Abstract
FAS (APO-1/CD95) and its physiological ligand, FASL, regulate apoptotic death of unwanted or dangerous cells in many tissues, functioning as a guardian against autoimmunity and cancer development1-4. Distinct cell types differ in the mechanisms by which the ‘death receptor’ FAS triggers their apoptosis1-4. In type I cells, such as lymphocytes, activation of ‘effector caspases’ by FAS-induced activation of caspase-8 suffices for cell killing whereas in type II cells, including hepatocytes and pancreatic β-cells, amplification of the caspase cascade through caspase-8 mediated activation of the pro-apoptotic BCL-2 family member BID5 is essential6-8. Here we show, that loss of X-chromosome linked inhibitor of apoptosis (XIAP)9,10 function by gene-targeting or treatment with a second mitochondria-derived activator of caspases (SMAC11, also called DIABLO12: direct IAP binding protein with low pI) mimetic drug rendered hepatocytes independent of BID for FAS-induced apoptosis signalling. These results show that XIAP is the critical discriminator between type I versus type II apoptosis signalling and suggest that IAP inhibitors should be used with caution in cancer patients with underlying liver conditions.
Hepatocytes are highly sensitive to FASL13 or agonistic antibodies14 and, based on the up-regulation of FAS on hepatocytes and invasion of FASL-expressing cytotoxic T lymphocytes or NK cells into hepatic sinusoids, FAS-induced apoptosis has been implicated as a cause of a variety of acute and chronic liver diseases, such as viral, drug or alcohol induced hepatitis15. Caspase-816 and its activator FADD/MORT117 are required for FAS-induced apoptosis in all cell types studied so far. Although initial studies with cell lines produced conflicting results13,18, it is now clear that amplification of apoptosis signalling through caspase-8-mediated proteolytic activation of the BH3-only protein BID leading to BAX/BAK-dependent activation of caspase-9 and effector caspases is essential in hepatocytes and pancreatic β-cells (type II cells) but dispensable in lymphocytes (type I cells; Supplementary Fig. 1)6-8.
It is unclear why FAS activates such substantially different apoptotic pathways in different cell types, but it has been postulated that this may be due to differences in the extent of FAS aggregation or internalisation, extent of caspase cascade activation, levels of caspase inhibitors (XIAP) and/or abundance of caspase substrates that need to be proteolysed for cells to die4,18-20. To begin to explore the differences between type I and type II cells, we compared the levels and activation status of apoptosis regulators and the processing of critical caspase substrates between characteristic type I cells, thymocytes, and type II cells, hepatocytes, after FAS stimulation (Supplementary Fig. 2a-c). Processing of caspase-8 into its cleaved form (p18) could be detected as early as 15 min after FAS stimulation by immunoblotting or pull-down of the active enzyme with biotinylated X-VAD-fmk (a compound that binds efficiently to active caspases) followed by immunoblotting with a caspase-8-specific antibody (Fig. 2e and Supplementary Fig. 2a). Caspase-8-mediated proteolysis of BID and activation of caspase-3 and -7 became evident by ~15 min and ~60 min, respectively (Supplementary Fig. 2a, b). Apoptosis induction was equivalent between WT thymocytes and hepatocytes (Supplementary Fig. 2a-c). As reported7, FASL elicited a similar extent of apoptosis in WT and Bid-/- thymocytes, regardless of whether they were kept in single-cell suspension cultures or fetal thymic organ culture (FTOC; Supplementary Fig. 2a, c). BID-deficient thymocytes and hepatocytes exhibited normal levels of early caspase-8 activation, but BID-deficient hepatocytes showed considerably less caspase-9 activation and a complete lack of effector caspase activation compared to their WT counterparts or thymocytes from WT as well as BID-deficient mice (Supplementary Fig. 2a, b). The levels of anti-apoptotic BCL-2, BCL-XL, MCL-1 as well as the pro-apoptotic SMAC/DIABLO were similar between thymocytes and hepatocytes and remained largely unchanged during FAS activation (Fig. 1a and Supplementary Fig. 2d, e).
