Abstract
GPR177, the mammalian ortholog of Drosophila Wntless/Evi/Sprinter, was recently identified as a novel mu-opioid receptor (MOR) interacting protein. GPR177 is a trans-membrane protein pivotal to mediating the secretion of Wnt signaling proteins. Wnt proteins, in turn, are essential in regulating neuronal development, a phenomenon inhibited upon chronic exposure to MOR agonists such as morphine and heroin. We previously showed that GPR177 and MOR are co-localized in the mouse dorsolateral striatum; however, the nature of this interaction was not fully elucidated. Therefore, in the present study, we examined cellular substrates for interactions between GPR177 and MOR using a combined immunogold-silver and peroxidase detection approach in coronal sections in the dorsolateral segment of the striatum. Semi-quantitative analysis of the ultrastructural distribution of GPR177 and MORin striatal somata and in dendritic processes showed that, of the somata and dendritic processes exhibiting GPR177, 32% contained MOR immunolabeling while for profiles exhibiting MOR, 37% also contained GPR177 immunoreactivity. GPR177-labeled particles were localized predominantly along both the plasma membrane and within the cytoplasm of MOR-labeled dendrites. Somata and dendritic processes that contained both GPR177 and MOR more often received symmetric (inhibitory-type) synapses from unlabeled axon terminals. To further define the phenotype of GPR177 and MOR-containing cellular profiles, triple immunofluorescence detection showed that GPR177 and MOR are localized in neurons containing the opioid peptide, enkephalin, within the dorsolateral striatum. The results provide an anatomical substrate for interactions between MOR and its interacting protein, GPR177, in striatal opioid-containing neurons that may underlie the morphological alterations produced in neurons by chronic opiate use.
Keywords: GPR177, mu opioid receptor, striatum, electron microscopy, confocal microscopy
1. Introduction
Dependence and addiction to opiate drugs (e.g. morphine and heroin) involve the activation of G-protein coupled opioid receptors (Contet et al., 2004). Specifically, the activation of mu-opioid receptors (MOR) results in adaptations that often lead to tolerance and dependence (Bailey and Connor, 2005). Along with the analgesic and rewarding effects of opiates primarily mediated by MOR (Kieffer, 1999), the addictive state is characterized by inhibitory effects of opiates on axon outgrowth, dendritic arborization, and neurogenesis in brain regions implicated in reward processing, learning, and memory (Robinson and Kolb, 1999; Eisch et al., 2000). Although the role of MOR in contributing to neuronal changes observed in opioid dependence remains complex, a growing literature on the multiple interacting proteins involved in the regulation of MOR appears promising.
GPR177, the mammalian ortholog of Drosophila Wntless/Evi/Sprinter, was recently identified as a MOR interacting protein that may possibly serve as a substrate underlying the alterations in neuronal structure and synaptic organization characteristics of opioid dependence (Jin et al., 2010). GPR177 is a seven-transmembrane protein essential in mediating the secretion of Wnt signaling proteins (Banziger et al., 2006; Bartscherer et al., 2006). Wnt proteins are, in turn, extracellular signaling molecules important for their neurotrophic properties (Ciani and Salinas, 2005; Banziger et al., 2006). Among their other functions in neuronal development, Wnt proteins stimulate synapse formation and dendritic morphogenesis, and control axon remodeling and guidance (Ciani and Salinas, 2005). GPR177 accompanies these Wnt proteins when it shuttles between the Golgi apparatus and the plasma membrane (Franch-Marro et al., 2008). It is precisely in this mechanism of secreting Wnt proteins that the interaction between GPR177 and MOR may potentially have an effect (Jin et al., 2010).
Known for its role in motor control and as a key structure in the formation of motor-related memories (Albin et al., 1989), the striatum is also involved in cognitive processes including executive function (Kehagia et al., 2010; Watson and Stanton, 2009). The striatum receives innervation from many brain regions including dopaminergic fibers from the pars compacta of the substantia nigra and glutamatergic innervation arising from cortical areas conveying sensorimotor, limbic and cognitive information (Graybiel, 1990; Calabresi et al., 1996). The striatal neuronal population is enriched with various peptidergic neurons including enkephalinergic, cholinergic, GABAergic and aminergic neurons (Fonnum et al., 1977; Beckstead and Kersey, 1985; Graybiel, 1990; Koos et al., 2004; Taverna et al., 2007).
