Abstract
Using key functional dissections, the synthesis of spirohexenolides is examined through a three component strategy that features a 1,2–addition to couple tetronate and aldehyde components forming the C2–C3 bond, and a Stille coupling to install the third sulfone–containing component. The macrocycle is completed by an intramolecular Julia–Kocienski reaction to form the C10–C11 trans–disubstituted olefin. Application of this strategy is described in progress towards the synthesis of (±)–spirohexenolide B.
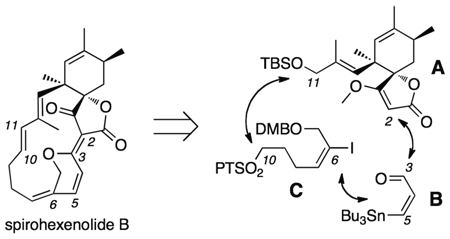
The spirohexenolides A (1a) and B (1b)1 comprise a family of structurally unique spirotetronate natural products2 isolated from strains of Streptomyces platensis (Scheme 1).3 Preliminary cytotoxic screening studies indicated that the spirohexenolides displayed a unique activity in the NCI–60 cell line analysis, while maintaining a low toxicity in mice.1 This data combined with a unique uptake and localization within tumor cells suggested a novel mode of action, which was recently shown to target the human macrophage migration inhibitory factor (hMIF).4 Exploratory studies on the natural material identified only one position, the C8 