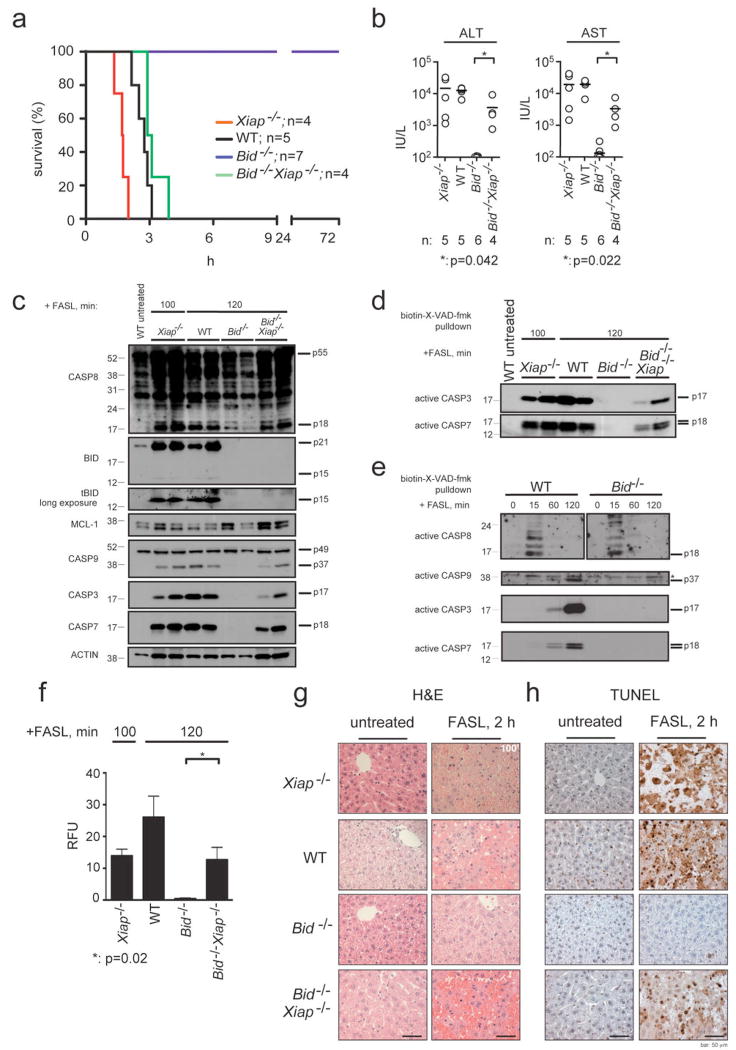
Figure 2. Loss of XIAP re-sensitises BID-deficient mice to FASL-induced fatal hepatitis.
a, Long-term survival of WT, Xiap-/-, Bid-/- and Bid-/-Xiap-/- mice injected i.v. with 0.25 mg/kg FLAG-tagged FASL crosslinked with anti-FLAG antibody (2 μg/μg of FASL) is shown, p (WT vs Bid-/-)<0.0003; p (Bid-/- vs Bid-/-Xiap-/-)= 0.0005; p (WT vs Bid-/-Xiap-/-)=0.1133. b, Serum levels of ALT and AST after 120 min of FASL treatment (100 min for Xiap-/- mice) were quantified in mice of the indicated genotypes. The horizontal bars indicate the mean; n: numbers of mice analysed. P values are indicated below graphs. c, Processing of caspases -3, -7, -8, -9, BID (truncated tBID panel exposed longer), MCL-1 and β-actin (loading control) in livers of the mice treated as described in a was examined by immunoblotting. Total protein lysates were prepared from livers of two experimental animals per genotype and time point of treatment. d, Activation of caspases -3 and -7 in livers of WT, Xiap-/-, Bid-/- and Bid-/-Xiap-/- mice treated as described in a and sacrificed at 120 min (100 min for Xiap-/- mice) was examined by pulldown with the caspase inhibitor biotin-X-VAD-fmk followed by detection by immunoblotting using caspase-specific antibodies. e, Activation of caspases -3, -7, -8 and -9 in liver extracts from WT or Bid-/- mice treated as described in a and sacrificed at the indicated time points, was examined as described in d. Asterix indicates a cross-reactive band. f, Fluorogenic measurement of the enzymatic activity of effector caspases (DEVDase activity) in the livers of the mice of the indicated genotypes treated as described in a. Four independent measurements of two individual mice per genotype and time point of treatment are shown. Data represent means +/-SEM. g, Histological analysis (H&E staining) of liver sections from animals of the indicated genotypes treated as described in a sacrificed after 120 min of FASL treatment (100 min for Xiap-/- mice) and untreated control animals. h, TUNEL staining of liver sections from the animals described in f. Images are representative of a least 3 mice per genotype and time point of treatment. Scale bar = 50 μm.