Our recent studies have demonstrated that GPR177 and MOR are co-localized in striatal perikarya and dendritic processes (Jin et al., 2010); thus, we have set out to elucidate the cellular substrates underlying interactions between GPR177 and MOR and define their phenotype in the striatum. In an effort to further characterize the interaction between GPR177 and MOR, we semiquantitatively analyzed data from dual electron microscopic immunocytochemical detection of GPR177 and MOR. Furthermore, this study used triple light microscopic immunocytochemical labeling for GPR177, MOR and enkephalin (ENK).
2. Results
2.1. GPR177 localization in MOR-containing cells in the striatum
Using immunofluorescence microscopy, GPR177 immunoreactivity was localized in striatal somata and in dendritic processes (Fig. 1) consistent with our recent report (Jin et al., 2010). Using ultrastructural analysis, GPR177 was visualized as irregularly shaped black deposits (Jin et al., 2010) indicative of immunogold-silver particles (Fig. 2). Dual electron microscopic immunolabeling whereGPR177 was visualized as immunogold-silver particles and MOR was identified by an electron-dense peroxidase reaction product showed that GPR177 was localized 1) along the plasma membrane only (Fig. 2A), 2) within the cytoplasmic compartment (Fig. 2B), and 3) along the plasma membrane as well as within the cytoplasmic compartment (Fig. 2C). In many instances, GPR177 was localized both within the cytoplasm and along the plasma membrane (Fig. 2C; 4). Quantification for the distribution of GPR177 immunoreactivity in MOR-labeled dendrites showed that 37% of these profiles exhibited GPR177 immunoreactivity within the cytoplasmic compartment only and 11% of these profiles exhibited GPR177 along the plasma membrane only. The remaining 52% of the MOR-labeled profiles exhibited GPR177 immunoreactivity both within the cytoplasmic compartment and along the plasma membrane (Fig. 2E). Of these profiles, however, there were more GPR177 in the cytoplasm as revealed by the higher percentage of immunogold-silver particles in the cytoplasm when compared to the total number of particles in a profile (66%).
Figure 1.
Confocal fluoroescence micrographs in the striatum showing GPR177 and MOR-opioid receptor immunoreactivities. A-B. GPR177 was detected using a rhodamine isothiocyanate-conjugated secondary antibody (red) and MOR was detected a fluorescein isothiocyanate-conjugated secondary antibody (green). C. Merged image. D-E. Photomicrographs of tissue sections that were processed in parallel without the primary antibodies. F. Schematic diagram adapted from the rat brain atlas of Paxinos and Watson (Paxinos and Watson, 1997) depicting the region sampled (trapezoid) for immunohistochemical analysis. Arrowheads point to individual GPR177-labeled perikarya that contain MOR. The single arrow points to a perikaryon containing GPR177 immunoreactivity only. In panels A and F, arrows indicate dorsal (D) and medial (M) orientation of the tissue section. ac, anterior commissure; cc, corpus callosum; LV, lateral ventricle; ox, optic chiasm. Scale bars, 100 μm.
Figure 2.
Electron photomicrographs showing immunogold-silver labeling for GPR177 and immunoperoxidase labeling for MOR in the striatum as well as an example where markers are reversed. A-C. Dually labeled GPR177 and MOR-dendrites (GPR177+MOR-d) containing GPR177 (arrowheads) along the plasma membrane only (A), within the cytoplasm only (B) and both within the cytoplasm as well as the plasma membrane (C). Arrowheads point to GPR177 while arrows point to MOR.D . Example of tissue sections processed where the detection methods were reversed. In this electron photomicrograph, GPR177 is labeled using immunoperoxidase detection(arrows) while MOR is indicated by immunogold-silver labeling (arrowheads). E. Schematic diagrams illustrate the distribution of GPR177 in MOR-labeled dendrites when GPR177 is localized to the plasma membrane only, within the cytoplasm only, or both within the cytoplasm and the plasma membrane. A higher percentage of MOR-labeled dendrites exhibited GPR177 labeling when GPR177 was present both on the plasma membrane as well as within the cytoplasm. m, mitochondria; ut, unlabeled terminal. Scale bars, 0.5 μm.
Figure 4.
Examples of synaptic contacts formed by unlabeled axon terminals with GPR177+MOR dendrites. Electron photomicrographs showing immunogold-silver labeling for GPR177 and immunoperoxidase labeling for MOR in the striatum. A–B. A dually labeled GPR177+MOR dendrite (GPR177+MOR-d) receives a symmetric synapse (black arrows) from an unlabeled terminal (ut). C. A dually labeled GPR177+MOR-d with a protruding spine receives an asymmetric synapse (curved arrow) from an unlabeled terminal (ut). Scale bars, 0.5 μm.