carbinol, which could be readily modified and retain activity.4 Efforts were focused on developing synthetic entry to the class in order to perform more complete structure–activity relationship (SAR) studies for preclinical evaluation.
Scheme 1.
Retrosynthetic analysis of spirohexenolide B (1b)
Given the comparable activity1,4 of spirohexenolide A (1a), B (1b) and the corresponding acetate 1c (Scheme 1), our studies focused on preparation of the putative biosynthetic deoxy–precursor 1b.
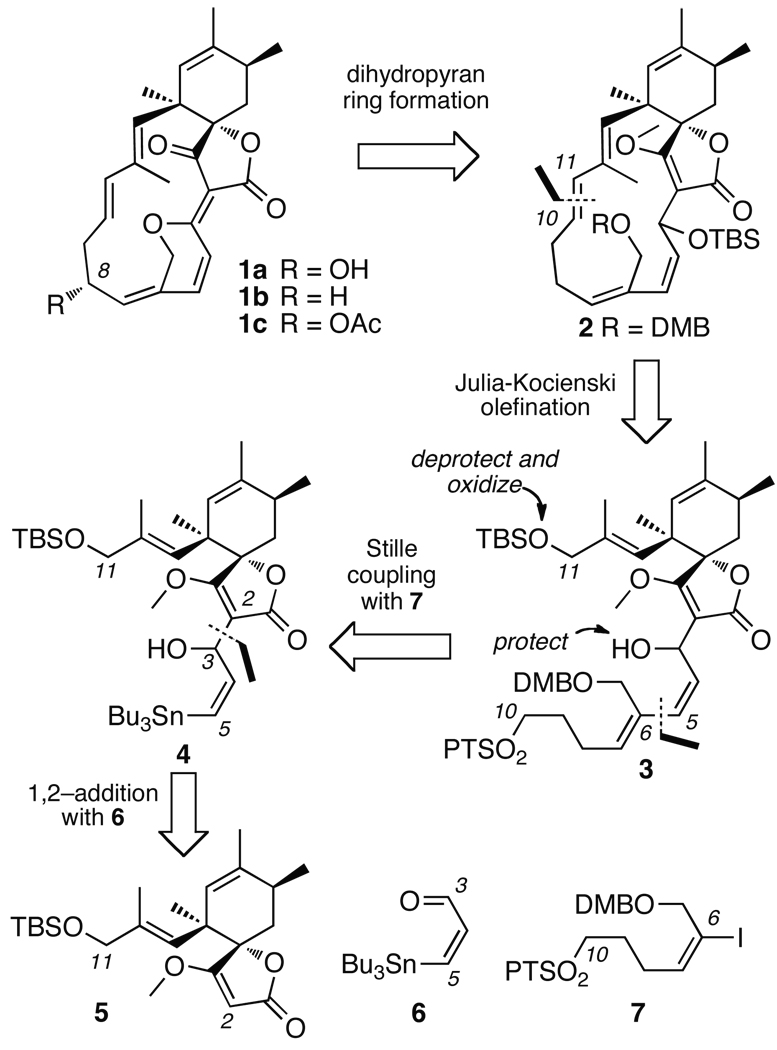
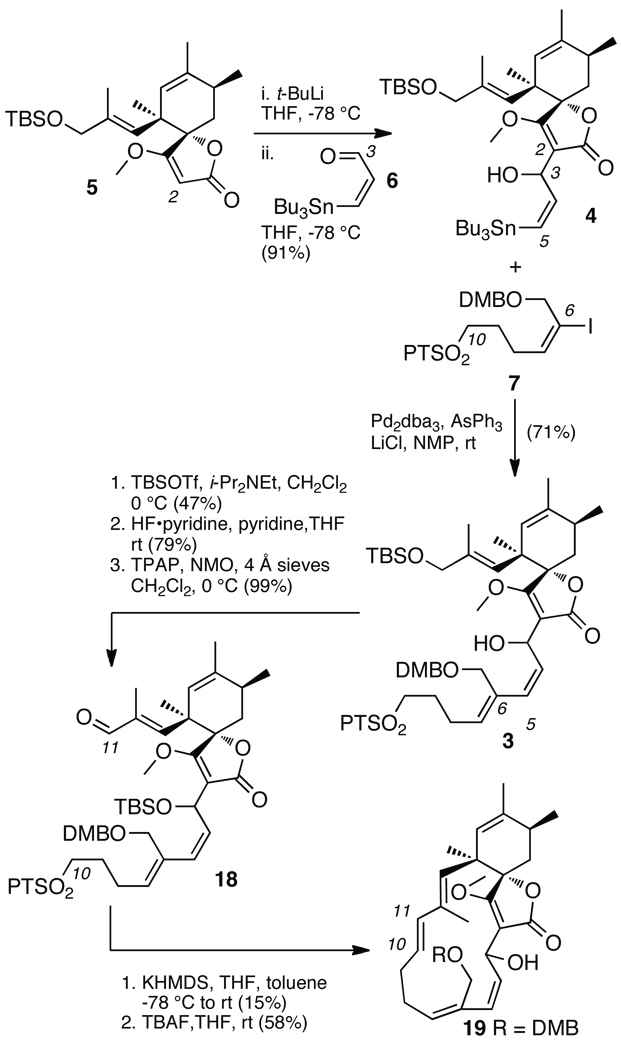
A modular strategy was implemented based on dissection of the molecule into three components: spirotetronate 5, aldehyde 6, and sulfone 7 (Scheme 1). A four–staged process was envisoned for their assembly that began by the addition of the lithium salt of 5 to aldehyde 6.5 The resulting adduct 4 had the required vinyl stannane handle for a Stille coupling with iodide component 7. With all three components in place, a Julia–Kocienski olefination would then complete the carbocyclic framework enabling completion of the synthesis through dehydrative pyran formation, as illustrated in the conversion of 2 to 1b (Scheme 1).