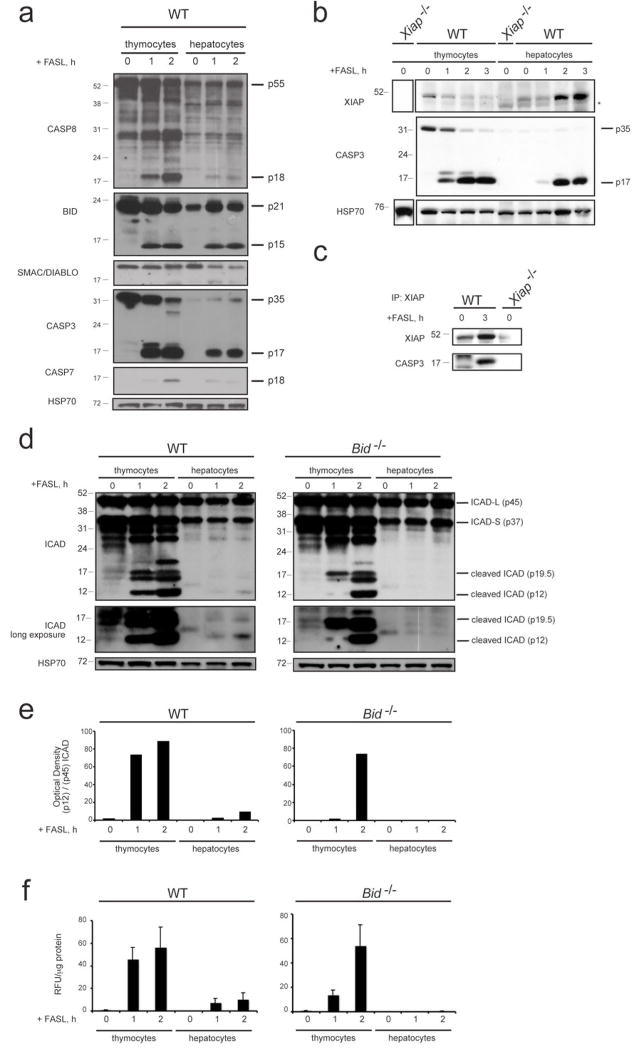
Figure 1. Comparison of the levels of XIAP, caspase activation and proteolysis of caspase substrates between FASL-treated thymocytes and hepatocytes.
a, Thymocytes from WT mice were treated in culture for the times indicated with 100 ng/mL FLAG-tagged FASL crosslinked with anti-FLAG antibody (2 μg/mL). Livers were harvested from WT mice that had been injected i.v. with 0.25 mg/kg FLAG-tagged FASL crosslinked with anti-FLAG antibody (2 μg/μg FASL) and sacrificed at the indicated time points. Lysates from thymocytes and livers were examined by immunoblotting for the expression and processing of caspase-8, BID, SMAC/DIABLO, caspase-3, caspase-7 and HSP70 (loading control). b, Lysates from thymocytes and hepatocytes of the indicated genotype treated as described in a were examined by immunoblotting using antibodies to XIAP, caspase-3 and HSP70 (loading control). Asterix indicates cross-reactive band. c, The binding of active caspase-3 to XIAP in liver lysates from mice of the indicated genotype treated as described in a was examined by co-immunoprecipitation with an antibody to XIAP followed by immunoblotting with antibodies to active caspase-3 or, as a loading control, with antibodies to XIAP. d, Lysates from thymocytes or hepatocytes of the indicated genotype treated as described in a were examined by immunoblotting using antibodies to the caspase substrate ICAD (lower panel exposed longer) and HSP70 (loading control). e, Relative optical density of bands (as shown in d) of cleaved ICAD (pl2) in relation to total ICAD-L (p45) is shown. f, Fluorogenic measurement of the enzymatic activity of effector caspases (DEVDase activity) in extracts from thymocytes and hepatocytes of the indicated genotype treated as described in a. Data shown are standardized per μg of protein. Three independent measurements of two individual mice per genotype and time point of treatment are shown. Error bars indicate standard error of the mean.
Interestingly, however, despite the fact that the basal levels of XIAP were comparable between both cell types, we found that in thymocytes treatment with FASL resulted in rapid loss of XIAP that paralleled cell death (Fig. 1b). Addition of the broad-spectrum caspase inhibitor QVD-oph blocked apoptosis and prevented disappearance of XIAP in FASL-treated thymocytes (Supplementary Fig. 3a), whereas inhibition of the proteasome had no impact on XIAP levels (Supplementary Fig. 3b). In striking contrast, in hepatocytes treatment with FASL resulted in an increase in XIAP protein levels over the first three hours, despite efficient initiation of apoptosis (Fig. 1b). The FASL treatment-induced increase in XIAP could be blocked by addition of QVD-oph or BID-deficiency, which prevents effector caspase activation (Supplementary Fig. 3c, d). Immunoprecipitation with antibodies to XIAP showed that treatment with FASL caused an increase in the amount of processed caspase-3 associated with its inhibitor XIAP in hepatocytes (Fig. 1c), indicating that XIAP is stabilised by binding and inhibiting proteolytically activated effector caspases. Furthermore, substantial differences in cleavage of the proto-typical effector caspase substrate ICAD (inhibitor of caspase-activated DNase, CAD)21,22 were detected between thymocytes and hepatocytes. Cleaved fragments of ICAD appeared after 60 min of FASL treatment in thymocytes from WT and Bid-/- animals. Notably, in WT hepatocytes ICAD processing was considerably less efficient compared to WT or Bid-/- thymocytes and in BID-deficient livers no ICAD cleavage could be detected (Fig. 1d, e). Accordingly, fluorogenic (DEVDase) assays showed that FASL-treated WT and Bid-/- thymocytes had ~6-fold higher levels of overall effector caspase activity compared to WT hepatocytes and no such enzymatic activity was detected in hepatocytes from FASL-injected Bid-/- mice (Fig. 1f). These results indicate that the ratio between proteolytically activated effector caspases, the caspase inhibitor XIAP and the caspase substrates that need to be proteolysed may determine whether cells undergo type I or type II FAS-induced apoptosis.
