Abstract
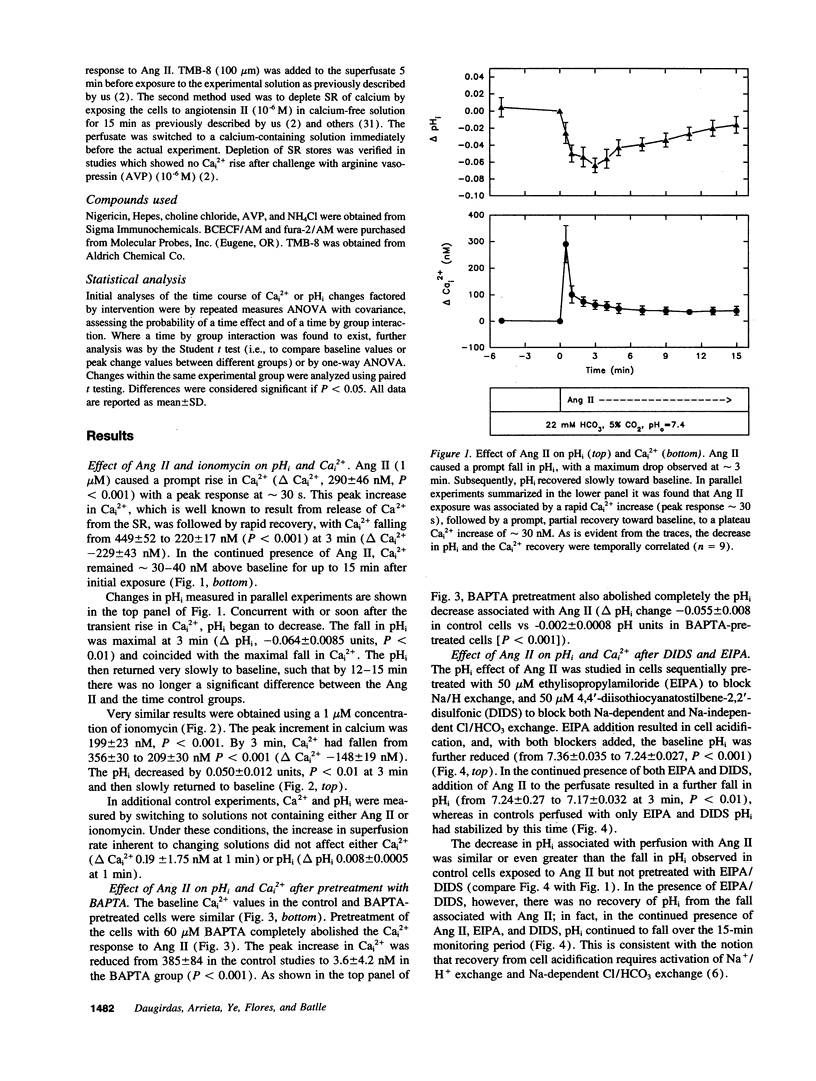
The purpose of this study was to define the mechanism whereby agonists that increase free cytosolic calcium (Cai2+) affect intracellular pH (pHi) in smooth muscle. Rat aortic vascular smooth muscle cells grown on coverslips were loaded with BCECF/AM or fura-2/AM for continuous monitoring of pHi or Cai2+, respectively, in a HCO3-/CO2- containing medium. Recovery from rapid increases in Cai2+ produced by 1 microM angiotensin (Ang) II (delta Cai2+ -229 +/- 43 nM) or 1 microM ionomycin (delta Cai2+ -148 +/- 19 nM) was accompanied by a fall in pHi (delta pHi, -0.064 +/- 0.0085 P < 0.01, and -0.05 +/- 0.012 pH units, P < 0.01, respectively). Neither the fall in pHi nor the rise in Cai2+ elicited by Ang II was prevented by pretreatment with agents which block the action of this agonist on pHi via the stimulation of the Cl/HCo3 exchangers (DIDS, 50 microM) or the Na+/H+ antiporter (EIPA, 50 microM). In the presence of DIDS and EIPA, Ang II produced a fall in pHi (delta pHi, -0.050 +/- 0.014, P < 0.01) and a rise in Cai2+ (delta Ca2+ 252 +/- 157 nM, P < 0.01). That the change in pHi was secondary to changes in Cai2+ was inferred from the finding that, when the rise in Cai2+ elicited by Ang II was prevented by preincubation with a Ca2+ buffer, BAPTA (60 microM), the fall in pHi was abolished as well (delta pHi, 0.0014 +/- 0.0046). The pHi fall produced by Ang II and ionomycin was prevented by cadmium at a very low concentration (20 nM) which is known to inhibit plasma membrane Ca(2+)-ATPase activity (delta pHi -0.002 +/- 0.0006 and -0.0016 pH units, respectively). Cadmium also blunted Cai2+ recovery after Ang II and ionomycin. These findings suggest that the fall in pHi produced by these agents is due to H+ entry coupled to Ca2+ extrusion via the plasma membrane Ca(2+)-ATPase. Our results indicate that agonists that increase Cai2+ cause intracellular acidification as a result of Ca2+/H+ exchange across the plasma membrane. This process appears to be mediated by a plasma membrane Ca(2+)-ATPase which, in the process of extruding Ca2+ from the cell, brings in [H+] and thus acidifies the cell.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerman K. E., Honkaniemi J., Scott I. G., Andersson L. C. Interaction of Cd2+ with the calmodulin-activated (Ca2+ + Mg2+)-ATPase activity of human erythrocyte ghosts. Biochim Biophys Acta. 1985 Apr 22;845(1):48–53. doi: 10.1016/0167-4889(85)90053-9. [DOI] [PubMed] [Google Scholar]
- Batlle D. C., Godinich M., LaPointe M. S., Munoz E., Carone F., Mehring N. Extracellular Na+ dependency of free cytosolic Ca2+ regulation in aortic vascular smooth muscle cells. Am J Physiol. 1991 Nov;261(5 Pt 1):C845–C856. doi: 10.1152/ajpcell.1991.261.5.C845. [DOI] [PubMed] [Google Scholar]
- Batlle D. C., Peces R., LaPointe M. S., Ye M., Daugirdas J. T. Cytosolic free calcium regulation in response to acute changes in intracellular pH in vascular smooth muscle. Am J Physiol. 1993 Apr;264(4 Pt 1):C932–C943. doi: 10.1152/ajpcell.1993.264.4.C932. [DOI] [PubMed] [Google Scholar]