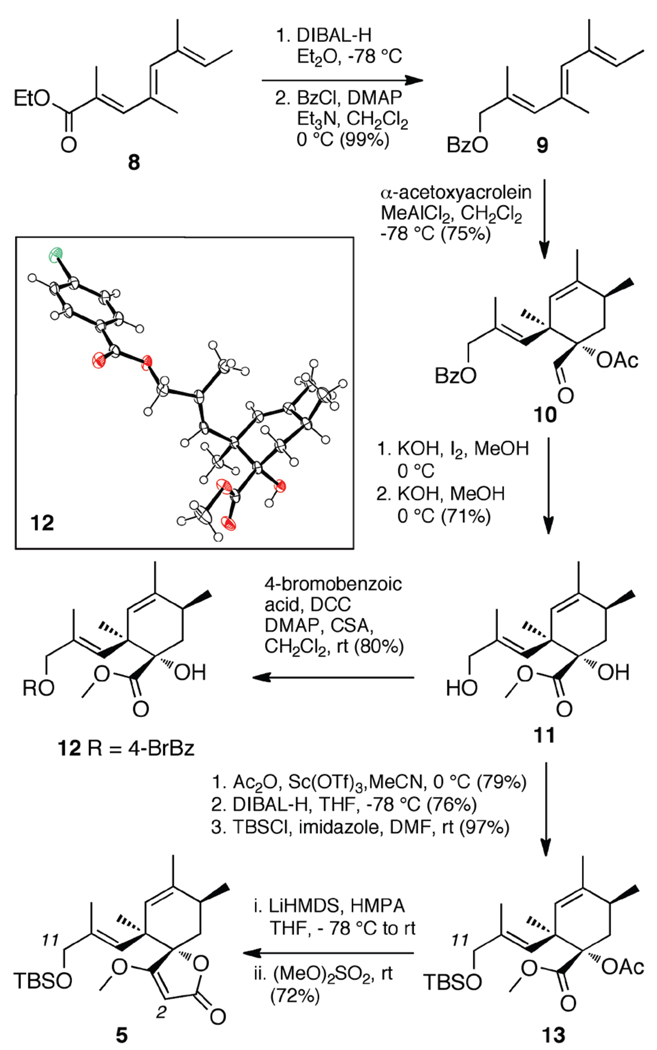
Our studies began by preparation of the northern component, spirotetronate 5 (Scheme 2). Diastereoselective construction of the cyclohexene system was accomplished by the Lewis acid catalyzed Diels–Alder reaction of triene 9, prepared from readily available ester 8,6 with α–acetoxyacrolein7 as the dienophile. The reaction favored the desired regioisomer, as precedented by a previously reported reaction using a similar triene substrate with α-bromoacrolein as the dienophile under Lewis acid catalysis.8a The adduct formed with high endo selectivity, as expected from previous studies on similar substrates.8 Adduct (±)–10 could be separated chromatographically from the other non-aldehydic byproducts, which constituted the majority of the remainder of the reaction mixture, when a benzoate protecting group was used on the primary alcohol. Using this procedure pure (±)–10 could be obtained in a 74% overall yield from 8.
Scheme 2.
Synthesis of the tetronate component 5
Oxidation of aldehyde 10 proved difficult; buffered KMnO4 9 and NaClO2 10 both failed to provide good conversion to the carboxylic acid. However, it was found that I2 in basic methanol11 offered acceptable conversion to the methyl ester. While effective, the highly alkaline nature of this reaction resulted in the removal of the acetate and partial cleavage of the benzoate group, which necessitated further processing to converge at diol 11. The structure of 11 was further confirmed by collection of an X–ray crystal structure of a derivatized p–bromobenzoate 12 (inset, Scheme 2).
With the structure of ester 11 confirmed, we turned to reinstall an acetate at the tertiary hydroxyl group in order to construct the tetronate ring by a Dieckmann cyclization. Lewis acid catalysis with Sc(OTf)3 provided excellent conversion to the corresponding bis–acetylated product,12 which was then converted to the primary TBS ether 13. Switching to TBS protection at C11 was key to achieving a satisfactory yield of the spirotetronate product 5. The desired Dieckmann cyclization was effected by the treatment of 13 with LiHMDS in a mixture of HMPA and THF,13,14 followed by methylation of the incipient tetronic acid salt with dimethylsulfate (Scheme 2). While not fully optimized, this route offered a 22% overall yield of 5 in 9 steps from 8.
