 134, 135]. Such molecules have been used succesfully to inhibit NF-κB activity and to study its role in tumor development [136, 137] and to sensitize tumor cells to apoptosis-inducing agents [134, 135]. Inhibiting NF-κB through the expression of an IκBα super-repressor (IκBαSR) has also been used to sensitize chemoresistant tumors to TNFα- and CPT-11-induced apoptosis, resulting in tumor regression [138], and to inhibit the proliferation of human head and neck carcinoma cells in vitro and in vivo [139]. However, IκBαSRs have also been shown to interact with and affect the activity of non-NF-κB pathway proteins including p53 [140], cyclin-dependent kinase 4 [141], and HDACs [142]. Furthermore, IκBαSR overexpression has been associated with the spontaneous development of squamous cell carcinoma in a murine model [143].
2.8.2. Antioxidants
Antioxidants were suggested as possible NF-κB inhibitors many years ago [144, 145]. Treatment with oxidants such as hydrogen peroxide can activate NF-κB in many cell types. In some cell types, antioxidants can inhibit the induction of NF-κB activity in response to a variety of stimuli (e.g., interleukin-1β, LPS, TNFα) [146, 147]. However, using antioxidants as NF-κB inhibitors is now regarded with increasing scepticism because the NF-κB-inhibiting properties of pyrrolidine dithiocarbamate, a thiol-containing compound, cannot be attributed to its antioxidant function but rather to its effects as an inhibitor of IκB ubiquitin ligase activity [148]. The ways in which antioxidants block NF-κB activation remain unclear, but it is likely that they act at different steps in the NF-κB pathway in different cell types. Antioxidants have been suggested to inhibit NF-κB activation by scavenging reactive oxygen intermediates that act as signaling molecules to activate the NF-κB pathway and by directly inhibiting IKK kinase activity by modifying critical Cys residues in the IKK kinase activation loop [146, 147]. Mitochondrial electron transport inhibitors that suppress reactive oxygen intermediate production (e.g., rotenone) and overexpression of antioxidizing enzymes (e.g., manganese superoxide dismutase and catalase) can block TNFα-induced NF-κB activation [149–151]. Caffeic acid phenethyl ester, a phenolic antioxidant, has been reported to cause direct interference with DNA binding by NF-κB [152] that can be reversed by dithiothreitol [78]. Other antioxidants, viz., N-acetylcysteine, calcium chelators (e.g., EGTA, lacidipine), and vitamin C and E derivatives have been reported to inhibit hydrogen peroxide- or stimulus-induced NF-κB activation.
2.8.3. Bacterial, fungal, and viral proteins
Several microorganisms and viruses encode proteins that can inhibit NF-κB activation. Many viruses have developed a number of mechanisms to inhibit NF-κB signaling [153], and three viruses—African swine fever virus (ASFV) [154], rabbit myxoma virus [155], and insect Microplitis demolitor bracovirus [156]—encode IκB-like NF-κB inhibitors. The ASFV encodes the A238L IκB-like protein, which can stably interact with RelA to inhibit TNFα-, IFN-γ-, and phorbol ester-induced NF-κB-DNA binding [157]. The poliovirus 3C protease cleaves RelA to reduce NF-κB signaling [158]. In addition, several viruses have adaptor-like or small proteins that inhibit IKK activity [153]. For example, the MC160 protein of Molluscum contagiosum [159] and the nonstructural 5B protein of the hepatitis C virus [160] appear to be IKKα-specific and thus may specifically inhibit the noncanonical NF-κB pathway.
The YopJ protein, a Src homology 2 domain protein encoded by Yersinia pseudotuberculosis, inhibits NF-κB activation by preventing the phosphorylation and degradation of IκBα [161]. YopJ has also been shown to bind directly to IKKβ in vitro and in vivo [162]. The Salmonella typhimurium AvrA protein also inhibits NF-κB activation, although its mechanism of action may be different than that of the YopJ protein [163].
Gliotoxin produced by the fungus Aspergillus fumigatus has been reported to inhibit NF-κB activation by preventing IκB degradation [164]. Several other small molecules synthesized by microorganisms or designed derivatives of such compounds that have NF-κB-inhibiting potential include panepoxydone (from Lentinus crinitus) [165], 5,6 epoxycyclohexenone compounds (from Amycolatopsis), and cycloepoxydon [166]. Such compounds may affect distinct parts of the NF-κB pathway including DNA binding, nuclear translocation, and IκBα phosphorylation and degradation.
2.8.4. Anti-inflammatory and immunosuppressive agents
Various anti-inflammatory agents including glucocorticoids, non-steroid anti-inflammatory drugs (NSAIDs), and immunosuppressants have been developed to block NF-κB activation. Glucocorticoids, which are commonly used as anti-inflammatory drugs, strongly inhibit NF-κB activation by mechanisms that are not completely understood but likely include inhibition of DNA binding, IKK activity and transactivation [167]. The glucocorticoids dexamethasone, prednisone and methylprednisolone have been reported to inhibit NF-κB activation. In addition, estrogen and selective estrogen receptor modulators (SERMs) such as raloxifene can act through the estrogen receptor to inhibit NF-κB activation [168, 169].
NSAIDs such as sodium salicylate (aspirin) and sulindac have been reported to inhibit NF-κB activation by inhibiting IκBα phosphorylation [170, 171]. At higher concentrations, aspirin has been shown to block NF-κB activity by directly binding to and inhibiting the kinase activity of IKKβ by reducing its ability to bind ATP [172]. More recently, aspirin was reported to inhibit proteasome activity [173]. As such, high-dose aspirin therapy may have applications in treating diseases in which NF-κB activity is involved, including cancer [174], diabetes [175], and heart disease [176]. Other NSAIDs such as ibuprofen and indomethacin have also been reported to inhibit NF-κB activation in cell culture [177–180].
Several well known immunosuppressants are known to target NF-κB by distinct mechanisms, some precluding NF-κB nuclear translocation [181], some through inhibiting calcineurin [182], some by binding heat-shock proteins [183] and some by modulating the DNA binding or transactivation potential of NF-κB [184–187]. Examples of immunosuppressants having inhibitory effect on NF-κB activation include cyclosporin A (CsA) [181], FK506 [188, 189], PG490 (diterpene triepoxide) [187] and deoxyspergualin [183].
2.8.5. p53 induction
It is known that p53 and NF-κB pathways play opposing roles in human cancer, with p53 acting as a tumor suppressor and NF-κB acting as a tumor activator. The crosstalk between p53 and NF-κB indicates that p53 and NF-κB repress each other’s activities owing to their competition for transcriptional coactivator proteins p300 and CBP [190]. A recent study has proposed an additional mechanism of how CBP phosphorylation by IKKα determines whether CBP binds to p53 or NF-κB [191]. Although a number of studies have focused on identifying p53 activators and NF-κB inhibitors individually, few studies have investigated the molecules that target both the pathways simultaneously. Identifying molecules that simultaneously activate p53 and inhibit NF-κB would have great potential in combination therapy for cancer and various other diseases and could provide helpful tools to better understand the crosstalk between the p53 and NF-κB pathways. Quinacrine, an antimalarial drug, and other derivatives of 9 aminoacridine have been shown to simultaneously repress NF-κB and activate p53 in renal cell carcinoma [192]. Other molecules with similar potential include nutlins [193, 194], cisplatin [195, 196], leptomycin B [197, 198], adenosine-2,3-dialdehyde [199], the NSAID JTE-522 [200], and the cyclin-dependent kinase inhibitors R-roscovitine [201, 202]; and flavopiridol [203, 204].
3. Conclusions and future perspective
NF-κB has been implicated in almost all chronic diseases, and more than 40,000 studies on NF-κB have been published with 9000 on its inhibitors. Although more than 700 different inhibitors (aspirin to IκBα super repressor) of this transcription factor have been reported, yet no NF-κB blocker has been approved for human use. Various steroids and NSAIDs have been found to block NF-κB, but their effects are highly pleiotropic. The molecules that block NF-κB activation lack specificity and thus interfere with NF-κB’s physiological roles in immunity, inflammation, and cellular homeostasis. Additionally, whether the concentrations of inhibitors used in tissue culture experiments can be applied in vivo is often unclear. Therefore, one of the major challenges facing researchers is to develop NF-κB inhibitors aimed at treating different diseases based on their ability to target specific pathways or cells, thereby avoiding the risk of undesired side effects. Future studies should also focus on validating in vitro data in vivo.