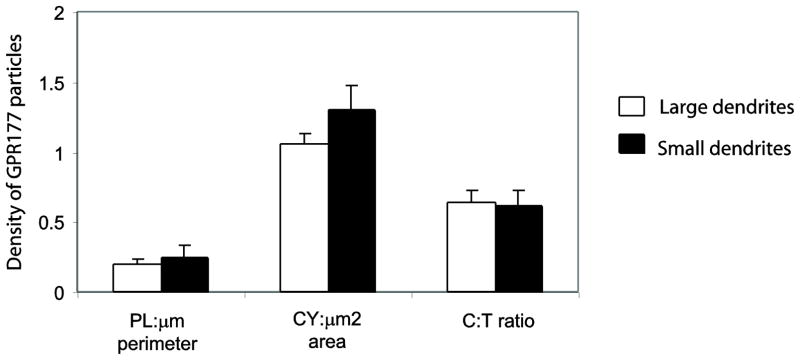
Along with the ratio of cytoplasmic to total immunogold-silver particles, the number of immunogold-silver particles per micron of plasmalemma and the number of immunogold-silver particles per micron squared of cytoplasm were also determined as presented in Figure 3. Statistical analysis showed that these associations did not significantly vary between large- (> 5 μm in perimeter)and small -(< 5 μm in perimeter) sized dendrites. Additionally, our results showed that the immunogold-silver particles were associated in a number of organelles within the cytoplasmic compartment. For this purpose, we did not intend to classify the size of dendritic profiles examined as our earlier results showed that the size of dendrites did not influence various criteria evaluated. Immunogold-silver particles were often associated with endosome-like vesicles (30%)and Golgi apparatus (12%). Some immunogold-silver particles were also associated with endoplasmic reticulum and multivesicular bodies while some were associated with organelles that could not be clearly classified.
Figure 3.
The distribution of GPR177 immunogold-silver particles in large and small dendrites in mice. The density of GPR177 immunogold-silver particles in large and small dendrites showed no significant difference. Also, the ratio of cytoplasmic to total immunogold-silver particles did not vary significantly between large and small dendrites.
2.2. GPR177 interactions with MORin the striatum
The results of the immunofluorescence studies were confirmed by the ultrastructural results where GPR177 was visualized by immunogold-silver detection and MOR was identified using an electron-dense peroxidase reaction product. Results from the electron microscopic analysis showed co-localization of GPR177 and MOR in common striatal profiles. A profile was considered to be positive for GPR177 only if two or more immunogold particles were present in the same profile. The immunoreactivities for GPR177 and MOR were observed in separate dendrites in the same neuropil, as well as in the same striatal dendrites (Fig. 2B–D;4A–C). Of the 412 dendrites containing GPR177, 32% (133/412) also contained MOR immunolabeling. On the other hand, of the 360 profiles containing MOR immunolabeling, 37% (133/360) also contained GPR177 labeling. Moreover, dendrites co-localized for GPR177 and MOR received synaptic contacts from unlabeled terminals (Fig.2C,D; 4A–C; Table 1). When synaptic associations were identifiable, they were either symmetric (Fig.2C,D; 4A,B; Table 1) or asymmetric (Fig. 3C; Table 1). However, symmetric synapses (37%; Fig. 2C,D; 3A,B) were more numerous compared to asymmetric synapses (10%; Fig. 3C; Table 1A). Nevertheless, most of the synaptic specializations formed by unlabeled axon terminals on co-localized dendrites could not be unequivocally classified as either symmetric or asymmetric, and were termed “undefined” possibly due to the fact that the plane of section analyzed was not through the portion of the active zone where this classification could be clearly discerned (53%; Table 1A).
Table 1A.
Synaptic specializations received by GPR177+MOR-labeled dendrites.
| Symmetric | Asymmetric | Undefined | |
|---|---|---|---|
| Number of profiles | 49 | 13 | 71 |
| Percentage | 37% | 10% | 53% |
To determine whether the synaptic specializations of GPR177 and MOR-dual labeled dendrites were selective to this subset of neuronal targets, we also investigated the synaptic specializations of GPR177 singly labeled dendrites. As shown in Table 1B,GPR177-singly labeled dendrites received either symmetric or asymmetric synapse from unlabeled axon terminals. Similar to GPR177 and MOR-dual labeled dendrites, symmetric synapses were more frequently observed (45%) as compared to asymmetric synapses (17%). There were less undefined synaptic specialization formed (51%) in GPR177-singly labeled dendrites as compared to GPR177 and MOR-dual labeled dendrites(71%).
Table 1B.