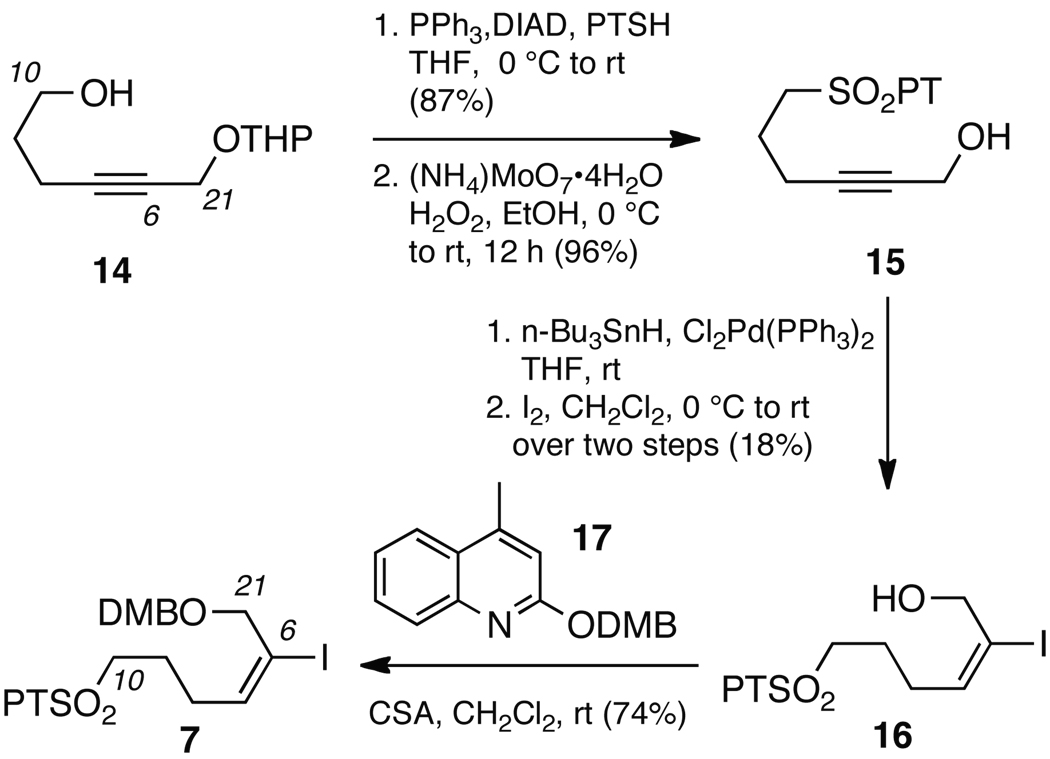
Our studies then turned to construction of the component 7. By applying methods of Bates,15 gram quantities of alkyne 14 were prepared from oxetane and then converted to the corresponding 1–phenyl–1H–tetrazol–5–yl (PT)16 sulfone 15 in two steps under conventional conditions (Scheme 3). At scales greater than 0.1 mol, the oxidation conditions and workup directly effected deprotection of the THP group, affording carbinol 15.
Scheme 3.
Synthesis of the vinyl iodide component 7.
We then turned to complete fragment 7 by installation of the vinyl iodide. While it was expected that the primary carbinol would direct the hydrostannation,17 the regioselectivity of this process was low, which after iodination afforded 16 in only 18% after purification. Given the rapid access to this grams of precursor 15 (an overall yield of 84% in 2 steps from 14), we did not focus on optimization. Rather, we turned our efforts to evaluate the component assembly. This necessitated protection of 16 as the 3,4-dimethoxybenzylether 7 using lepidine 17 in a comparable manner to that developed by Dudley for the formation of PMB ethers.18
The assembly process began with the coupling of components 5 and 6. Using established protocols,19 lithium salt of tetronate 5 was prepared and added to aldehyde 6 to afford stannane 4 as an inseparable 1 : 1 mixture of diastereomers (Scheme 4). The resulting product was then coupled under modified Stille conditions20 to vinyl iodide 7 to afford adduct 3. Protecting group manipulations, followed by oxidation with TPAP,21 delivered aldehyde 18, as a 1 : 1: mixture of inseparable diastereomers. Macrocyclization was achieved by the intramolecular Julia–Kocienski olefination22 of one of the diastereomers of 18 to afford E–olefin 2 (Scheme 1), which was desilylated to provide alcohol 19. Evidence for the trans–configuration at C10–C11 bond was indicated by a 3JH10–H11 = 15.5 Hz in 2 and 16.0 Hz in 19.23
Scheme 4.