Acknowledgements
We thank Joe Munch for carefully proof-reading the manuscript and providing valuable comments. Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research. This work was supported by a grant from the Clayton Foundation for Research (B.B.A.), a core grant from the National Institutes of Health (CA-16 672), a program project grant from National Institutes of Health (NIH CA-124787-01A2), and grant from Center for Targeted Therapy of M.D. Anderson Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 2.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat. Rev. Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 3.Haefner B. NF-kappa B: arresting a major culprit in cancer. Drug Discov. Today. 2002;7:653–663. doi: 10.1016/s1359-6446(02)02309-7. [DOI] [PubMed] [Google Scholar]
- 4.Richmond A. Nf-kappa B, chemokine gene transcription and tumour growth. Nat. Rev. Immunol. 2002;2:664–674. doi: 10.1038/nri887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Clevers H. At the crossroads of inflammation and cancer. Cell. 2004;118:671–674. doi: 10.1016/j.cell.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Withoff S, Verma IM. Inflammation-associated cancer: NF-kappaB is the lynchpin. Trends Immunol. 2005;26:318–325. doi: 10.1016/j.it.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Luo JL, Kamata H, Karin M. IKK/NF-kappaB signaling: balancing life and death--a new approach to cancer therapy. J. Clin. Invest. 2005;115:2625–2632. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaffer AL, Rosenwald A, Staudt LM. Lymphoid malignancies: the dark side of B-cell differentiation. Nat. Rev. Immunol. 2002;2:920–932. doi: 10.1038/nri953. [DOI] [PubMed] [Google Scholar]
- 12.Panwalkar A, Verstovsek S, Giles F. Nuclear factor-kappaB modulation as a therapeutic approach in hematologic malignancies. Cancer. 2004;100:1578–1589. doi: 10.1002/cncr.20182. [DOI] [PubMed] [Google Scholar]
- 13.Shishodia S, Aggarwal BB. Nuclear factor-kappaB activation mediates cellular transformation, proliferation, invasion angiogenesis and metastasis of cancer. Cancer Treat. Res. 2004;119:139–173. doi: 10.1007/1-4020-7847-1_8. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109 Suppl:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 16.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 17.Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, Karin M. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 18.Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, Li ZW, Karin M, Ware CF, Green DR. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002;17:525–535. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 19.Dejardin E. The alternative NF-kappaB pathway from biochemistry to biology: pitfalls and promises for future drug development. Biochem. Pharmacol. 2006;72:1161–1179. doi: 10.1016/j.bcp.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S. The transcriptional activity of NF-kappaB is regulated by the IkappaB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell. 1997;89:413–424. doi: 10.1016/s0092-8674(00)80222-6. [DOI] [PubMed] [Google Scholar]
- 21.Wang D, Westerheide SD, Hanson JL, Baldwin AS., Jr Tumor necrosis factor alpha-induced phosphorylation of RelA/p65 on Ser529 is controlled by casein kinase II. J. Biol. Chem. 2000;275:32592–32597. doi: 10.1074/jbc.M001358200. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Fan GH, Wadzinski BE, Sakurai H, Richmond A. Protein phosphatase 2A interacts with and directly dephosphorylates RelA. J. Biol. Chem. 2001;276:47828–47833. doi: 10.1074/jbc.M106103200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- 24.Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J. 2002;21:6539–6548. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang XD, Huang B, Li M, Lamb A, Kelleher NL, Chen LF. Negative regulation of NF-kappaB action by Set9-mediated lysine methylation of the RelA subunit. EMBO J. 2009;28:1055–1066. doi: 10.1038/emboj.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sethi G, Sung B, Aggarwal BB. Nuclear factor-kappaB activation: from bench to bedside. Exp. Biol. Med. (Maywood) 2008;233:21–31. doi: 10.3181/0707-MR-196. [DOI] [PubMed] [Google Scholar]
- 27.Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat. Rev. Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- 28.Nagashima K, Sasseville VG, Wen D, Bielecki A, Yang H, Simpson C, Grant E, Hepperle M, Harriman G, Jaffee B, Ocain T, Xu Y, Fraser CC. Rapid TNFR1-dependent lymphocyte depletion in vivo with a selective chemical inhibitor of IKKbeta. Blood. 2006;107:4266–4273. doi: 10.1182/blood-2005-09-3852. [DOI] [PubMed] [Google Scholar]
- 29.Karin M. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene. 1999;18:6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 30.Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, Donehower LA, Elledge SJ. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- 31.Pande V, Ramos MJ. NF-kappaB in human disease: current inhibitors and prospects for de novo structure based design of inhibitors. Curr. Med. Chem. 2005;12:357–374. doi: 10.2174/0929867053363180. [DOI] [PubMed] [Google Scholar]
- 32.Burke JR, Pattoli MA, Gregor KR, Brassil PJ, MacMaster JF, McIntyre KW, Yang X, Iotzova VS, Clarke W, Strnad J, Qiu Y, Zusi FC. BMS-345541 is a highly selective inhibitor of I kappa B kinase that binds at an allosteric site of the enzyme and blocks NF-kappa B-dependent transcription in mice. J. Biol. Chem. 2003;278:1450–1456. doi: 10.1074/jbc.M209677200. [DOI] [PubMed] [Google Scholar]
- 33.Kapahi P, Takahashi T, Natoli G, Adams SR, Chen Y, Tsien RY, Karin M. Inhibition of NF-kappa B activation by arsenite through reaction with a critical cysteine in the activation loop of Ikappa B kinase. J. Biol. Chem. 2000;275:36062–36066. doi: 10.1074/jbc.M007204200. [DOI] [PubMed] [Google Scholar]
- 34.Kwok BH, Koh B, Ndubuisi MI, Elofsson M, Crews CM. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem. Biol. 2001;8:759–766. doi: 10.1016/s1074-5521(01)00049-7. [DOI] [PubMed] [Google Scholar]
- 35.Liang MC, Bardhan S, Li C, Pace EA, Porco JA, Jr, Gilmore TD. Jesterone dimer, a synthetic derivative of the fungal metabolite jesterone, blocks activation of transcription factor nuclear factor kappaB by inhibiting the inhibitor of kappaB kinase. Mol. Pharmacol. 2003;64:123–131. doi: 10.1124/mol.64.1.123. [DOI] [PubMed] [Google Scholar]
- 36.Liang MC, Bardhan S, Pace EA, Rosman D, Beutler JA, Porco JA, Jr, Gilmore TD. Inhibition of transcription factor NF-kappaB signaling proteins IKKbeta and p65 through specific cysteine residues by epoxyquinone A monomer: correlation with its anti-cancer cell growth activity. Biochem. Pharmacol. 2006;71:634–645. doi: 10.1016/j.bcp.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 37.Perkins ND. Post-translational modifications regulating the activity and function of the nuclear factor kappa B pathway. Oncogene. 2006;25:6717–6730. doi: 10.1038/sj.onc.1209937. [DOI] [PubMed] [Google Scholar]
- 38.Scheidereit C. IkappaB kinase complexes: gateways to NF-kappaB activation and transcription. Oncogene. 2006;25:6685–6705. doi: 10.1038/sj.onc.1209934. [DOI] [PubMed] [Google Scholar]
- 39.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 40.Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 41.Regnier CH, Song HY, Gao X, Goeddel DV, Cao Z, Rothe M. Identification and characterization of an IkappaB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 42.Woronicz JD, Gao X, Cao Z, Rothe M, Goeddel DV. IkappaB kinase-beta: NF-kappaB activation and complex formation with IkappaB kinase-alpha and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 43.Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 44.Shikama Y, Yamada M, Miyashita T. Caspase-8 and caspase-10 activate NF-kappaB through RIP, NIK and IKKalpha kinases. Eur. J. Immunol. 2003;33:1998–2006. doi: 10.1002/eji.200324013. [DOI] [PubMed] [Google Scholar]