Therefore and because XIAP is a potent inhibitor of caspase-9 and effector caspases9,10 and because XIAP over-expression delayed FAS-induced apoptosis in mouse thymocytes23, we explored the role of XIAP in FASL- (Fig. 2) and anti-FAS antibody-induced (not shown) hepatocyte killing in vivo. As reported13,14, WT mice succumbed to these treatments within 2.5-3 h (FASL, Fig. 2a) or 3-4 h (anti-FAS antibody), respectively. Interestingly, all XIAP-deficient mice died considerably earlier (within 1-2 h for FASL; Fig. 2a and within 2 h for anti-FAS antibody), presenting with elevated serum levels of the liver associated transaminases ALT and AST (Fig. 2b) and severe liver damage (Fig. 2g, h). Thus, XIAP is a critical attenuator of FAS-induced hepatocyte apoptosis. In contrast, loss of XIAP did not increase FAS-mediated apoptosis in thymocytes (type I cells; data not shown and24).
To examine whether XIAP is the crucial discriminator between type I versus type II FAS-induced apoptosis signalling, we generated BID/XIAP double-deficient mice, which proved to be fertile and presented with no obvious abnormalities under unstressed conditions. As previously reported6,7, BID-deficient mice survived injection with FASL or anti-FAS antibody without developing signs of illness and showed no abnormal increase in serum ALT or AST levels or disruption of liver architecture (Fig. 2 and Supplementary Fig. 4a). In contrast, all BID/XIAP double-deficient animals succumbed to this treatment (albeit slightly later than WT mice; ~3-3.5 h vs 2.5-3 h), presenting with elevated liver enzyme levels in their sera and severe liver damage (Fig. 2a, b, g, h and Supplementary Fig. 4a). Immunoblotting demonstrated that FAS stimulation caused similar levels of caspase-8 processing in livers of mice of all genotypes, but caspase-9 and effector caspases were only substantially activated in livers of the sensitive animals (WT, Xiap-/- and Bid-/-Xiap-/-) but not in livers from the resistant Bid-/- mice (Fig. 2c). This was confirmed by pull-down of active caspases -8, -9, -3 and -7 with biotinylated X-VAD-fmk followed by immunoblotting with caspase-specific antibodies (Fig. 2d, e) and by fluorogenic effector caspase activity (DEVDase) assays (Fig. 2f). The hepatocytes in the FASL- or anti-FAS antibody-injected Bid-/-Xiap-/- mice exhibited severe disruption of liver architecture (Fig. 2g) and classical signs of apoptosis, including chromatin condensation and DNA cleavage, the latter revealed by TUNEL staining (Fig. 2h and Supplementary Fig. 4a).
In accordance with our analyses of hepatocytes, pancreatic β-cells, which also undergo type II Fas-induced apoptosis signalling8, deficient for BID were resistant to FASL, but concomitant loss of XIAP (in Bid-/-Xiap-/- mice) restored sensitivity (Supplementary Fig. 5).
