Abstract
The plasma cholesteryl ester transfer protein (CETP) mediates the exchange of HDL cholesteryl esters (CE) and VLDL triglycerides leading to catabolism of HDL. There is some evidence that HDL ameliorates the toxicity of LPS, and LPS is known to influence several enzymes affecting HDL metabolism. Therefore, the effects of LPS on CETP and plasma lipoproteins were examined in human CETP transgenic mice. Administration of LPS to mice expressing a CETP transgene linked to its natural flanking sequences (NFR-CETP Tg) resulted in a rapid marked decrease in hepatic CETP mRNA and plasma CETP concentration. Corticosteroid injection produced a similar decrease in hepatic CETP mRNA and adrenalectomy abolished this response to LPS. LPS caused disproportionate reductions in plasma CETP activity compared to mass, and was found to be a potent inhibitor of CETP activity when added directly to plasma. LPS was injected into mice expressing (A) a human apoA-I transgene, (B) apoA-I and NFR-CETP transgenes, or (C) apoA-I and LPS-inducible metallothionein promoter-driven CETP transgenes, producing (A) minimal changes in HDL cholesterol, (B) decreased plasma CETP and increased HDL cholesterol, and (C) increased plasma CETP and decreased HDL cholesterol. Thus, LPS administration produces a profound decrease in hepatic CETP mRNA, primarily as a result of adrenal corticosteroid release. The decrease in plasma CETP activity after LPS administration may reflect both this effect as well as a direct interaction between CETP and LPS. The decrease of CETP in response to LPS has major effects on HDL levels, and may represent an adaptive response to preserve or increase HDL and thereby modify the response to LPS.
Full text
PDF

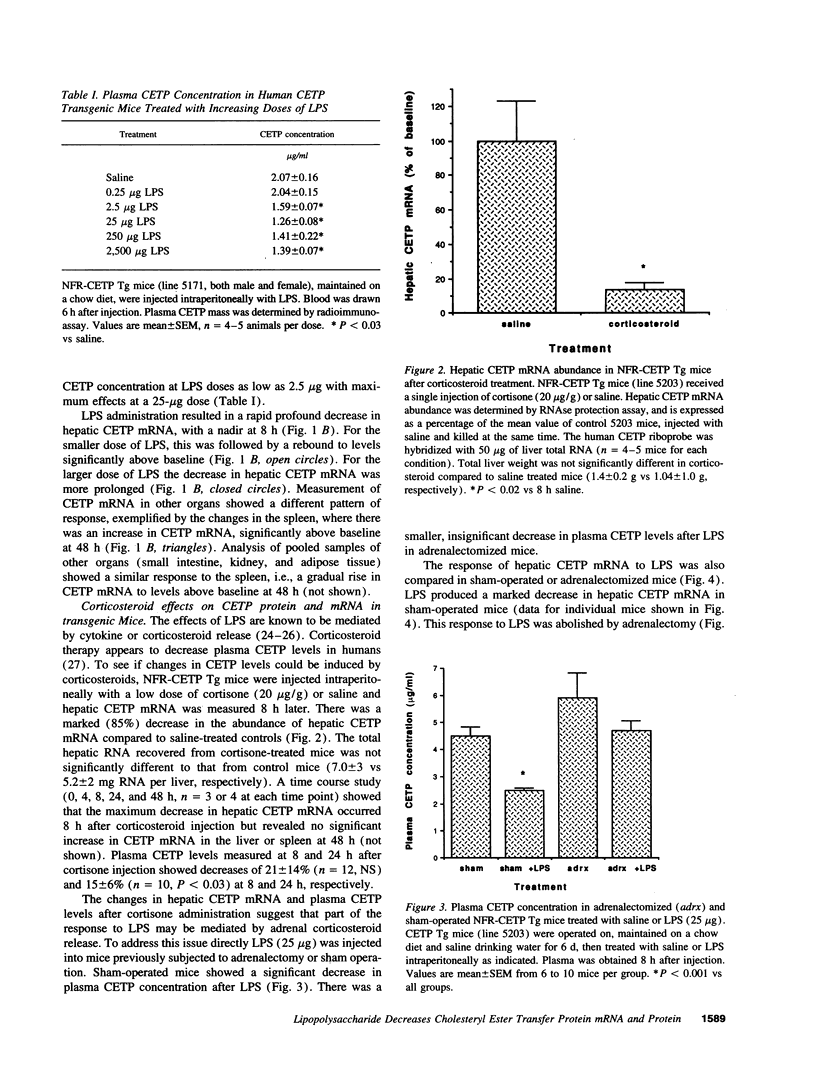




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agellon L. B., Walsh A., Hayek T., Moulin P., Jiang X. C., Shelanski S. A., Breslow J. L., Tall A. R. Reduced high density lipoprotein cholesterol in human cholesteryl ester transfer protein transgenic mice. J Biol Chem. 1991 Jun 15;266(17):10796–10801. [PubMed] [Google Scholar]
- Andersen J. M., Dietschy J. M. Relative importance of high and low density lipoproteins in the regulation of cholesterol synthesis in the adrenal gland, ovary, and testis of the rat. J Biol Chem. 1978 Dec 25;253(24):9024–9032. [PubMed] [Google Scholar]
- Auerbach B. J., Parks J. S. Lipoprotein abnormalities associated with lipopolysaccharide-induced lecithin: cholesterol acyltransferase and lipase deficiency. J Biol Chem. 1989 Jun 15;264(17):10264–10270. [PubMed] [Google Scholar]
- Baumann H., Richards C., Gauldie J. Interaction among hepatocyte-stimulating factors, interleukin 1, and glucocorticoids for regulation of acute phase plasma proteins in human hepatoma (HepG2) cells. J Immunol. 1987 Dec 15;139(12):4122–4128. [PubMed] [Google Scholar]
- Beisel W. R. Magnitude of the host nutritional responses to infection. Am J Clin Nutr. 1977 Aug;30(8):1236–1247. doi: 10.1093/ajcn/30.8.1236. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., Grunfeld C. Tumor necrosis factor-alpha stimulates hepatic lipogenesis in the rat in vivo. J Clin Invest. 1987 Jul;80(1):184–190. doi: 10.1172/JCI113046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass C., Pittman R. C., Civen M., Steinberg D. Uptake of high-density lipoprotein-associated apoprotein A-I and cholesterol esters by 16 tissues of the rat in vivo and by adrenal cells and hepatocytes in vitro. J Biol Chem. 1985 Jan 25;260(2):744–750. [PubMed] [Google Scholar]
- Glomset J. A., Norum K. R. The metabolic role of lecithin: cholesterol acyltransferase: perspectives form pathology. Adv Lipid Res. 1973;11:1–65. [PubMed] [Google Scholar]