- 45.Broide DH, Lawrence T, Doherty T, Cho JY, Miller M, McElwain K, McElwain S, Karin M. Allergen-induced peribronchial fibrosis and mucus production mediated by IkappaB kinase beta-dependent genes in airway epithelium. Proc. Natl. Acad. Sci. U.S.A. 2005;102:17723–17728. doi: 10.1073/pnas.0509235102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Catley MC, Chivers JE, Holden NS, Barnes PJ, Newton R. Validation of IKK beta as therapeutic target in airway inflammatory disease by adenoviral-mediated delivery of dominant-negative IKK beta to pulmonary epithelial cells. Br. J. Pharmacol. 2005;145:114–122. doi: 10.1038/sj.bjp.0706170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.May MJ, D'Acquisto F, Madge LA, Glockner J, Pober JS, Ghosh S. Selective inhibition of NF-kappaB activation by a peptide that blocks the interaction of NEMO with the IkappaB kinase complex. Science. 2000;289:1550–1554. doi: 10.1126/science.289.5484.1550. [DOI] [PubMed] [Google Scholar]
- 48.Battistutta R, Sarno S, De Moliner E, Papinutto E, Zanotti G, Pinna LA. The replacement of ATP by the competitive inhibitor emodin induces conformational modifications in the catalytic site of protein kinase CK2. J. Biol. Chem. 2000;275:29618–29622. doi: 10.1074/jbc.M004257200. [DOI] [PubMed] [Google Scholar]
- 49.Demarchi F, Bertoli C, Sandy P, Schneider C. Glycogen synthase kinase-3 beta regulates NF-kappa B1/p105 stability. J. Biol. Chem. 2003;278:39583–39590. doi: 10.1074/jbc.M305676200. [DOI] [PubMed] [Google Scholar]
- 50.Schwabe RF, Brenner DA. Role of glycogen synthase kinase-3 in TNF-alpha-induced NF-kappaB activation and apoptosis in hepatocytes. Am. J. Physiol. 2002;283:G204–G211. doi: 10.1152/ajpgi.00016.2002. [DOI] [PubMed] [Google Scholar]
- 51.Fujita F, Taniguchi Y, Kato T, Narita Y, Furuya A, Ogawa T, Sakurai H, Joh T, Itoh M, Delhase M, Karin M, Nakanishi M. Identification of NAP1, a regulatory subunit of IkappaB kinase-related kinases that potentiates NF-kappaB signaling. Mol. Cell. Biol. 2003;23:7780–7793. doi: 10.1128/MCB.23.21.7780-7793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegeman G. Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1) EMBO J. 2003;22:1313–1324. doi: 10.1093/emboj/cdg139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang X, Takahashi N, Matsui N, Tetsuka T, Okamoto T. The NF-kappa B activation in lymphotoxin beta receptor signaling depends on the phosphorylation of p65 at serine 536. J. Biol. Chem. 2003;278:919–926. doi: 10.1074/jbc.M208696200. [DOI] [PubMed] [Google Scholar]
- 54.Mattioli I, Sebald A, Bucher C, Charles RP, Nakano H, Doi T, Kracht M, Schmitz ML. Transient and selective NF-kappa B p65 serine 536 phosphorylation induced by T cell costimulation is mediated by I kappa B kinase beta and controls the kinetics of p65 nuclear import. J. Immunol. 2004;172:6336–6344. doi: 10.4049/jimmunol.172.10.6336. [DOI] [PubMed] [Google Scholar]
- 55.Sizemore N, Leung S, Stark GR. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-kappaB p65/RelA subunit. Mol. Cell. Biol. 1999;19:4798–4805. doi: 10.1128/mcb.19.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Madrid LV, Mayo MW, Reuther JY, Baldwin AS., Jr Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J. Biol. Chem. 2001;276:18934–18940. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- 57.Sizemore N, Lerner N, Dombrowski N, Sakurai H, Stark GR. Distinct roles of the Ikappa B kinase alpha and beta subunits in liberating nuclear factor kappa B (NF-kappa B) from Ikappa B and in phosphorylating the p65 subunit of NF-kappa B. J. Biol. Chem. 2002;277:3863–3869. doi: 10.1074/jbc.M110572200. [DOI] [PubMed] [Google Scholar]
- 58.Vanden Berghe W, Plaisance S, Boone E, De Bosscher K, Schmitz ML, Fiers W, Haegeman G. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-kappaB p65 transactivation mediated by tumor necrosis factor. J. Biol. Chem. 1998;273:3285–3290. doi: 10.1074/jbc.273.6.3285. [DOI] [PubMed] [Google Scholar]
- 59.Bhatt KH, Pandey RK, Dahiya Y, Sodhi A. Protein kinase Cdelta and protein tyrosine kinase regulate peptidoglycan-induced nuclear factor-kappaB activation and inducible nitric oxide synthase expression in mouse peritoneal macrophages in vitro. Mol. Immunol. 2010;47:861–870. doi: 10.1016/j.molimm.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 60.Shimada H, Rajagopalan LE. RHO kinase-2 activation in human endothelial cells drives LPA-mediated expression of cell adhesion molecules via NF-{kappa}B p65. J. Biol. Chem. doi: 10.1074/jbc.M109.099630. (2010, in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun W, Ge N, Yu Y, Burlingame S, Li X, Zhang M, Ye S, Fu S, Yang J. Phosphorylation of Thr-516 and Ser-520 in the Kinase Activation Loop of MEKK3 Is Required for Lysophosphatidic Acid-mediated Optimal I{kappa}B Kinase {beta} (IKK{beta})/Nuclear Factor-{kappa}B (NF-{kappa}B) Activation. J. Biol. Chem. 2010;285:7911–7918. doi: 10.1074/jbc.M109.051219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ravi R, Bedi A. NF-kappaB in cancer--a friend turned foe. Drug Resist. Updat. 2004;7:53–67. doi: 10.1016/j.drup.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 63.Sanchez-Duffhues G, Calzado MA, de Vinuesa AG, Appendino G, Fiebich BL, Loock U, Lefarth-Risse A, Krohn K, Munoz E. Denbinobin inhibits nuclear factor-kappaB and induces apoptosis via reactive oxygen species generation in human leukemic cells. Biochem. Pharmacol. 2009;77:1401–1409. doi: 10.1016/j.bcp.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 64.Martin D, Daulny A, Decoville M, Locker D. Mutagenesis analysis of the interaction between the dorsal rel homology domain and HMG boxes of DSP1 protein. J. Biochem. 2003;134:583–589. doi: 10.1093/jb/mvg177. [DOI] [PubMed] [Google Scholar]
- 65.Domagala F, Martin G, Bogdanowicz P, Ficheux H, Pujol JP. Inhibition of interleukin-1beta-induced activation of MEK/ERK pathway and DNA binding of NF-kappaB and AP-1: potential mechanism for Diacerein effects in osteoarthritis. Biorheology. 2006;43:577–587. [PubMed] [Google Scholar]
- 66.Hu Y, Wang HF, Sun WQ, Xie CS, Wei WN, Zheng JE, Yao JX. Regulation of tissue factor expression in brain microvascular endothelial cells by PLA nanoparticles coating NF-kappaB decoy oligonucleotides. Zhonghua Xue Ye Xue Za Zhi. 2005;26:534–538. [PubMed] [Google Scholar]
- 67.Manna SK, Bueso-Ramos C, Alvarado F, Aggarwal BB. Calagualine inhibits nuclear transcription factors-kappaB activated by various inflammatory and tumor promoting agents. Cancer Lett. 2003;190:171–182. doi: 10.1016/s0304-3835(02)00618-3. [DOI] [PubMed] [Google Scholar]
- 68.Go EK, Jung KJ, Kim JM, Lim H, Lim HK, Yu BP, Chung HY. Betaine modulates age-related NF-kappaB by thiol-enhancing action. Biol. Pharm. Bull. 2007;30:2244–2249. doi: 10.1248/bpb.30.2244. [DOI] [PubMed] [Google Scholar]
- 69.Kamiyama H, Usui T, Sakurai H, Shoji M, Hayashi Y, Kakeya H, Osada H. Epoxyquinol B, a naturally occurring pentaketide dimer, inhibits NF-kappaB signaling by crosslinking TAK1. Biosci. Biotechnol. Biochem. 2008;72:1894–1900. doi: 10.1271/bbb.80142. [DOI] [PubMed] [Google Scholar]