- Berk B. C., Brock T. A., Gimbrone M. A., Jr, Alexander R. W. Early agonist-mediated ionic events in cultured vascular smooth muscle cells. Calcium mobilization is associated with intracellular acidification. J Biol Chem. 1987 Apr 15;262(11):5065–5072. [PubMed] [Google Scholar]
- Boyarsky G., Ganz M. B., Sterzel R. B., Boron W. F. pH regulation in single glomerular mesangial cells. II. Na+-dependent and -independent Cl(-)-HCO3- exchangers. Am J Physiol. 1988 Dec;255(6 Pt 1):C857–C869. doi: 10.1152/ajpcell.1988.255.6.C857. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Cheek T. R., Morgan A., O'Sullivan A. J., Moreton R. B., Berridge M. J., Mata A. M., Colyer J., Lee A. G., East J. M. Distribution of two distinct Ca2+-ATPase-like proteins and their relationships to the agonist-sensitive calcium store in adrenal chromaffin cells. Nature. 1989 Nov 2;342(6245):72–74. doi: 10.1038/342072a0. [DOI] [PubMed] [Google Scholar]
- Capponi A. M., Lew P. D., Vallotton M. B. Cytosolic free calcium levels in monolayers of cultured rat aortic smooth muscle cells. Effects of angiotensin II and vasopressin. J Biol Chem. 1985 Jul 5;260(13):7836–7842. [PubMed] [Google Scholar]
- Caroni P., Carafoli E. The Ca2+-pumping ATPase of heart sarcolemma. Characterization, calmodulin dependence, and partial purification. J Biol Chem. 1981 Apr 10;256(7):3263–3270. [PubMed] [Google Scholar]
- Dean W. L. Structure, function and subcellular localization of a human platelet Ca2+-ATPase. Cell Calcium. 1989 Jul;10(5):289–297. doi: 10.1016/0143-4160(89)90055-9. [DOI] [PubMed] [Google Scholar]
- Dixon D. A., Haynes D. H. The pH dependence of the cardiac sarcolemmal Ca2(+)-transporting ATPase: evidence that the Ca2+ translocator bears a doubly negative charge. Biochim Biophys Acta. 1990 Nov 16;1029(2):274–284. doi: 10.1016/0005-2736(90)90164-j. [DOI] [PubMed] [Google Scholar]
- Eggermont J. A., Vrolix M., Raeymaekers L., Wuytack F., Casteels R. Ca2+-transport ATPases of vascular smooth muscle. Circ Res. 1988 Feb;62(2):266–278. doi: 10.1161/01.res.62.2.266. [DOI] [PubMed] [Google Scholar]
- Eggermont J. A., Vrolix M., Wuytack F., Raeymaekers L., Casteels R. The (Ca2+-Mg2+)-ATPases of the plasma membrane and of the endoplasmic reticulum in smooth muscle cells and their regulation. J Cardiovasc Pharmacol. 1988;12 (Suppl 5):S51–S55. [PubMed] [Google Scholar]
- Furukawa K., Nakamura H. Characterization of the (Ca2+-Mg2+)ATPase purified by calmodulin-affinity chromatography from bovine aortic smooth muscle. J Biochem. 1984 Nov;96(5):1343–1350. doi: 10.1093/oxfordjournals.jbchem.a134962. [DOI] [PubMed] [Google Scholar]
- Ganz M. B., Boyarsky G., Sterzel R. B., Boron W. F. Arginine vasopressin enhances pHi regulation in the presence of HCO3- by stimulating three acid-base transport systems. Nature. 1989 Feb 16;337(6208):648–651. doi: 10.1038/337648a0. [DOI] [PubMed] [Google Scholar]