- Harris H. W., Grunfeld C., Feingold K. R., Rapp J. H. Human very low density lipoproteins and chylomicrons can protect against endotoxin-induced death in mice. J Clin Invest. 1990 Sep;86(3):696–702. doi: 10.1172/JCI114765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayek T., Azrolan N., Verdery R. B., Walsh A., Chajek-Shaul T., Agellon L. B., Tall A. R., Breslow J. L. Hypertriglyceridemia and cholesteryl ester transfer protein interact to dramatically alter high density lipoprotein levels, particle sizes, and metabolism. Studies in transgenic mice. J Clin Invest. 1993 Sep;92(3):1143–1152. doi: 10.1172/JCI116683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayek T., Chajek-Shaul T., Walsh A., Agellon L. B., Moulin P., Tall A. R., Breslow J. L. An interaction between the human cholesteryl ester transfer protein (CETP) and apolipoprotein A-I genes in transgenic mice results in a profound CETP-mediated depression of high density lipoprotein cholesterol levels. J Clin Invest. 1992 Aug;90(2):505–510. doi: 10.1172/JCI115887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X. C., Agellon L. B., Walsh A., Breslow J. L., Tall A. Dietary cholesterol increases transcription of the human cholesteryl ester transfer protein gene in transgenic mice. Dependence on natural flanking sequences. J Clin Invest. 1992 Oct;90(4):1290–1295. doi: 10.1172/JCI115993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X. C., Masucci-Magoulas L., Mar J., Lin M., Walsh A., Breslow J. L., Tall A. Down-regulation of mRNA for the low density lipoprotein receptor in transgenic mice containing the gene for human cholesteryl ester transfer protein. Mechanism to explain accumulation of lipoprotein B particles. J Biol Chem. 1993 Dec 25;268(36):27406–27412. [PubMed] [Google Scholar]
- Kawakami M., Cerami A. Studies of endotoxin-induced decrease in lipoprotein lipase activity. J Exp Med. 1981 Sep 1;154(3):631–639. doi: 10.1084/jem.154.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVIN J., CLUFF L. E. ENDOTOXEMIA AND ADRENAL HEMORRHAGE. A MECHANISM FOR THE WATERHOUSE-FRIDERICHSEN SYNDROME. J Exp Med. 1965 Feb 1;121:247–260. doi: 10.1084/jem.121.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine D. M., Parker T. S., Donnelly T. M., Walsh A., Rubin A. L. In vivo protection against endotoxin by plasma high density lipoprotein. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):12040–12044. doi: 10.1073/pnas.90.24.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loppnow H., Libby P., Freudenberg M., Krauss J. H., Weckesser J., Mayer H. Cytokine induction by lipopolysaccharide (LPS) corresponds to lethal toxicity and is inhibited by nontoxic Rhodobacter capsulatus LPS. Infect Immun. 1990 Nov;58(11):3743–3750. doi: 10.1128/iai.58.11.3743-3750.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann C. J., Yen F. T., Grant A. M., Bihain B. E. Mechanism of plasma cholesteryl ester transfer in hypertriglyceridemia. J Clin Invest. 1991 Dec;88(6):2059–2066. doi: 10.1172/JCI115535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcel Y. L., McPherson R., Hogue M., Czarnecka H., Zawadzki Z., Weech P. K., Whitlock M. E., Tall A. R., Milne R. W. Distribution and concentration of cholesteryl ester transfer protein in plasma of normolipemic subjects. J Clin Invest. 1990 Jan;85(1):10–17. doi: 10.1172/JCI114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo K. E., Palmiter R. D. Glucocorticoid regulation of metallothionein-I mRNA synthesis in cultured mouse cells. J Biol Chem. 1981 Mar 25;256(6):2621–2624. [PubMed] [Google Scholar]
- McPherson R., Hogue M., Milne R. W., Tall A. R., Marcel Y. L. Increase in plasma cholesteryl ester transfer protein during probucol treatment. Relation to changes in high density lipoprotein composition. Arterioscler Thromb. 1991 May-Jun;11(3):476–481. doi: 10.1161/01.atv.11.3.476. [DOI] [PubMed] [Google Scholar]