- 70.Gedey R, Jin XL, Hinthong O, Shisler JL. Poxviral regulation of the host NF-kappaB response: the vaccinia virus M2L protein inhibits induction of NF-kappaB activation via an ERK2 pathway in virus-infected human embryonic kidney cells. J. Virol. 2006;80:8676–8685. doi: 10.1128/JVI.00935-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hinthong O, Jin XL, Shisler JL. Characterization of wild-type and mutant vaccinia virus M2L proteins' abilities to localize to the endoplasmic reticulum and to inhibit NF-kappaB activation during infection. Virology. 2008;373:248–262. doi: 10.1016/j.virol.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Y, Chu N, Hu A, Gran B, Rostami A, Zhang GX. Inducible IL-23p19 expression in human microglia via p38 MAPK and NF-kappaB signal pathways. Exp. Mol. Pathol. 2008;84:1–8. doi: 10.1016/j.yexmp.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Channavajhala PL, Rao VR, Spaulding V, Lin LL, Zhang YG. hKSR-2 inhibits MEKK3-activated MAP kinase and NF-kappaB pathways in inflammation. Biochem. Biophys. Res. Commun. 2005;334:1214–1218. doi: 10.1016/j.bbrc.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 74.Feng B, Cheng S, Pear WS, Liou HC. NF-kB inhibitor blocks B cell development at two checkpoints. Med. Immunol. 2004;3:1. doi: 10.1186/1476-9433-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barisic S, Strozyk E, Peters N, Walczak H, Kulms D. Identification of PP2A as a crucial regulator of the NF-kappaB feedback loop: its inhibition by UVB turns NF-kappaB into a pro-apoptotic factor. Cell Death Differ. 2008;15:1681–1690. doi: 10.1038/cdd.2008.98. [DOI] [PubMed] [Google Scholar]
- 76.Sreenivasan Y, Sarkar A, Manna SK. Mechanism of cytosine arabinoside-mediated apoptosis: role of Rel A (p65) dephosphorylation. Oncogene. 2003;22:4356–4369. doi: 10.1038/sj.onc.1206486. [DOI] [PubMed] [Google Scholar]
- 77.Arbibe L, Kim DW, Batsche E, Pedron T, Mateescu B, Muchardt C, Parsot C, Sansonetti PJ. An injected bacterial effector targets chromatin access for transcription factor NF-kappaB to alter transcription of host genes involved in immune responses. Nat. Immunol. 2007;8:47–56. doi: 10.1038/ni1423. [DOI] [PubMed] [Google Scholar]
- 78.Singh S, Aggarwal BB. Protein-tyrosine phosphatase inhibitors block tumor necrosis factor-dependent activation of the nuclear transcription factor NF-kappa B. J. Biol. Chem. 1995;270:10631–10639. doi: 10.1074/jbc.270.18.10631. [DOI] [PubMed] [Google Scholar]
- 79.Koul D, Yao Y, Abbruzzese JL, Yung WK, Reddy SA. Tumor suppressor MMAC/PTEN inhibits cytokine-induced NFkappaB activation without interfering with the IkappaB degradation pathway. J. Biol. Chem. 2001;276:11402–11408. doi: 10.1074/jbc.M007806200. [DOI] [PubMed] [Google Scholar]
- 80.Chew J, Biswas S, Shreeram S, Humaidi M, Wong ET, Dhillion MK, Teo H, Hazra A, Fang CC, Lopez-Collazo E, Bulavin DV, Tergaonkar V. WIP1 phosphatase is a negative regulator of NF-kappaB signalling. Nat. Cell Biol. 2009;11:659–666. doi: 10.1038/ncb1873. [DOI] [PubMed] [Google Scholar]
- 81.Iqbal M, Chatterjee S, Kauer JC, Das M, Messina P, Freed B, Biazzo W, Siman R. Potent inhibitors of proteasome. J. Med. Chem. 1995;38:2276–2277. doi: 10.1021/jm00013a002. [DOI] [PubMed] [Google Scholar]
- 82.Staudt LM. Gene expression profiling of lymphoid malignancies. Annu. Rev. Med. 2002;53:303–318. doi: 10.1146/annurev.med.53.082901.103941. [DOI] [PubMed] [Google Scholar]
- 83.Sunwoo JB, Chen Z, Dong G, Yeh N, Crowl Bancroft C, Sausville E, Adams J, Elliott P, Van Waes C. Novel proteasome inhibitor PS-341 inhibits activation of nuclear factor-kappa B, cell survival, tumor growth, and angiogenesis in squamous cell carcinoma. Clin. Cancer Res. 2001;7:1419–1428. [PubMed] [Google Scholar]
- 84.Lun M, Zhang PL, Pellitteri PK, Law A, Kennedy TL, Brown RE. Nuclear factor-kappaB pathway as a therapeutic target in head and neck squamous cell carcinoma: pharmaceutical and molecular validation in human cell lines using Velcade and siRNA/NF-kappaB. Ann. Clin. Lab. Sci. 2005;35:251–258. [PubMed] [Google Scholar]
- 85.Allen C, Saigal K, Nottingham L, Arun P, Chen Z, Van Waes C. Bortezomib-induced apoptosis with limited clinical response is accompanied by inhibition of canonical but not alternative nuclear factor-{kappa}B subunits in head and neck cancer. Clin. Cancer Res. 2008;14:4175–4185. doi: 10.1158/1078-0432.CCR-07-4470. [DOI] [PubMed] [Google Scholar]
- 86.Williams S, Pettaway C, Song R, Papandreou C, Logothetis C, McConkey DJ. Differential effects of the proteasome inhibitor bortezomib on apoptosis and angiogenesis in human prostate tumor xenografts. Mol. Cancer Ther. 2003;2:835–843. [PubMed] [Google Scholar]
- 87.Nawrocki ST, Carew JS, Pino MS, Highshaw RA, Andtbacka RH, Dunner K, Jr, Pal A, Bornmann WG, Chiao PJ, Huang P, Xiong H, Abbruzzese JL, McConkey DJ. Aggresome disruption: a novel strategy to enhance bortezomib-induced apoptosis in pancreatic cancer cells. Cancer Res. 2006;66:3773–3781. doi: 10.1158/0008-5472.CAN-05-2961. [DOI] [PubMed] [Google Scholar]
- 88.Adachi M, Zhang Y, Zhao X, Minami T, Kawamura R, Hinoda Y, Imai K. Synergistic effect of histone deacetylase inhibitors FK228 and m-carboxycinnamic acid bis-hydroxamide with proteasome inhibitors PSI and PS-341 against gastrointestinal adenocarcinoma cells. Clin. Cancer Res. 2004;10:3853–3862. doi: 10.1158/1078-0432.CCR-03-0806. [DOI] [PubMed] [Google Scholar]
- 89.Zhu H, Zhang L, Dong F, Guo W, Wu S, Teraishi F, Davis JJ, Chiao PJ, Fang B. Bik/NBK accumulation correlates with apoptosis-induction by bortezomib (PS-341, Velcade) and other proteasome inhibitors. Oncogene. 2005;24:4993–4999. doi: 10.1038/sj.onc.1208683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lun M, Zhang PL, Siegelmann-Danieli N, Blasick TM, Brown RE. Intracellular inhibitory effects of Velcade correlate with morphoproteomic expression of phosphorylated-nuclear factor-kappaB and p53 in breast cancer cell lines. Ann. Clin. Lab. Sci. 2005;35:15–24. [PubMed] [Google Scholar]
- 91.Ishii Y, Waxman S, Germain D. Targeting the ubiquitin-proteasome pathway in cancer therapy. Anti-cancer Agents Med. Chem. 2007;7:359–365. doi: 10.2174/187152007780618180. [DOI] [PubMed] [Google Scholar]
- 92.Ahn KS, Sethi G, Chao TH, Neuteboom ST, Chaturvedi MM, Palladino MA, Younes A, Aggarwal BB. Aggarwal, Salinosporamide A (NPI-0052) potentiates apoptosis, suppresses osteoclastogenesis, and inhibits invasion through down-modulation of NF-kappaB regulated gene products. Blood. 2007;110:2286–2295. doi: 10.1182/blood-2007-04-084996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Higuchi M, Singh S, Chan H, Aggarwal BB. Aggarwal, Protease inhibitors differentially regulate tumor necrosis factor-induced apoptosis, nuclear factor-kappa B activation, cytotoxicity, and differentiation. Blood. 1995;86:2248–2256. [PubMed] [Google Scholar]
- 94.D'Acquisto F, Sautebin L, Iuvone T, Di Rosa M, Carnuccio R. Prostaglandins prevent inducible nitric oxide synthase protein expression by inhibiting nuclear factor-kappaB activation in J774 macrophages. FEBS Lett. 1998;440:76–80. doi: 10.1016/s0014-5793(98)01407-0. [DOI] [PubMed] [Google Scholar]
- 95.Rossi A, Elia G, Santoro MG. Activation of the heat shock factor 1 by serine protease inhibitors. An effect associated with nuclear factor-kappaB inhibition. J. Biol. Chem. 1998;273:16446–16452. doi: 10.1074/jbc.273.26.16446. [DOI] [PubMed] [Google Scholar]
- 96.Zhou H, Monack DM, Kayagaki N, Wertz I, Yin J, Wolf B, Dixit VM. Yersinia virulence factor YopJ acts as a deubiquitinase to inhibit NF-kappa B activation. J. Exp. Med. 2005;202:1327–1332. doi: 10.1084/jem.20051194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Swinney DC, Xu YZ, Scarafia LE, Lee I, Mak AY, Gan QF, Ramesha CS, Mulkins MA, Dunn J, So OY, Biegel T, Dinh M, Volkel P, Barnett J, Dalrymple SA, Lee S, Huber M. A small molecule ubiquitination inhibitor blocks NF-kappa B-dependent cytokine expression in cells and rats. J. Biol. Chem. 2002;277:23573–23581. doi: 10.1074/jbc.M200842200. [DOI] [PubMed] [Google Scholar]