Component assembly.
Initial attempts to purify the macrocycle 18 were hindered by coelution of oligomeric byproducts, presumably from the diastereomer of 18 that did not cyclize. We eventually isolated pure 1922 by improved chromatographic conditions.
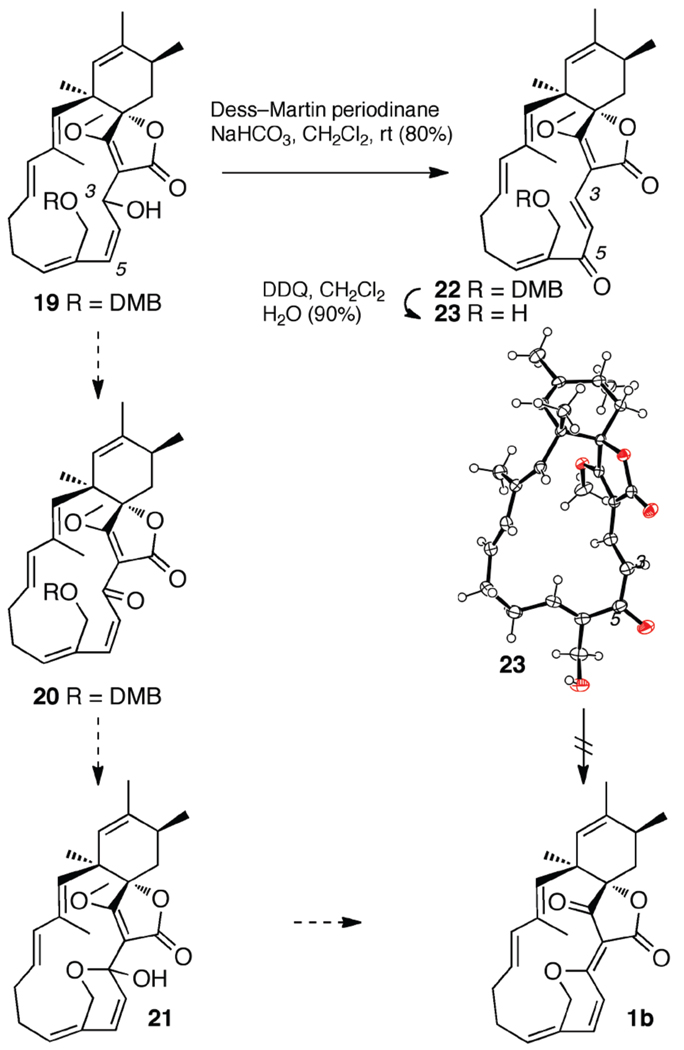
With the full carbocyclic framework intact, conversion to spirohexenolide B (1b) seemed to be just at hand through a three–step sequence (left, Scheme 5). As outlined, the plan called for oxidation at C3 followed by removing the DMB protecting group at C21, and a dehydrative deprotection of tetronate 2.
Scheme 5.
Attempted conversion to spirohexenolide B (1b)
Unfortunately, the oxidation of allylic alcohol 19 was problematic, with only Dess–Martin periodinane or IBX providing a single ketone product (see Supporting Information). However, inspection of the resulting 1H–NMR spectrum showed that the disubstituted olefin in the enone chemical shift region was trans, with a 3JH-H = 16.0 Hz, indicating that the product was not ketone 20.