- Green J., Yamaguchi D. T., Kleeman C. R., Muallem S. Cytosolic pH regulation in osteoblasts. Regulation of anion exchange by intracellular pH and Ca2+ ions. J Gen Physiol. 1990 Jan;95(1):121–145. doi: 10.1085/jgp.95.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hatori N., Fine B. P., Nakamura A., Cragoe E., Jr, Aviv A. Angiotensin II effect on cytosolic pH in cultured rat vascular smooth muscle cells. J Biol Chem. 1987 Apr 15;262(11):5073–5078. [PubMed] [Google Scholar]
- Ives H. E., Daniel T. O. Interrelationship between growth factor-induced pH changes and intracellular Ca2+. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1950–1954. doi: 10.1073/pnas.84.7.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn A. M., Cragoe E. J., Jr, Allen J. C., Seidel C. L., Shelat H. Effects of pHi on Na(+)-H+, Na(+)-dependent, and Na(+)-independent C1(-)-HCO3-exchangers in vascular smooth muscle. Am J Physiol. 1991 Nov;261(5 Pt 1):C837–C844. doi: 10.1152/ajpcell.1991.261.5.C837. [DOI] [PubMed] [Google Scholar]
- Kapus A., Szászi K., Ligeti E. Phorbol 12-myristate 13-acetate activates an electrogenic H(+)-conducting pathway in the membrane of neutrophils. Biochem J. 1992 Feb 1;281(Pt 3):697–701. doi: 10.1042/bj2810697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikeri D., Zeidel M. L., Ballermann B. J., Brenner B. M., Hebert S. C. pH regulation and response to AVP in A10 cells differ markedly in the presence vs. absence of CO2-HCO3-. Am J Physiol. 1990 Sep;259(3 Pt 1):C471–C483. doi: 10.1152/ajpcell.1990.259.3.C471. [DOI] [PubMed] [Google Scholar]
- Levy D., Seigneuret M., Bluzat A., Rigaud J. L. Evidence for proton countertransport by the sarcoplasmic reticulum Ca2(+)-ATPase during calcium transport in reconstituted proteoliposomes with low ionic permeability. J Biol Chem. 1990 Nov 15;265(32):19524–19534. [PubMed] [Google Scholar]
- Lyall V., Belcher T. S., Biber T. U. Effect of changes in extracellular potassium on intracellular pH in principal cells of frog skin. Am J Physiol. 1992 Oct;263(4 Pt 2):F722–F730. doi: 10.1152/ajprenal.1992.263.4.F722. [DOI] [PubMed] [Google Scholar]
- Milanick M. A. Proton fluxes associated with the Ca pump in human red blood cells. Am J Physiol. 1990 Mar;258(3 Pt 1):C552–C562. doi: 10.1152/ajpcell.1990.258.3.C552. [DOI] [PubMed] [Google Scholar]
- Missiaen L., Taylor C. W., Berridge M. J. Spontaneous calcium release from inositol trisphosphate-sensitive calcium stores. Nature. 1991 Jul 18;352(6332):241–244. doi: 10.1038/352241a0. [DOI] [PubMed] [Google Scholar]
- Muallem S., Pandol S. J., Beeker T. G. Modulation of agonist-activated calcium influx by extracellular pH in rat pancreatic acini. Am J Physiol. 1989 Dec;257(6 Pt 1):G917–G924. doi: 10.1152/ajpgi.1989.257.6.G917. [DOI] [PubMed] [Google Scholar]
- Nabika T., Velletri P. A., Lovenberg W., Beaven M. A. Increase in cytosolic calcium and phosphoinositide metabolism induced by angiotensin II and [Arg]vasopressin in vascular smooth muscle cells. J Biol Chem. 1985 Apr 25;260(8):4661–4670. [PubMed] [Google Scholar]