- 98.Yaron A, Gonen H, Alkalay I, Hatzubai A, Jung S, Beyth S, Mercurio F, Manning AM, Ciechanover A, Ben-Neriah Y. Inhibition of NF-kappa-B cellular function via specific targeting of the I-kappa-B-ubiquitin ligase. EMBO J. 1997;16:6486–6494. doi: 10.1093/emboj/16.21.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tang W, Li Y, Yu D, Thomas-Tikhonenko A, Spiegelman VS, Fuchs SY. Targeting beta-transducin repeat-containing protein E3 ubiquitin ligase augments the effects of antitumor drugs on breast cancer cells. Cancer Res. 2005;65:1904–1908. doi: 10.1158/0008-5472.CAN-04-2597. [DOI] [PubMed] [Google Scholar]
- 100.Shembade N, Ma A, Harhaj EW. Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes. Science. 2010;327:1135–1139. doi: 10.1126/science.1182364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J. Biol. Chem. 1995;270:14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- 102.Torgerson TR, Colosia AD, Donahue JP, Lin YZ, Hawiger J. Regulation of NFkappa B, AP-1, NFAT, and STAT1 nuclear import in T lymphocytes by noninvasive delivery of peptide carrying the nuclear localization sequence of NF-kappa B p50. J. Immunol. 1998;161:6084–6092. [PubMed] [Google Scholar]
- 103.Letoha T, Somlai C, Takacs T, Szabolcs A, Jarmay K, Rakonczay Z, Jr, Hegyi P, Varga I, Kaszaki J, Krizbai I, Boros I, Duda E, Kusz E, Penke B. A nuclear import inhibitory peptide ameliorates the severity of cholecystokinin-induced acute pancreatitis. World J. Gastroenterol. 2005;11:990–999. doi: 10.3748/wjg.v11.i7.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abate A, Oberle S, Schroder H. Lipopolysaccharide-induced expression of cyclooxygenase-2 in mouse macrophages is inhibited by chloromethylketones and a direct inhibitor of NF-kappa B translocation. Prostaglandins Other Lipid Med. 1998;56:277–290. doi: 10.1016/s0090-6980(98)00061-6. [DOI] [PubMed] [Google Scholar]
- 105.Kolenko V, Bloom T, Rayman P, Bukowski R, Hsi E, Finke J. Inhibition of NF-kappa B activity in human T lymphocytes induces caspase-dependent apoptosis without detectable activation of caspase-1 and −3. J. Immunol. 1999;163:590–598. [PubMed] [Google Scholar]
- 106.Mohan RR, Mohan RR, Kim WJ, Wilson SE. Modulation of TNF-alpha-induced apoptosis in corneal fibroblasts by transcription factor NF-kappaB. Invest. Ophthalmol. Vis. Sci. 2000;41:1327–1336. [PubMed] [Google Scholar]
- 107.Koulich E, Nguyen T, Johnson K, Giardina C, D'Mello S. NF-kappaB is involved in the survival of cerebellar granule neurons: association of IkappaBbeta [correction of Ikappabeta] phosphorylation with cell survival. J. Neurochem. 2001;76:1188–1198. doi: 10.1046/j.1471-4159.2001.00134.x. [DOI] [PubMed] [Google Scholar]
- 108.D'Acquisto F, Ialenti A, Ianaro A, Di Vaio R, Carnuccio R. Local administration of transcription factor decoy oligonucleotides to nuclear factor-kappaB prevents carrageenin-induced inflammation in rat hind paw. Gene Ther. 2000;7:1731–1737. doi: 10.1038/sj.gt.3301295. [DOI] [PubMed] [Google Scholar]
- 109.D'Acquisto F, Ianaro A, Ialenti A, Maffia P, Maiuri MC, Carnuccio R. Transcription factor decoy oligodeoxynucleotides to nuclear factor-kappaB inhibit reverse passive Arthus reaction in rat. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;364:422–429. doi: 10.1007/s002100100472. [DOI] [PubMed] [Google Scholar]
- 110.Umezawa K. Inhibition of tumor growth by NF-kappaB inhibitors. Cancer Sci. 2006;97:990–995. doi: 10.1111/j.1349-7006.2006.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kiernan R, Bres V, Ng RW, Coudart MP, El Messaoudi S, Sardet C, Jin DY, Emiliani S, Benkirane M. Post-activation turn-off of NF-kappa B-dependent transcription is regulated by acetylation of p65. J. Biol. Chem. 2003;278:2758–2766. doi: 10.1074/jbc.M209572200. [DOI] [PubMed] [Google Scholar]
- 112.Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat. Rev. Mol. Cell. Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 113.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Greene WC, Chen LF. Regulation of NF-kappaB action by reversible acetylation. Novartis Found. Symp. 2004;259:208–217. discussion 218–225. [PubMed] [Google Scholar]
- 115.Choi KC, Lee YH, Jung MG, Kwon SH, Kim MJ, Jun WJ, Lee J, Lee JM, Yoon HG. Gallic acid suppresses lipopolysaccharide-induced nuclear factor-kappaB signaling by preventing RelA acetylation in A549 lung cancer cells. Mol. Cancer Res. 2009;7:2011–2021. doi: 10.1158/1541-7786.MCR-09-0239. [DOI] [PubMed] [Google Scholar]
- 116.Park J, Lee JH, La M, Jang MJ, Chae GW, Kim SB, Tak H, Jung Y, Byun B, Ahn JK, Joe CO. Inhibition of NF-kappaB acetylation and its transcriptional activity by Daxx. J. Mol. Biol. 2007;368:388–397. doi: 10.1016/j.jmb.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 117.Sung B, Pandey MK, Ahn KS, Yi T, Chaturvedi MM, Liu M, Aggarwal BB. Anacardic acid (6-nonadecyl salicylic acid), an inhibitor of histone acetyltransferase, suppresses expression of nuclear factor-kappaB-regulated gene products involved in cell survival, proliferation, invasion, and inflammation through inhibition of the inhibitory subunit of nuclear factor-kappaBalpha kinase, leading to potentiation of apoptosis. Blood. 2008;111:4880–4891. doi: 10.1182/blood-2007-10-117994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang XD, Tajkhorshid E, Chen LF. Functional Interplay between Acetylation and Methylation of the RelA Subunit of NF-{kappa}B. Mol. Cell. Biol. doi: 10.1128/MCB.01343-09. (2010, in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang S, Won YK, Ong CN, Shen HM. Anti-cancer potential of sesquiterpene lactones: bioactivity and molecular mechanisms. Curr. Med. Chem. Anticancer Agents. 2005;5:239–249. doi: 10.2174/1568011053765976. [DOI] [PubMed] [Google Scholar]
- 120.Garcia-Pineres AJ, Castro V, Mora G, Schmidt TJ, Strunck E, Pahl HL, Merfort I. Cysteine 38 in p65/NF-kappaB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J. Biol. Chem. 2001;276:39713–39720. doi: 10.1074/jbc.M101985200. [DOI] [PubMed] [Google Scholar]
- 121.Garcia-Pineres AJ, Lindenmeyer MT, Merfort I. Role of cysteine residues of p65/NFkappaB on the inhibition by the sesquiterpene lactone parthenolide and N-ethyl maleimide, and on its transactivating potential. Life Sci. 2004;75:841–856. doi: 10.1016/j.lfs.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 122.Wagner S, Hofmann A, Siedle B, Terfloth L, Merfort I, Gasteiger J. Development of a structural model for NF-kappaB inhibition of sesquiterpene lactones using self-organizing neural networks. J. Med. Chem. 2006;49:2241–2252. doi: 10.1021/jm051125n. [DOI] [PubMed] [Google Scholar]
- 123.Morishita R, Sugimoto T, Aoki M, Kida I, Tomita N, Moriguchi A, Maeda K, Sawa Y, Kaneda Y, Higaki J, Ogihara T. In vivo transfection of cis element "decoy" against nuclear factor-kappaB binding site prevents myocardial infarction. Nature medicine. 1997;3:894–899. doi: 10.1038/nm0897-894. [DOI] [PubMed] [Google Scholar]
- 124.Khaled AR, Butfiloski EJ, Sobel ES, Schiffenbauer J. Use of phosphorothioate-modified oligodeoxynucleotides to inhibit NF-kappaB expression and lymphocyte function. Clin. Immunol. Immunopathol. 1998;86:170–179. doi: 10.1006/clin.1997.4486. [DOI] [PubMed] [Google Scholar]
- 125.Kupatt C, Wichels R, Deiss M, Molnar A, Lebherz C, Raake P, von Degenfeld G, Hahnel D, Boekstegers P. Retroinfusion of NFkappaB decoy oligonucleotide extends cardioprotection achieved by CD18 inhibition in a preclinical study of myocardial ischemia and retroinfusion in pigs. Gene Ther. 2002;9:518–526. doi: 10.1038/sj.gt.3301673. [DOI] [PubMed] [Google Scholar]
- 126.Tomita N, Ogihara T, Morishita R. Transcription factors as molecular targets: molecular mechanisms of decoy ODN and their design. Curr. Drug Targets. 2003;4:603–608. doi: 10.2174/1389450033490803. [DOI] [PubMed] [Google Scholar]
- 127.Crinelli R, Bianchi M, Gentilini L, Palma L, Magnani M. Locked nucleic acids (LNA): versatile tools for designing oligonucleotide decoys with high stability and affinity. Curr. Drug Targets. 2004;5:745–752. doi: 10.2174/1389450043345083. [DOI] [PubMed] [Google Scholar]