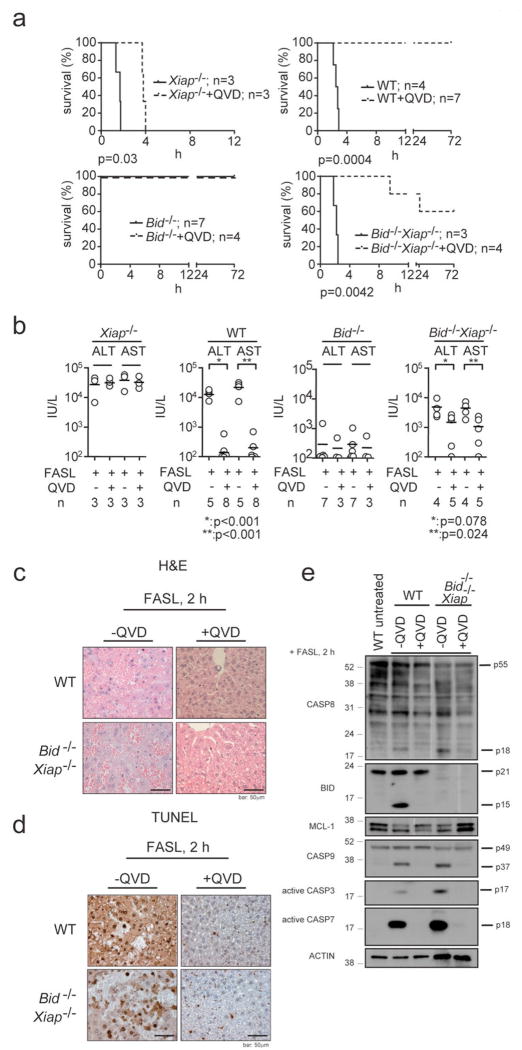
Remarkably, pre-treatment with the broad spectrum caspase inhibitor QVD-oph significantly (p=0.0042) protected Bid-/-Xiap-/- mice from FASL-induced fatal hepatitis and these animals contained lower ALT (p=0.078) and AST (p=0.024) levels in their sera and less hepatocyte destruction compared to Bid-/-Xiap-/- animals that had only been treated with FASL (Fig. 3a-d and Supplementary Fig. 4b). As expected, pre-treatment with QVD-oph inhibited FASL-induced processing of caspase-8 and subsequent proteolytic activation of BID and caspases -3, -7 and -9 (Fig. 3e). Although QVD-oph was not able to rescue all Bid-/-Xiap-/- mice from FASL-induced fatal hepatitis (presumably because the inhibitor could not completely block caspase activation long-term), these results show that FAS stimulation kills BID/XIAP double-deficient hepatocytes through caspase-dependent apoptosis and not through some caspase-independent process. Thus, XIAP functions as the critical regulator that discriminates between type I versus type II FAS-induced apoptosis signalling.
Figure 3. Caspase inhibitors protect Bid-/-Xiap-/- hepatocytes from FASL-induced apoptosis.
a, Long-term survival of WT, Xiap-/-, Bid-/- and Bid-/-Xiap-/- mice injected i.v. with 0.25 mg/kg FLAG-tagged FASL crosslinked with anti-FLAG antibody (2 μg/μg of FASL) with or without pre-treatment with the broad spectrum caspase inhibitor Q-VD-oph (20 mg/kg, i.p., 30 min prior to FASL administration) is shown. P values are indicated below individual panels. b, Levels of transaminases ALT and AST were quantified in sera of mice of the indicated genotypes after 120 min of FASL treatment (100 min for Xiap-/- mice). The horizontal bars indicate the mean; n: numbers of mice analysed. P values as indicated by asterix. c, Histological analysis (H&E staining) of liver sections from animals of the indicated genotypes treated as described in a and sacrificed after 120 min (100 min for Xiap-/- mice). d, TUNEL staining of liver sections from animals described in c. Images are representative of a least 3 mice per genotype and time point of FASL treatment. Scale bar = 50 μm. e, Processing of caspases -3, -7, -8, -9, BID, MCL-1 and β-actin (loading control) in extracts of livers from WT and Bid-/-Xiap-/- mice treated as described in a was examined by immunoblotting.
An important question is whether cytochrome c/APAF-1 mediated activation of caspase-9 is necessary for type II FAS-induced apoptosis, or whether the release of IAP antagonists (e.g. SMAC/DIABLO) from mitochondria and their effect on XIAP, leading to the release of active effector caspases, is sufficient. Notably, apoptotic nuclei (Fig. 2h) and caspase -3 and -7 activation (Fig. 2c, d) were detectable in livers from Bid-/-Xiap-/- mice 120 min after FASL injection, although at that time the cleaved form of caspase-9 was observed at lower levels in Bid-/-Xiap-/- mice compared to WT mice (Fig. 2c). The caspase-9 processing seen in Bid-/-Xiap-/- hepatocytes (Fig. 2c) might thus be a consequence of cleavage of pro-caspase-9 by already activated effector caspases, indicating that caspase-9 may not be necessary for effector caspase activation in FAS-induced hepatocyte apoptosis. Consistent with this hypothesis, gene-targeted mice harbouring a mutation in cytochrome c that allows respiration but prevents apoptosome formation (and hence caspase-9 activation), were found to be normally sensitive to FAS-induced hepatocyte killing25, whereas combined loss of caspases-3 and -7 rendered cells resistant to FASL26.