- Moulin P., Appel G. B., Ginsberg H. N., Tall A. R. Increased concentration of plasma cholesteryl ester transfer protein in nephrotic syndrome: role in dyslipidemia. J Lipid Res. 1992 Dec;33(12):1817–1822. [PubMed] [Google Scholar]
- Munford R. S., Andersen J. M., Dietschy J. M. Sites of tissue binding and uptake in vivo of bacterial lipopolysaccharide-high density lipoprotein complexes: studies in the rat and squirrel monkey. J Clin Invest. 1981 Dec;68(6):1503–1513. doi: 10.1172/JCI110404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newnham H. H., Barter P. J. Synergistic effects of lipid transfers and hepatic lipase in the formation of very small high-density lipoproteins during incubation of human plasma. Biochim Biophys Acta. 1990 May 1;1044(1):57–64. doi: 10.1016/0005-2760(90)90218-m. [DOI] [PubMed] [Google Scholar]
- Papaioannou V. E., Fox J. G. Efficacy of tribromoethanol anesthesia in mice. Lab Anim Sci. 1993 Apr;43(2):189–192. [PubMed] [Google Scholar]
- Quinet E. M., Agellon L. B., Kroon P. A., Marcel Y. L., Lee Y. C., Whitlock M. E., Tall A. R. Atherogenic diet increases cholesteryl ester transfer protein messenger RNA levels in rabbit liver. J Clin Invest. 1990 Feb;85(2):357–363. doi: 10.1172/JCI114446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinet E., Tall A., Ramakrishnan R., Rudel L. Plasma lipid transfer protein as a determinant of the atherogenicity of monkey plasma lipoproteins. J Clin Invest. 1991 May;87(5):1559–1566. doi: 10.1172/JCI115169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkowski C. A., Vogel S. N. Lipopolysaccharide increases glucocorticoid receptor expression in murine macrophages. A possible mechanism for glucocorticoid-mediated suppression of endotoxicity. J Immunol. 1992 Dec 15;149(12):4041–4047. [PubMed] [Google Scholar]
- Schumann R. R., Leong S. R., Flaggs G. W., Gray P. W., Wright S. D., Mathison J. C., Tobias P. S., Ulevitch R. J. Structure and function of lipopolysaccharide binding protein. Science. 1990 Sep 21;249(4975):1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- Sobocinski P. Z., Canterbury W. J., Jr Hepatic metallothionein induction in inflammation. Ann N Y Acad Sci. 1982;389:354–367. doi: 10.1111/j.1749-6632.1982.tb22149.x. [DOI] [PubMed] [Google Scholar]
- Tall A. R. Plasma high density lipoproteins. Metabolism and relationship to atherogenesis. J Clin Invest. 1990 Aug;86(2):379–384. doi: 10.1172/JCI114722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R. The modification of biophysical and endotoxic properties of bacterial lipopolysaccharides by serum. J Clin Invest. 1978 Dec;62(6):1313–1324. doi: 10.1172/JCI109252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Isolation and characterization of a bacterial lipopolysaccharide-high density lipoprotein complex formed in rabbit plasma. J Clin Invest. 1981 Mar;67(3):827–837. doi: 10.1172/JCI110100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Their participation in intravascular reactions of bacterial lipopolysaccharides. J Clin Invest. 1979 Nov;64(5):1516–1524. doi: 10.1172/JCI109610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh A., Ito Y., Breslow J. L. High levels of human apolipoprotein A-I in transgenic mice result in increased plasma levels of small high density lipoprotein (HDL) particles comparable to human HDL3. J Biol Chem. 1989 Apr 15;264(11):6488–6494. [PubMed] [Google Scholar]
- Whitlock M. E., Swenson T. L., Ramakrishnan R., Leonard M. T., Marcel Y. L., Milne R. W., Tall A. R. Monoclonal antibody inhibition of cholesteryl ester transfer protein activity in the rabbit. Effects on lipoprotein composition and high density lipoprotein cholesteryl ester metabolism. J Clin Invest. 1989 Jul;84(1):129–137. doi: 10.1172/JCI114132. [DOI] [PMC free article] [PubMed] [Google Scholar]