In the process of further evaluating the structure of 22, we removed the DMB protecting group with DDQ. Samples of the resulting carbinol 23 were crystallized (mp = 170–172 °C). The X–ray crystallographic structure was determined (Scheme 5), indicating that transposition of the allylic alcohol from C3 to C5 had occurred during the oxidation of 19, which then oxidized to afford dienone 22 and not the desired isomer 20. To confirm that the rearrangement had occurred at the oxidation step and not during some previous operation, we conducted gCOSY, gHSQC and gHMBC analyses of compound 19, all of which support the proposed structure. This NMR evidence, combined with the crystal structure of dienone 23, indicates that the macrolide core of 1b was complete.
To date, we have demonstrated methods to prepare the intact, racemic, skeleton of the spirohexenolides. Efforts are now underway to establish methods to install the pyran motif at an earlier stage within the synthesis as well as to develop routes that could deliver intermediates 20 or 21 en route to a total synthesis of 1b.
Supplementary Material
Acknowledgment
Financial support from the American Cancer Society (RSG–06–011–01–CDD) is gratefully acknowledged. B.D.J. was supported by Ruth L Kirschstein National Research Service Award T32 CA009523 (NCI/NIH). We thank Dr. Yongxuan Su (UC San Diego) for mass spectral analyses, and Drs. Anthony Mrse (UC San Diego) and Prof. James Golen (UMass Dartmouth) for assistance with collection of the NMR data and the X-ray data on 12, respectively,
Footnotes
Supporting Information Available: Experimental procedures and copies of NMR spectra from compounds 2–5, 7, 8–13, 15–19, cif files for the X-ray structures of 12 and 23, as well as a further description of the oxidation of 19 and related model studies are available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Kang M, Jones BD, Mandel AL, Hammons JC, DiPasquale AG, Rheingold AL, La Clair JJ, Burkart MD. J. Org. Chem. 2009;74:9054–9061. doi: 10.1021/jo901826d. [DOI] [PubMed] [Google Scholar]
- 2.For reviews on the spirotetronate natural products see: Zografos AL, Georgiadis D. Synthesis. 2006:3157–3188. Schobert R, Schlenk A. Bioorg. Med. Chem. 2008;16:4203–4221. doi: 10.1016/j.bmc.2008.02.069. Athanasellis G, Igglessi–Markopoulou O, Markopoulos J. Bioinorg. Chem. Appl. 2010 doi: 10.1155/2010/315056. doi:10.1155/2010/315056..
- 3.For other natural products isolated from S. platensis, see: Hochlowski JE, Whittern DN, Hill P, McAlpine JB. J. Antibiot. 1994;47:870–874. doi: 10.7164/antibiotics.47.870. Woo EJ, Starks CM, Carney JR, Arslanian R, Cadapan L, Zavala S, Licari P. J. Antibiot. 2002;55:141–146. doi: 10.7164/antibiotics.55.141. Asai N, Kotake Y, Niijima J, Fukuda Y, Uehara T, Sakai T. J. Antibiot. 2007;60:364–369. doi: 10.1038/ja.2007.49. Kohama T, Maeda H, Sakai JI, Shiraishi A, Yamashita K. J. Antibiot. 1996;49:91–94. doi: 10.7164/antibiotics.49.91. Furumai T, Eto K, Sasaki T, Higuchi H, Onaka H, Saito N, Fujita T, Naoki H, Igarashi Y. J. Antibiot. 2002;55:873–880. doi: 10.7164/antibiotics.55.873. Herath KB, Attygalle AB, Singh SB. J. Am. Chem. Soc. 2007;129:15422–15423. doi: 10.1021/ja0758943..