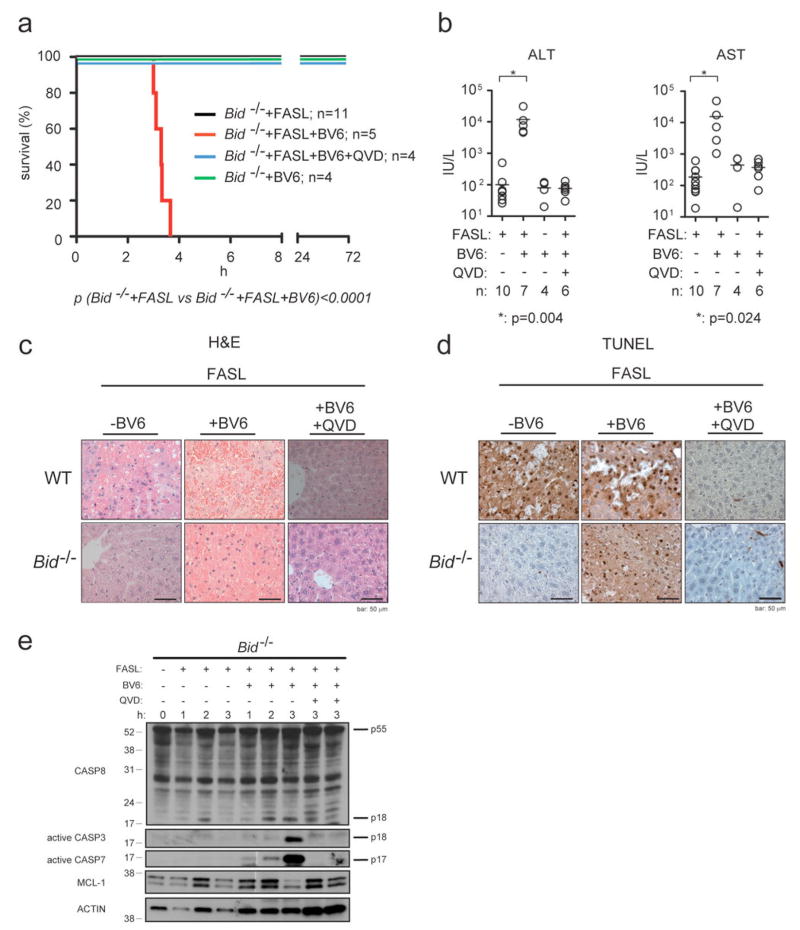
XIAP and related IAPs (cIAP1/2) are negatively regulated by SMAC/DIABLO11,12 and OMI/HTRA2 proteins that are released from the mitochondria during apoptosis9,10. SMAC/DIABLO mimetics are currently in development for cancer therapy and were shown to kill tumour cells (at least in part) by enhancing autocrine TNF>TNF-R1 signalling27-30. We examined the impact of the IAP inhibitor BV627 on FASL-induced hepatocyte killing and hepatitis. WT mice injected with BV6 plus FASL died significantly earlier than WT mice treated with FASL alone, presenting with elevated serum levels of ALT and AST similar to Xiap-/- mice (Supplementary Fig. 6). Remarkably, all Bid-/- mice injected with FASL plus BV6 rapidly developed fatal hepatitis (Fig. 4a), presenting with abnormally increased serum ALT and AST levels (Fig. 4b) and extensive hepatocyte destruction (Fig. 4c, d and Supplementary Fig. 4c), whereas all Bid-/- mice injected with FASL alone or BV6 alone remained healthy. In agreement with the observed morbidity and liver damage, and despite initial activation of caspase-8 in all FASL-treated mice, significant activation of effector caspases -3 and -7 was only seen in Bid-/- mice that were injected with both FASL and BV6 but not in those treated with FASL or BV6 alone (Fig. 4e and data not shown). Importantly, pre-treatment with QVD-oph protected Bid-/- mice from FASL plus BV6 induced fatal hepatitis (Fig. 4a) and, accordingly, reduced serum levels of ALT and AST to those seen in animals treated with FASL alone (Fig. 4b). Moreover, histological examination and TUNEL staining revealed normal liver architecture and only few apoptotic hepatocytes in FASL plus BV6 plus QVD-oph treated animals (Fig. 4c, d and Supplementary Fig. 4c). This demonstrates that the combination of FASL plus BV6 triggers classical, caspase-dependent hepatocyte apoptosis in Bid-/- mice. It could be argued that BV6 treatment may promote FASL induced hepatocyte killing by blocking cIAP1 and/or cIAP, which would be expected to require autocrine/paracrine TNFα>TNF-Rl signalling27-30. ELISA showed, however, that treatment with FASL, BV6 or both had no impact on the levels of TNFα in the livers of mice (Supplementary Figs. 6c and 7c). Pertinently, TNFα neutralising antibodies had no impact on FASL plus BV6 induced hepatocyte killing in Bid-/- mice (Supplementary Fig. 7a, b) although they could abrogate TNFα-induced killing of L929 cells in culture (Supplementary Fig. 8a) and prevent lipopolysacchride plus galactosamine (LPS+Ga1N) induced fatal hepatitis in mice (Supplementary Fig. 8b, c), a process dependent of TNFα>TNF-Rl signalling7. These results demonstrate that BV6 does not need to activate TNF>TNF-R1 signalling to promote FASL induced hepatocyte killing, but functions by blocking the anti-apoptotic activity of XIAP. The notion that XIAP rather than cIAP1/cIAP2 is the critical target of BV6 in FAS-induced hepatocyte apoptosis is underscored by the finding that only Xiap-/- mice (Fig. 2a and Supplementary Figure 6a), but not mice lacking cIAP1, cIAP2, or both in their livers succumbed more rapidly to FASL induced hepatitis than WT animals (not shown). The critical role of XIAP in regulating FAS-induced apoptosis predicts that endogenous IAP antagonists, such as SMAC/DIABLO or OMI/HTRA2, play critical roles in this process. Since Bid-/-Xiap-/- mice succumb to FASL-induced hepatitis more slowly than WT controls, apoptosis inhibitors in addition to XIAP, such as ML-IAP10, may contribute to the regulation of this process. Finally, while IAP inhibition represents a promising new strategy for the treatment of cancer, our data indicate that IAP inhibitors should be used with caution in cancer patients in combination with liver toxic chemotherapy or with underlying liver conditions, particularly those associated with significant infiltration of intra-hepatic FASL-producing cells.