Synaptic specializations received by GPR177-labeled dendrites.
| Symmetric | Asymmetric | Undefined | |
|---|---|---|---|
| Number of profiles | 61 | 23 | 51 |
| Percentage | 45% | 17% | 38% |
2.3. GPR177 and MOR are co-localized in ENK-containing perikarya in the striatum
Using confocal microscopy, light microscopic immunocytochemical labeling for GPR177, MOR and ENK was conducted on the same tissue section. Immunoreactivities for GPR177, MOR and ENK were found distributed in the same tissue section through the dorsolateral striatum (Fig. 5). GPR177 immunoreactivity was identified in the dendrites and perikarya (Fig. 5B, D). Likewise, MOR immunoreactivity was found localized within the dendrites and perikarya (Fig. 5A, D). while ENK immunoreactivity was found distributed in the perikarya and fibers (Fig, 5C, D). GPR177 and MOR colocalized within perikarya that also exhibited ENK immunoreactivity (Fig. 5D).
Figure 5.
Triple-immunofluorescence labeling in the striatum showing μ-opioid receptor (μ-OR; blue), GPR177 (red), and enkephalin (ENK; green). A. μOR immunoreactivity was detected using Cy5-tagged secondary antibody. Arrows point to μOR-labeling. B. GPR177 immunoreactivity was detected using TRITC-tagged secondary antibody. Arrows point to GPR177-labeling. C. ENK immunoreactivity was detected using FITC-tagged secondary antibody. Arrows point to ENK-labeling. D. Photomicrograph showing merged images μOR (blue), GPR177 (red), and ENK (green). Arrowheads point ENK-labeled perikaryon containing both GPR177 and μOR immunoreactivities. Scale bars, 20 μm.
3. Discussion
This study presents findings that advance our understanding of the mechanism by which MOR and its interacting protein GPR177 may potentially alter neuronal development in opioid dependence. Specifically, the results provide ultrastructural evidence for dual localization of GPR177 and MOR in a population of striatal neurons that are primarily under inhibitory control in the dorsolateral striatum. In addition, the results provide an anatomical substrate for co-localization of GPR177 and MOR in ENK-containing neurons in the dorsolateral striatum.
Localization of GPR177
In mammalian cells, GPR177has been shown to regulate Wnt protein secretion (Banziger et al., 2006; Bartscherer et al., 2006; Franch-Marro et al., 2008). While mice heterozygous for GPR177lacZappeared normal and were fertile , homozygous null mutants died in utero during early embryogenesis (Fu et al., 2009). The number and location of the transmembrane segment of GPR177 is somehow controversial with models predicting four (Goodman et al., 2006), seven (Banziger et al., 2006) or eight (Bartscherer et al., 2006) membrane-spanning domains. Nevertheless, sequence comparisons indicate that GPR177 is highly conserved in mammalian species such that human GPR177 is 96% identical to mouse GPR177and 78% identical to the zebra protein. In neural epithelial progenitors and neurosphere cells, GPR177 was highly concentrated in vesicles and perinuclear area resembling Golgi (Fu et al., 2009).
Co-localization of GPR177 and MOR within striatal perikarya and dendrites confirms and extends our previous microscopic studies showing that GPR177 and MOR are distributed in this brain region (Jin et al., 2010; Mansour et al., 1994a; Mansour et al., 1994b). GPR177 and MOR interactions have also been described using co-immunoprecipitation experiments from HEK 293 cells stably expressing MOR (293-MOR cells) and transiently transfected with a His/FLAG-tagged GPR177 construct. From these lysates prepared from transfected cells with an anti-GPR177 antibody, an immunoreactive band of approximately 50kDA, the predicted molecular weight of human GPR177, was detected (Jin et al., 2010). The interaction of GPR177 and MOR was also found in the brain as confirmed when MOR was immunoprecipitated from rat brain lysates and immunocomplexes were probed for the immunoreactive detection of GPR177 where the presence of approximately 50kDA band was also observed (Jin et al., 2010). Taken together, these results suggest a potential interaction between GPR177 and MOR in a subset of striatal neurons that may serve as a substrate through which opiate drugs exert morphological alterations as a consequence of chronic use.