- 4.Yu W–L, Jones BD, Kang MJ, Hammons JC, La Clair JJ, Burkart MD. J. Am. Chem. Soc. 2010 submitted. [Google Scholar]
- 5.Franci X, Martina SLX, McGrady JE, Webb MR, Donald C, Taylor RJK. Tetrahedron Lett. 2003;44:7735–7740. [Google Scholar]
- 6.(a) Moses JE, Baldwin JE, Brueckner S, Eade SerenaJ, Adlington RM. Org. Biomol. Chem. 2003;1:3670–3684. doi: 10.1039/b306933h. [DOI] [PubMed] [Google Scholar]; (b) Patel P, Pattenden G. Tetrahedron Lett. 1985;26:4789–4792. [Google Scholar]
- 7.Funk RL, Yost KJ. J. Org. Chem. 1996;61:2598–2599. doi: 10.1021/jo960340w. [DOI] [PubMed] [Google Scholar]
- 8.(a) Marshall JA, Xie SP. J. Org. Chem. 1992;57:2987–2989. [Google Scholar]; (b) Zografos AL, Yiotakis A, Georgiadis D. Org. Lett. 2005;7:4515–4518. doi: 10.1021/ol051872e. [DOI] [PubMed] [Google Scholar]; (c) Roush WR, Limberakis C, Kunz RK, Barda DA. Org. Lett. 2002;4:1543–1546. doi: 10.1021/ol025772+. [DOI] [PubMed] [Google Scholar]; (d) Roush WR, Barda DA. J. Am. Chem. Soc. 1997;119:7402–7403. [Google Scholar]
- 9.Abiko A, Roberts JC, Takemasa T, Masamune S. Tetrahedron Lett. 1986;27:4537–4540. [Google Scholar]
- 10.Lindgren BO, Nilsson T. Acta Chem. Scand. 1973;27:888–890. [Google Scholar]
- 11.Yamada S, Morizono D, Yamamoto K. Tetrahedron Lett. 1992;33:4329–4332. [Google Scholar]
- 12.Ishihara K, Kubota M, Kurihara H, Yamamoto H. J. Org. Chem. 1996;61:4560–4567. doi: 10.1021/jo952237x. [DOI] [PubMed] [Google Scholar]
- 13.Roush WR, Reilly ML, Koyama K, Brown BB. J. Org. Chem. 1997;62:8708–8721. [Google Scholar]
- 14.Ireland RE, Thompson WJ. J. Org. Chem. 1979;44:3041–3052. [Google Scholar]
- 15.(a) Bates RW, Sridhar S. J. Org. Chem. 2008;73:8104–8105. doi: 10.1021/jo801433f. [DOI] [PubMed] [Google Scholar]; (b) Yamaguchi M, Nobayashi Y, Hirao I. Tetrahedron. 1984;40:4261–4266. [Google Scholar]
- 16.Blakemore PR, Cole WJ, Kocienski PJ, Morley A. Synlett. 1998:26–28. [Google Scholar]
- 17.Marshall JA, Bourbeau MP. Tetrahedron Lett. 2003;44:1087–1089. [Google Scholar]
- 18.(a) Albiniak PA, Dudley GB. Synlett. 2010:841–841. [Google Scholar]; (b) Nwoye EO, Dudley GB. Chem. Commun. 2007:1436–1437. doi: 10.1039/b617926f. [DOI] [PubMed] [Google Scholar]; (c) Stewart CA, Peng XW, Paquette LA. Synthesis. 2008:433–437. [Google Scholar]
- 19.Takeda K, Kawanishi E, Nakamura H, Yoshii E. Tetrahedron Lett. 1991;32:4925–4928. [Google Scholar]
- 20.Marshall JA, Adams ND. J. Org. Chem. 2002;67:733–740. doi: 10.1021/jo015864x. [DOI] [PubMed] [Google Scholar]
- 21.Ley SV, Norman J, Griffith WP, Marsden SP. Synthesis. 1994:639–666. [Google Scholar]
- 22.This is one of a few examples of an intramolecular Julia–Kocienski olefination, see: Jakubec P, Cockfield DM, Dixon DJ. J. Am. Chem. Soc. 2009;131:16632–16633. doi: 10.1021/ja908399s. Aissa C. J. Org. Chem. 2006;71:360–363. doi: 10.1021/jo051693a. Giesbrecht HE, Knight BJ, Tanguileg NR, Emerson CR, Blakemore PR. Synlett. 2010:374–378..
- 23.Assignment of the relative stereochemistry at C3 in 2 or 19 was not attempted, but may be possible through NOESY studies or modification of the C3 -OH of 19 to form a crystalline derivative.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.