Figure 4. The SMAC/DIABLO mimetic drug BV6 sensitises BID-deficient mice to FASL-induced hepatocyte destruction.
a, Long-term survival of Bid-/- mice injected i.v. with 0.25 mg/kg FLAG-tagged FASL crosslinked with anti-FLAG antibody (2 μg/μg of FASL) with or without co-injection of the SMAC/DIABLO mimetic drug BV6 (10 mg/kg, i.p., 30 min prior to FASL administration) and with or without pre-treatment with the caspase inhibitor QVD-oph (20 mg/kg, i.p., 30 min prior to FASL administration) is shown. P value is indicated below graph. b, Serum levels of ALT and AST were quantified after 120 min of the indicated treatment. The horizontal bars indicate the mean; n: numbers of mice analysed. P values as indicated by asterix. c, Histological analysis (H&E staining) of liver sections from WT and Bid-/- mice that had been treated as described in a and sacrificed after 180 min of FASL treatment (120 min for all images of sections from animals that were treated with BV6 alone). d, TUNEL staining of liver sections from mice described in c. Images are representative of a least 3 mice per genotype and time point of treatment. Scale bar = 50 μm. e, Processing of caspases -3, -7, -8, MCL-1 and β-actin (loading control) in liver extracts of Bid-/- mice that had been treated as described in a and sacrificed at the indicated time points were examined by immunoblotting.
METHODS SUMMARY
FASL-induced hepatitis
Mice were injected intravenously (i.v.) with 0.25 mg/kg body weight recombinant soluble FASL (FLAG® tagged FASL, Alexis) that had been crosslinked with 2 μg anti-FLAG® antibody (M2, SIGMA) per μg of FASL. The pancaspase inhibitor QVD-oph (MP Biomedicals) was injected at a concentration of 20 mg/kg body weight i.p. 30 min prior to treatment with FASL. Pre-treatment with the SMAC/DIABLO mimetic compound BV6 (a kind gift from Genentech) was performed by i.p. injection at a concentration of 10 mg/kg body weight 30 min prior to injection of FASL. BV6 was dissolved in freshly prepared, sterile-filtered (0.2 μM membrane) 15% hydroxy-propyl-β-cyclodextrin in 20 mM succinic acid (pH 5.5). Mice were sacrificed at various time points, bled for serum analysis of the liver-associated transaminases ALT and AST and the livers removed for biochemical and histological analysis. Statistical analyses were performed applying a two-tailed unpaired t test. Statistical analysis of animal survival was performed using a Log-rank (Mantel-Cox) test.
Full Methods, Mice and Reagents and any associated references are available in the online version of the paper at www.nature.com/nature.
Supplementary Material
Acknowledgments
We thank Drs D Vaux, J Tschopp, S Cory, J Adams, S Nagata and Y Lazebnik for gifts of mice and reagents, K Vella, D Cooper and G Siciliano for animal care, B Helbert for genotyping, R Czajko from the Biochemistry Department of the Royal Melbourne Hospital for ALT/AST measurements, C Young and D Chau for excellent technical assistance and Drs D Vaux, M Van Delft and L O’Reilly for advice and critical comments on the manuscript. This work was supported by grants (programs #257502 and #251608 and project #384404) and fellowships from the NHMRC (Canberra), the NCI (NIH, USA; # CA 80188 and #CA 43540), the Leukemia and Lymphoma Society of America (SCOR grant #7015), the JDRF/NHMRC, the Cancer Council Victoria, the Leukemia Foundation of Australia, the Swiss National Science Foundation (Fellowships to TK and UN), Novartis Jubilaeumsstiftung (Fellowship to UN), HepatoSys programme of the BMBF, the German Jose Carreras Leukemia Foundation (DJCLS R 06/09) and the Spemann Graduate School of Biology and Medicine (SGBM) (GSC-4), an institution of the Excellence Initiative of the Deutsche Forschungsgemeinschaft (DFG) (to CB) and the Dr. Mildred-Scheel Stiftung/Deutsche Krebshilfe (Fellowship to PJJ).