- 128.Isomura I, Morita A. Regulation of NF-kappaB signaling by decoy oligodeoxynucleotides. Microbiol. Immunol. 2006;50:559–563. doi: 10.1111/j.1348-0421.2006.tb03827.x. [DOI] [PubMed] [Google Scholar]
- 129.Kawamura I, Morishita R, Tomita N, Lacey E, Aketa M, Tsujimoto S, Manda T, Tomoi M, Kida I, Higaki J, Kaneda Y, Shimomura K, Ogihara T. Intratumoral injection of oligonucleotides to the NF kappa B binding site inhibits cachexia in a mouse tumor model. Gene Ther. 1999;6:91–97. doi: 10.1038/sj.gt.3300819. [DOI] [PubMed] [Google Scholar]
- 130.Van Antwerp DJ, Verma IM. Signal-induced degradation of I(kappa)B(alpha): association with NF-kappaB and the PEST sequence in I(kappa)B(alpha) are not required. Mol. Cell. Biol. 1996;16:6037–6045. doi: 10.1128/mcb.16.11.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jobin C, Panja A, Hellerbrand C, Iimuro Y, Didonato J, Brenner DA, Sartor RB. Inhibition of proinflammatory molecule production by adenovirus-mediated expression of a nuclear factor kappaB super-repressor in human intestinal epithelial cells. J. Immunol. 1998;160:410–418. [PubMed] [Google Scholar]
- 132.Bentires-Alj M, Hellin AC, Ameyar M, Chouaib S, Merville MP, Bours V. Stable inhibition of nuclear factor kappaB in cancer cells does not increase sensitivity to cytotoxic drugs. Cancer Res. 1999;59:811–815. [PubMed] [Google Scholar]
- 133.Abu-Amer Y, Dowdy SF, Ross FP, Clohisy JC, Teitelbaum SL. TAT fusion proteins containing tyrosine 42-deleted IkappaBalpha arrest osteoclastogenesis. J. Biol. Chem. 2001;276:30499–30503. doi: 10.1074/jbc.M104725200. [DOI] [PubMed] [Google Scholar]
- 134.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 135.Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 136.Bushdid PB, Brantley DM, Yull FE, Blaeuer GL, Hoffman LH, Niswander L, Kerr LD. Inhibition of NF-kappaB activity results in disruption of the apical ectodermal ridge and aberrant limb morphogenesis. Nature. 1998;392:615–618. doi: 10.1038/33435. [DOI] [PubMed] [Google Scholar]
- 137.Kanegae Y, Tavares AT, Izpisua Belmonte JC, Verma IM. Role of Rel/NF-kappaB transcription factors during the outgrowth of the vertebrate limb. Nature. 1998;392:611–614. doi: 10.1038/33429. [DOI] [PubMed] [Google Scholar]
- 138.Wang CY, Cusack JC, Jr, Liu R, Baldwin AS., Jr Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat. Med. 1999;5:412–417. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- 139.Duffey DC, Chen Z, Dong G, Ondrey FG, Wolf JS, Brown K, Siebenlist U, Van Waes C. Expression of a dominant-negative mutant inhibitor-kappaBalpha of nuclear factor-kappaB in human head and neck squamous cell carcinoma inhibits survival, proinflammatory cytokine expression, and tumor growth in vivo. Cancer Res. 1999;59:3468–3474. [PubMed] [Google Scholar]
- 140.Chang NS. The non-ankyrin C terminus of Ikappa Balpha physically interacts with p53 in vivo and dissociates in response to apoptotic stress, hypoxia, DNA damage, and transforming growth factor-beta 1-mediated growth suppression. J. Biol. Chem. 2002;277:10323–10331. doi: 10.1074/jbc.M106607200. [DOI] [PubMed] [Google Scholar]
- 141.Li J, Joo SH, Tsai MD. An NF-kappaB-specific inhibitor, IkappaBalpha, binds to and inhibits cyclin-dependent kinase 4. Biochemistry. 2003;42:13476–13483. doi: 10.1021/bi035390r. [DOI] [PubMed] [Google Scholar]
- 142.Aguilera C, Hoya-Arias R, Haegeman G, Espinosa L, Bigas A. Recruitment of IkappaBalpha to the hes1 promoter is associated with transcriptional repression. Proc. Natl. Acad. Sci. U.S.A. 2004;101:16537–16542. doi: 10.1073/pnas.0404429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.van Hogerlinden M, Rozell BL, Ahrlund-Richter L, Toftgard R. Squamous cell carcinomas and increased apoptosis in skin with inhibited Rel/nuclear factor-kappaB signaling. Cancer Res. 1999;59:3299–3303. [PubMed] [Google Scholar]
- 144.Staal FJ, Roederer M, Herzenberg LA, Herzenberg LA. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proc. Natl. Acad. Sci. U.S.A. 1990;87:9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mihm S, Ennen J, Pessara U, Kurth R, Droge W. Inhibition of HIV-1 replication and NF-kappa B activity by cysteine and cysteine derivatives. AIDS. 1991;5:497–503. doi: 10.1097/00002030-199105000-00004. [DOI] [PubMed] [Google Scholar]
- 146.Bubici C, Papa S, Dean K, Franzoso G. Mutual cross-talk between reactive oxygen species and nuclear factor-kappa B: molecular basis and biological significance. Oncogene. 2006;25:6731–6748. doi: 10.1038/sj.onc.1209936. [DOI] [PubMed] [Google Scholar]
- 147.Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem. Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 148.Hayakawa M, Miyashita H, Sakamoto I, Kitagawa M, Tanaka H, Yasuda H, Karin M, Kikugawa K. Evidence that reactive oxygen species do not mediate NF-kappaB activation. EMBO J. 2003;22:3356–3366. doi: 10.1093/emboj/cdg332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schulze-Osthoff K, Beyaert R, Vandevoorde V, Haegeman G, Fiers W. Depletion of the mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF. EMBO J. 1993;12:3095–3104. doi: 10.1002/j.1460-2075.1993.tb05978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Manna SK, Zhang HJ, Yan T, Oberley LW, Aggarwal BB. Overexpression of manganese superoxide dismutase suppresses tumor necrosis factor-induced apoptosis and activation of nuclear transcription factor-kappaB and activated protein-1. J. Biol. Chem. 1998;273:13245–13254. doi: 10.1074/jbc.273.21.13245. [DOI] [PubMed] [Google Scholar]
- 151.Manna SK, Kuo MT, Aggarwal BB. Overexpression of gamma-glutamylcysteine synthetase suppresses tumor necrosis factor-induced apoptosis and activation of nuclear transcription factor-kappa B and activator protein-1. Oncogene. 1999;18:4371–4382. doi: 10.1038/sj.onc.1202811. [DOI] [PubMed] [Google Scholar]
- 152.Natarajan K, Singh S, Burke TR, Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hiscott J, Nguyen TL, Arguello M, Nakhaei P, Paz S. Manipulation of the nuclear factor-kappaB pathway and the innate immune response by viruses. Oncogene. 2006;25:6844–6867. doi: 10.1038/sj.onc.1209941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Powell PP, Dixon LK, Parkhouse RM. An IkappaB homolog encoded by African swine fever virus provides a novel mechanism for downregulation of proinflammatory cytokine responses in host macrophages. J. Virol. 1996;70:8527–8533. doi: 10.1128/jvi.70.12.8527-8533.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Camus-Bouclainville C, Fiette L, Bouchiha S, Pignolet B, Counor D, Filipe C, Gelfi J, Messud-Petit F. A virulence factor of myxoma virus colocalizes with NF-kappaB in the nucleus and interferes with inflammation. J. Virol. 2004;78:2510–2516. doi: 10.1128/JVI.78.5.2510-2516.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Thoetkiattikul H, Beck MH, Strand MR. Inhibitor kappaB-like proteins from a polydnavirus inhibit NF-kappaB activation and suppress the insect immune response. Proc. Natl. Acad. Sci. U.S.A. 2005;102:11426–11431. doi: 10.1073/pnas.0505240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Revilla Y, Callejo M, Rodriguez JM, Culebras E, Nogal ML, Salas ML, Vinuela E, Fresno M. Inhibition of nuclear factor kappaB activation by a virus-encoded IkappaB-like protein. J. Biol. Chem. 1998;273:5405–5411. doi: 10.1074/jbc.273.9.5405. [DOI] [PubMed] [Google Scholar]
- 158.Neznanov N, Chumakov KM, Neznanova L, Almasan A, Banerjee AK, Gudkov AV. Proteolytic cleavage of the p65-RelA subunit of NF-kappaB during poliovirus infection. J. Biol. Chem. 2005;280:24153–24158. doi: 10.1074/jbc.M502303200. [DOI] [PubMed] [Google Scholar]