- Nakamura J. pH and temperature resolve the kinetics of two pools of calcium bound to the sarcoplasmic reticulum Ca2+ -ATPase. J Biol Chem. 1989 Oct 15;264(29):17029–17031. [PubMed] [Google Scholar]
- Negulescu P. A., Machen T. E. Lowering extracellular sodium or pH raises intracellular calcium in gastric cells. J Membr Biol. 1990 Jul;116(3):239–248. doi: 10.1007/BF01868463. [DOI] [PubMed] [Google Scholar]
- Owen N. E., Villereal M. L. Efflux of 45Ca2+ from human fibroblasts in response to serum or growth factors. J Cell Physiol. 1983 Oct;117(1):23–29. doi: 10.1002/jcp.1041170105. [DOI] [PubMed] [Google Scholar]
- Redon J., Batlle D. Regulation of intracellular pH in the spontaneously hypertensive rat. Role of bicarbonate-dependent transporters. Hypertension. 1994 Apr;23(4):503–512. doi: 10.1161/01.hyp.23.4.503. [DOI] [PubMed] [Google Scholar]
- Rossi J. P., Schatzmann H. J. Trypsin activation of the red cell Ca2+-pump ATPase is calcium-sensitive. Cell Calcium. 1982 Dec;3(6):583–590. doi: 10.1016/0143-4160(82)90046-x. [DOI] [PubMed] [Google Scholar]
- Saleh A. M., Batlle D. C. Kinetic properties of the Na+/H+ antiporter of lymphocytes from the spontaneously hypertensive rat: role of intracellular pH. J Clin Invest. 1990 Jun;85(6):1734–1739. doi: 10.1172/JCI114629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J. I., Waisman D. M., Lafreniere D., Rasmussen H. Evidence that the erythrocyte calcium pump catalyzes a Ca2+:nH+ exchange. J Biol Chem. 1983 Sep 25;258(18):11092–11097. [PubMed] [Google Scholar]
- Smith J. B., Zheng T., Lyu R. M. Ionomycin releases calcium from the sarcoplasmic reticulum and activates Na+/Ca2+ exchange in vascular smooth muscle cells. Cell Calcium. 1989 Apr;10(3):125–134. doi: 10.1016/0143-4160(89)90066-3. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Verbost P. M., Flik G., Lock R. A., Wendelaar Bonga S. E. Cadmium inhibits plasma membrane calcium transport. J Membr Biol. 1988 May;102(2):97–104. doi: 10.1007/BF01870448. [DOI] [PubMed] [Google Scholar]
- Verbost P. M., Flik G., Pang P. K., Lock R. A., Wendelaar Bonga S. E. Cadmium inhibition of the erythrocyte Ca2+ pump. A molecular interpretation. J Biol Chem. 1989 Apr 5;264(10):5613–5615. [PubMed] [Google Scholar]
- Wray S. Smooth muscle intracellular pH: measurement, regulation, and function. Am J Physiol. 1988 Feb;254(2 Pt 1):C213–C225. doi: 10.1152/ajpcell.1988.254.2.C213. [DOI] [PubMed] [Google Scholar]
- Wuytack F., Raeymaekers L. The Ca(2+)-transport ATPases from the plasma membrane. J Bioenerg Biomembr. 1992 Jun;24(3):285–300. doi: 10.1007/BF00768849. [DOI] [PubMed] [Google Scholar]
- Xu Y. H., Roufogalis B. D. Asymmetric effects of divalent cations and protons on active Ca2+ efflux and Ca2+-ATPase in intact red blood cells. J Membr Biol. 1988 Oct;105(2):155–164. doi: 10.1007/BF02009168. [DOI] [PubMed] [Google Scholar]