Footnotes
Author Contributions: P.J.J, T.K. designed and performed the experiments, A.S. designed experiments and supervised the project, S.G., D.G., M.D.M., J.S. and U.N. performed some experiments, D.C.S.H., P. B., C.B., H.E.T. generated essential tools.
Supplementary Information is linked to the online version of the paper available at www.nature.com/nature
References
- 1.Nagata S. Fas ligand-induced apoptosis. Annual Review of Genetics. 1999;33:29–55. doi: 10.1146/annurev.genet.33.1.29. [DOI] [PubMed] [Google Scholar]
- 2.Krammer PH. CD95’s deadly mission in the immune system. Nature. 2000;407:789–795. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- 3.Peter ME, et al. The CD95 receptor: apoptosis revisited. Cell. 2007;129:447–450. doi: 10.1016/j.cell.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 4.Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30:180–192. doi: 10.1016/j.immuni.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang K, Yin X-M, Chao DT, Milliman CL, Korsmeyer SJ. BID: a novel BH3 domain-only death agonist. Genes and Development. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 6.Yin X-M, et al. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- 7.Kaufmann T, et al. The BH3-Only Protein Bid Is Dispensable for DNA Damage-and Replicative Stress-Induced Apoptosis or Cell-Cycle Arrest. Cell. 2007;129:423–433. doi: 10.1016/j.cell.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 8.McKenzie MD, et al. Proapoptotic BH3-only protein Bid is essential for death receptor-induced apoptosis of pancreatic beta-cells. Diabetes. 2008;57:1284–1292. doi: 10.2337/db07-1692. [DOI] [PubMed] [Google Scholar]
- 9.Holcik M, Korneluk RG. XIAP, the guardian angel. Nature Reviews Mol Cell Biol. 2001;2:550–556. doi: 10.1038/35080103. [DOI] [PubMed] [Google Scholar]
- 10.Vaux DL, Silke J. IAPs, RINGs and ubiquitylation. Nat Rev Mol Cell Biol. 2005;6:287–297. doi: 10.1038/nrm1621. [DOI] [PubMed] [Google Scholar]
- 11.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 12.Verhagen AM, et al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing inhibitor of apoptosis (IAP) proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 13.Huang DC, et al. Activation of Fas by FasL induces apoptosis by a mechanism that cannot be blocked by Bcl-2 or Bcl-xL. Proc Natl Acad Sci U S A. 1999;96:14871–14876. doi: 10.1073/pnas.96.26.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogasawara J, et al. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 15.Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134:1641–1654. doi: 10.1053/j.gastro.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varfolomeev EE, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apol, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 17.Newton K, Harris AW, Bath ML, Smith KGC, Strasser A. A dominant interfering mutant of FADD/Mortl enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO Journal. 1998;17:706–718. doi: 10.1093/emboj/17.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scaffidi C, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO Journal. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Algeciras-Schimnich A, et al. Molecular ordering of the initial signaling events of CD95. Molecular and Cellular Biology. 2002;22:207–220. doi: 10.1128/MCB.22.1.207-220.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li S, et al. Relief of extrinsic pathway inhibition by the Bid-dependent mitochondrial release of Smac in Fas-mediated hepatocyte apoptosis. J Biol Chem. 2002;277:26912–26920. doi: 10.1074/jbc.M200726200. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Zou H, Slaughter C, Wang X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- 22.Enari M, et al. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 23.Conte D, Liston P, Wong JW, Wright KE, Korneluk RG. Thymocyte-targeted overexpression of xiap transgene disrupts T lymphoid apoptosis and maturation. Proc Natl Acad Sci U S A. 2001;98:5049–5054. doi: 10.1073/pnas.081547998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harlin H, Reffey SB, Duckett CS, Lindsten T, Thompson CB. Characterization of XIAP-deficient mice. Molecular and Cellular Biology. 2001;21:3604–3608. doi: 10.1128/MCB.21.10.3604-3608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hao Z, et al. Specific ablation of the apoptotic functions of cytochrome C reveals a differential requirement for cytochrome C and Apaf-1 in apoptosis. Cell. 2005;121:579–591. doi: 10.1016/j.cell.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 26.Lakhani SA, et al. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 2006;311:847–851. doi: 10.1126/science.1115035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varfolomeev E, et al. IAP Antagonists Induce Autoubiquitination of c-IAPs, NF-kappaB Activation, and TNFalpha-Dependent Apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 28.Vince JE, et al. IAP Antagonists Target cIAPl to Induce TNFalpha-Dependent Apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 29.Petersen SL, et al. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaither A, et al. A Smac mimetic rescue screen reveals roles for inhibitor of apoptosis proteins in tumor necrosis factor-alpha signaling. Cancer Res. 2007;67:11493–11498. doi: 10.1158/0008-5472.CAN-07-5173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.