- 159.Nichols DB, Shisler JL. The MC160 protein expressed by the dermatotropic poxvirus molluscum contagiosum virus prevents tumor necrosis factor alpha-induced NF-kappaB activation via inhibition of I kappa kinase complex formation. J. Virol. 2006;80:578–586. doi: 10.1128/JVI.80.2.578-586.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Choi SH, Park KJ, Ahn BY, Jung G, Lai MM, Hwang SB. Hepatitis C virus nonstructural 5B protein regulates tumor necrosis factor alpha signaling through effects on cellular IkappaB kinase. Mol. Cell. Biol. 2006;26:3048–3059. doi: 10.1128/MCB.26.8.3048-3059.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schesser K, Spiik AK, Dukuzumuremyi JM, Neurath MF, Pettersson S, Wolf-Watz H. The yopJ locus is required for Yersinia-mediated inhibition of NF-kappaB activation and cytokine expression: YopJ contains a eukaryotic SH2-like domain that is essential for its repressive activity. Mol. Microbiol. 1998;28:1067–1079. doi: 10.1046/j.1365-2958.1998.00851.x. [DOI] [PubMed] [Google Scholar]
- 162.Orth K, Palmer LE, Bao ZQ, Stewart S, Rudolph AE, Bliska JB, Dixon JE. Inhibition of the mitogen-activated protein kinase kinase superfamily by a Yersinia effector. Science. 1999;285:1920–1923. doi: 10.1126/science.285.5435.1920. [DOI] [PubMed] [Google Scholar]
- 163.Collier-Hyams LS, Zeng H, Sun J, Tomlinson AD, Bao ZQ, Chen H, Madara JL, Orth K, Neish AS. Cutting edge: Salmonella AvrA effector inhibits the key proinflammatory, anti-apoptotic NF-kappa B pathway. J. Immunol. 2002;169:2846–2850. doi: 10.4049/jimmunol.169.6.2846. [DOI] [PubMed] [Google Scholar]
- 164.Pahl HL, Krauss B, Schulze-Osthoff K, Decker T, Traenckner EB, Vogt M, Myers C, Parks T, Warring P, Muhlbacher A, Czernilofsky AP, Baeuerle PA. The immunosuppressive fungal metabolite gliotoxin specifically inhibits transcription factor NF-kappaB. J. Exp. Med. 1996;183:1829–1840. doi: 10.1084/jem.183.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Erkel G, Anke T, Sterner O. Inhibition of NF-kappa B activation by panepoxydone. Biochem. Biophys. Res. Commun. 1996;226:214–221. doi: 10.1006/bbrc.1996.1335. [DOI] [PubMed] [Google Scholar]
- 166.Umezawa K, Ariga A, Matsumoto N. Naturally occurring and synthetic inhibitors of NF-kappaB functions. Anti-cancer Drug Des. 2000;15:239–244. [PubMed] [Google Scholar]
- 167.De Bosscher K, Vanden Berghe W, Haegeman G. Cross-talk between nuclear receptors and nuclear factor kappaB. Oncogene. 2006;25:6868–6886. doi: 10.1038/sj.onc.1209935. [DOI] [PubMed] [Google Scholar]
- 168.Kalaitzidis D, Gilmore TD. Transcription factor cross-talk: the estrogen receptor and NF-kappaB. Trends Endocrinol. Metabol. 2005;16:46–52. doi: 10.1016/j.tem.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 169.Olivier S, Close P, Castermans E, de Leval L, Tabruyn S, Chariot A, Malaise M, Merville MP, Bours V, Franchimont N. Raloxifene-induced myeloma cell apoptosis: a study of nuclear factor-kappaB inhibition and gene expression signature. Mol. Pharmacol. 2006;69:1615–1623. doi: 10.1124/mol.105.020479. [DOI] [PubMed] [Google Scholar]
- 170.McDade TP, Perugini RA, Vittimberga FJ, Jr, Carrigan RC, Callery MP. Salicylates inhibit NF-kappaB activation and enhance TNF-alpha-induced apoptosis in human pancreatic cancer cells. J. Surg. Res. 1999;83:56–61. doi: 10.1006/jsre.1998.5560. [DOI] [PubMed] [Google Scholar]
- 171.Yamamoto Y, Yin MJ, Lin KM, Gaynor RB. Sulindac inhibits activation of the NF-kappaB pathway. J. Biol. Chem. 1999;274:27307–27314. doi: 10.1074/jbc.274.38.27307. [DOI] [PubMed] [Google Scholar]
- 172.Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 173.Dikshit P, Chatterjee M, Goswami A, Mishra A, Jana NR. Aspirin induces apoptosis through the inhibition of proteasome function. J. Biol. Chem. 2006;281:29228–29235. doi: 10.1074/jbc.M602629200. [DOI] [PubMed] [Google Scholar]
- 174.McCarty MF, Block KI. Preadministration of high-dose salicylates, suppressors of NFkappaB activation, may increase the chemosensitivity of many cancers: an example of proapoptotic signal modulation therapy. Integr. Cancer Ther. 2006;5:252–268. doi: 10.1177/1534735406291499. [DOI] [PubMed] [Google Scholar]
- 175.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 176.Li JJ, Fang CH. C-reactive protein is not only an inflammatory marker but also a direct cause of cardiovascular diseases. Med. Hypotheses. 2004;62:499–506. doi: 10.1016/j.mehy.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 177.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 178.Grilli M, Pizzi M, Memo M, Spano P. Neuroprotection by aspirin and sodium salicylate through blockade of NF-kappaB activation. Science. 1996;274:1383–1385. doi: 10.1126/science.274.5291.1383. [DOI] [PubMed] [Google Scholar]
- 179.Palayoor ST, Bump EA, Calderwood SK, Bartol S, Coleman CN. Combined antitumor effect of radiation and ibuprofen in human prostate carcinoma cells. Clin. Cancer Res. 1998;4:763–771. [PubMed] [Google Scholar]
- 180.Takada Y, Bhardwaj A, Potdar P, Aggarwal BB. Aggarwal, Nonsteroidal anti-inflammatory agents differ in their ability to suppress NF-kappaB activation, inhibition of expression of cyclooxygenase-2 and cyclin D1, and abrogation of tumor cell proliferation. Oncogene. 2004;23:9247–9258. doi: 10.1038/sj.onc.1208169. [DOI] [PubMed] [Google Scholar]
- 181.Meyer S, Kohler NG, Joly A. Cyclosporine A is an uncompetitive inhibitor of proteasome activity and prevents NF-kappaB activation. FEBS Lett. 1997;413:354–358. doi: 10.1016/s0014-5793(97)00930-7. [DOI] [PubMed] [Google Scholar]
- 182.Frantz B, Nordby EC, Bren G, Steffan N, Paya CV, Kincaid RL, Tocci MJ, O'Keefe SJ, O'Neill EA. Calcineurin acts in synergy with PMA to inactivate I kappa B/MAD3, an inhibitor of NF-kappa B. EMBO J. 1994;13:861–870. doi: 10.1002/j.1460-2075.1994.tb06329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tepper MA, Nadler SG, Esselstyn JM, Sterbenz KG. Deoxyspergualin inhibits kappa light chain expression in 70Z/3 pre-B cells by blocking lipopolysaccharide-induced NF-kappa B activation. J. Immunol. 1995;155:2427–2436. [PubMed] [Google Scholar]
- 184.McCaffrey PG, Kim PK, Valge-Archer VE, Sen R, Rao A. Cyclosporin A sensitivity of the NF-kappa B site of the IL2R alpha promoter in untransformed murine T cells. Nucleic Acids Res. 1994;22:2134–2142. doi: 10.1093/nar/22.11.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wechsler AS, Gordon MC, Dendorfer U, LeClair KP. Induction of IL-8 expression in T cells uses the CD28 costimulatory pathway. J. Immunol. 1994;153:2515–2523. [PubMed] [Google Scholar]
- 186.Kunz D, Walker G, Eberhardt W, Nitsch D, Pfeilschifter J. Interleukin 1 beta-induced expression of nitric oxide synthase in rat renal mesangial cells is suppressed by cyclosporin A. Biochem. Biophys. Res. Commun. 1995;216:438–446. doi: 10.1006/bbrc.1995.2642. [DOI] [PubMed] [Google Scholar]
- 187.Qiu D, Zhao G, Aoki Y, Shi L, Uyei A, Nazarian S, Ng JC, Kao PN. Immunosuppressant PG490 (triptolide) inhibits T-cell interleukin-2 expression at the level of purine-box/nuclear factor of activated T-cells and NF-kappaB transcriptional activation. J. Biol. Chem. 1999;274:13443–13450. doi: 10.1074/jbc.274.19.13443. [DOI] [PubMed] [Google Scholar]
- 188.Sen J, Venkataraman L, Shinkai Y, Pierce JW, Alt FW, Burakoff SJ, Sen R. Expression and induction of nuclear factor-kappa B-related proteins in thymocytes. J. Immunol. 1995;154:3213–3221. [PubMed] [Google Scholar]
- 189.Venkataraman L, Burakoff SJ, Sen R. FK506 inhibits antigen receptor-mediated induction of c-rel in B and T lymphoid cells. J. Exp. Med. 1995;181:1091–1099. doi: 10.1084/jem.181.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Webster GA, Perkins ND. Transcriptional cross talk between NF-kappaB and p53. Mol. Cell. Biol. 1999;19:3485–3495. doi: 10.1128/mcb.19.5.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Huang WC, Ju TK, Hung MC, Chen CC. Phosphorylation of CBP by IKKalpha promotes cell growth by switching the binding preference of CBP from p53 to NF-kappaB. Mol. Cell. 2007;26:75–87. doi: 10.1016/j.molcel.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gurova KV, Hill JE, Guo C, Prokvolit A, Burdelya LG, Samoylova E, Khodyakova AV, Ganapathi R, Ganapathi M, Tararova ND, Bosykh D, Lvovskiy D, Webb TR, Stark GR, Gudkov AV. Small molecules that reactivate p53 in renal cell carcinoma reveal a NF-kappaB-dependent mechanism of p53 suppression in tumors. Proc. Natl. Acad. Sci. U.S.A. 2005;102:17448–17453. doi: 10.1073/pnas.0508888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tovar C, Rosinski J, Filipovic Z, Higgins B, Kolinsky K, Hilton H, Zhao X, Vu BT, Qing W, Packman K, Myklebost O, Heimbrook DC, Vassilev LT. Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy. Proc. Natl. Acad. Sci. U.S.A. 2006;103:1888–1893. doi: 10.1073/pnas.0507493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dey A, Wong ET, Bist P, Tergaonkar V, Lane DP. Nutlin-3 inhibits the NFkappaB pathway in a p53-dependent manner: implications in lung cancer therapy. Cell cycle. 2007;6:2178–2185. doi: 10.4161/cc.6.17.4643. [DOI] [PubMed] [Google Scholar]
- 195.Campbell KJ, Witty JM, Rocha S, Perkins ND. Cisplatin mimics ARF tumor suppressor regulation of RelA (p65) nuclear factor-kappaB transactivation. Cancer Res. 2006;66:929–935. doi: 10.1158/0008-5472.CAN-05-2234. [DOI] [PubMed] [Google Scholar]
- 196.Dey A, Tergaonkar V, Lane DP. Double-edged swords as cancer therapeutics: simultaneously targeting p53 and NF-kappaB pathways. Nat. Rev. Drug Discov. 2008;7:1031–1040. doi: 10.1038/nrd2759. [DOI] [PubMed] [Google Scholar]
- 197.Rodriguez MS, Thompson J, Hay RT, Dargemont C. Nuclear retention of IkappaBalpha protects it from signal-induced degradation and inhibits nuclear factor kappaB transcriptional activation. J. Biol. Chem. 1999;274:9108–9115. doi: 10.1074/jbc.274.13.9108. [DOI] [PubMed] [Google Scholar]
- 198.Kau TR, Silver PA. Nuclear transport as a target for cell growth. Drug Discov. Today. 2003;8:78–85. doi: 10.1016/s1359-6446(02)02562-x. [DOI] [PubMed] [Google Scholar]
- 199.Dasgupta A, Jung KJ, Jeong SJ, Brady JN. Inhibition of methyltransferases results in induction of g2/m checkpoint and programmed cell death in human T-lymphotropic virus type 1-transformed cells. J. Virol. 2008;82:49–59. doi: 10.1128/JVI.01497-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li HL, Chen DD, Li XH, Zhang HW, Lu YQ, Ye CL, Ren XD. Changes of NFkB, p53, Bcl-2 and caspase in apoptosis induced by JTE-522 in human gastric adenocarcinoma cell line AGS cells: role of reactive oxygen species. World J. Gastroenterol. 2002;8:431–435. doi: 10.3748/wjg.v8.i3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lu W, Chen L, Peng Y, Chen J. Activation of p53 by roscovitine-mediated suppression of MDM2 expression. Oncogene. 2001;20:3206–3216. doi: 10.1038/sj.onc.1204412. [DOI] [PubMed] [Google Scholar]
- 202.Dey A, Wong ET, Cheok CF, Tergaonkar V, Lane DP. R-Roscovitine simultaneously targets both the p53 and NF-kappaB pathways and causes potentiation of apoptosis: implications in cancer therapy. Cell Death Differ. 2008;15:263–273. doi: 10.1038/sj.cdd.4402257. [DOI] [PubMed] [Google Scholar]
- 203.Demidenko ZN, Blagosklonny MV. Flavopiridol induces p53 via initial inhibition of Mdm2 and p21 and, independently of p53, sensitizes apoptosis-reluctant cells to tumor necrosis factor. Cancer Res. 2004;64:3653–3660. doi: 10.1158/0008-5472.CAN-04-0204. [DOI] [PubMed] [Google Scholar]
- 204.Takada Y, Aggarwal BB. Flavopiridol inhibits NF-kappaB activation induced by various carcinogens and inflammatory agents through inhibition of IkappaBalpha kinase and p65 phosphorylation: abrogation of cyclin D1, cyclooxygenase-2, and matrix metalloprotease-9. J. Biol. Chem. 2004;279:4750–4759. doi: 10.1074/jbc.M304546200. [DOI] [PubMed] [Google Scholar]
- 205.Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin. Immunol. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- 206.Belich MP, Salmeron A, Johnston LH, Ley SC. TPL-2 kinase regulates the proteolysis of the NF-kappaB-inhibitory protein NF-kappaB1 p105. Nature. 1999;397:363–368. doi: 10.1038/16946. [DOI] [PubMed] [Google Scholar]
- 207.Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem. Sci. 2005;30:43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 208.Li N, Karin M. Ionizing radiation and short wavelength UV activate NF-kappaB through two distinct mechanisms. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13012–13017. doi: 10.1073/pnas.95.22.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kato T, Jr, Delhase M, Hoffmann A, Karin M. CK2 Is a C-Terminal IkappaB Kinase Responsible for NF-kappaB Activation during the UV Response. Mol. Cell. 2003;12:829–839. doi: 10.1016/s1097-2765(03)00358-7. [DOI] [PubMed] [Google Scholar]
- 210.Sethi G, Ahn KS, Chaturvedi MM, Aggarwal BB. Epidermal growth factor (EGF) activates nuclear factor-kappaB through IkappaBalpha kinase-independent but EGF receptor-kinase dependent tyrosine 42 phosphorylation of IkappaBalpha. Oncogene. 2007;26:7324–7332. doi: 10.1038/sj.onc.1210544. [DOI] [PubMed] [Google Scholar]
- 211.West AP, Koblansky AA, Ghosh S. Recognition and signaling by toll-like receptors. Annu. Rev. Cell Dev. Biol. 2006;22:409–437. doi: 10.1146/annurev.cellbio.21.122303.115827. [DOI] [PubMed] [Google Scholar]
- 212.Takada Y, Mukhopadhyay A, Kundu GC, Mahabeleshwar GH, Singh S, Aggarwal BB. Hydrogen peroxide activates NF-kappa B through tyrosine phosphorylation of I kappa B alpha and serine phosphorylation of p65: evidence for the involvement of I kappa B alpha kinase and Syk protein-tyrosine kinase. J. Biol. Chem. 2003;278:24233–24241. doi: 10.1074/jbc.M212389200. [DOI] [PubMed] [Google Scholar]
- 213.Imbert V, Rupec RA, Livolsi A, Pahl HL, Traenckner EB, Mueller-Dieckmann C, Farahifar D, Rossi B, Auberger P, Baeuerle PA, Peyron JF. Tyrosine phosphorylation of I kappa B-alpha activates NF-kappa B without proteolytic degradation of I kappa B-alpha. Cell. 1996;86:787–798. doi: 10.1016/s0092-8674(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 214.Schulze-Luehrmann J, Ghosh S. Antigen-receptor signaling to nuclear factor kappa B. Immunity. 2006;25:701–715. doi: 10.1016/j.immuni.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 215.Noels H, van Loo G, Hagens S, Broeckx V, Beyaert R, Marynen P, Baens M. A Novel TRAF6 binding site in MALT1 defines distinct mechanisms of NF-kappaB activation by API2middle dotMALT1 fusions. J. Biol. Chem. 2007;282:10180–10189. doi: 10.1074/jbc.M611038200. [DOI] [PubMed] [Google Scholar]
- 216.Rosato RR, Kolla SS, Hock SK, Almenara JA, Patel A, Amin S, Atadja P, Dent P, Grant S. HDAC inhibitors activate NF-{kappa}B in human leukemia cells through an ATM/nemo-related pathway. J. Biol. Chem. 2010;285:10064–10077. doi: 10.1074/jbc.M109.095208. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 217.Wu ZH, Shi Y, Tibbetts RS, Miyamoto S. Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science. 2006;311:1141–1146. doi: 10.1126/science.1121513. [DOI] [PubMed] [Google Scholar]
- 218.Janssens S, Tinel A, Lippens S, Tschopp J. PIDD mediates NF-kappaB activation in response to DNA damage. Cell. 2005;123:1079–1092. doi: 10.1016/j.cell.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 219.Hur GM, Lewis J, Yang Q, Lin Y, Nakano H, Nedospasov S, Liu ZG. The death domain kinase RIP has an essential role in DNA damage-induced NF-kappa B activation. Genes Dev. 2003;17:873–882. doi: 10.1101/gad.1062403. [DOI] [PMC free article] [PubMed] [Google Scholar]