MOR interacting proteins and potential roles in addiction
Repeated exposure to opiate drugs leads to dependence and addiction, and at the cellular level opiate drugs exert their effects via the activation of G-protein coupled receptors (Contet et al., 2004). As a G-protein coupled receptor, MOR is activated upon exposure to morphine (Koch et al., 2001; Koch et al., 2005), and its regulation is governed by various mechanisms that play significant role in the development of opioid dependence and addiction (Waldhoer et al., 2004; Raehal and Bohn, 2005). Desensitization and internalization of MOR following exposure to opiates represent cellular mechanisms pivotal in physiological adaptations that are apparent in the development of opioid dependence and addiction (von Zastrow et al., 2003). In vivo studies showed that in brain regions involved in reward processing, learning and memory, chronic morphine treatment significantly reduced cellular proliferation and neurogenesis and dramatically altered neuronal phenotypes (Eisch et al., 2000; Kahn et al., 2005; Harburg et al., 2007). In addition, chronic morphine treatment profoundly decreased the complexity of dendritic branching and number of dendritic spines (Robinson and Kolb, 1999) and caused morphological alterations in dopaminergic neurons (Sklair-Tavron et al., 1996). The mechanism by which MOR is involved in neuronal plasticity following chronic opiate exposure is not clearly understood and requires further investigation.
Recently, proteins that interact directly with the MOR have been identified and shown to influence MOR biosynthesis, trafficking and signaling (Milligan, 2005). Thus, MOR interacting proteins could regulate multiple mechanisms including signaling and trafficking. As a MOR interacting protein, GPR177 has been shown to be essential in mediating the secretion of Wnt signaling proteins (Banziger et al., 2006; Bartscherer et al., 2006; Goodman et al., 2006). While Wnt proteins are known for their role in neuronal development (Salinas and Zou, 2008), evidence has shown that GPR177 is required in mediating their secretion in the signal producing cells (Fu et al., 2009). Using immunohistochemistry and co-immunoprecipitation, we recently showed that in MOR-expressing HEK293 cells, morphine significantly decreased Wnt secretion and that the inhibition appears to be specific to the activation of MORs considering that DAMGO treatment did not affect Wnt secretion from 293-MOR cells (Jin et al., 2010). Interestingly, the morphine-mediated inhibition of Wnt2 secretion was reversed by overexpression of GPR177, indicating that while morphine specifically blocks Wnt secretion from MOR expressing cells, this inhibition can be overcome by overexpression of GPR177 (Jin et al., 2010).
In the present study, dendritic processes that co-localized GPR177 and MOR exhibited differential patterns of localization within individual profiles and frequently received heterogeneous synapses from unlabeled axon terminals. GPR177 immunoreactivity was localized in MOR-labeled perikaryaas well as in MOR-labeled dendrites(Jin et al., 2010) . Within dendrites, GPR177 immunoreactivity was distributed within the cytoplasm and along the plasma membrane of MOR-labeled dendrites. A higher percentage of profiles exhibiting MOR and GPR177 showed that the distribution of GPR177 was more common within the cytoplasm and along the plasma membrane as opposed to being restricted to any of these individual cellular compartments.
Unlabeled axon terminals formed symmetric, asymmetric or “undefined” synapses (e.g. did not form recognizable junctions in the plane of section examined) with GPR177 and MOR-labeled dendrites. Semi-quantitative analysis showed a preponderance of symmetric synapses with GPR177 and MOR-labeled dendrites. While asymmetric synapses have been associated with excitatory transmission, symmetric synapses have been associated with inhibitory transmission (Gray, 1959; Peters et al., 1991). Electrophysiological studies have shown that mu selective opioid peptides act to post-synaptically inhibit striatal neurons(Pickel et al., 1992). The striatum is known to be enriched with GABA-containing neurons (Graveland and DiFiglia, 1985; Gerfen and Wilson, 1996)and these GABAergic neurons serve as the primary source of synaptic inhibition in the striatum (Koos et al., 2004; Taverna et al., 2007). Likewise, ENK-containing neurons are known to be localized in the striatal neurons (Pickel et al., 1992). These data suggest that unlabeled axon terminals targeting GPR177 and MOR-labeled dendrites may represent inhibitory (ENK-or GABA ) axon terminals that may hyperpolarize striatal neuronal activity .
Functional implications
Since striatal neurons have been shown to contain multiple peptidergic and aminergic neuromodulators (Fonnum et al., 1977; Graybiel, 1990; Chen et al., 2008), we sought to determine the phenotype of striatal neurons containing both GPR177 and MOR. Our triple immunofluorescence data show that colocalization of GPR177 and MOR is contained in enkephalinergic individual striatal neurons. Our data extend previous electron microscopic studies that GPR177 (Jin et al., 2010), MOR (Wang et al., 1996; Wang et al., 1999) and ENK (Pickel et al., 1992) are contained in striatal neurons that have cortical connections critical for complex cognitive functions (Alexander et al., 1986). ENK is known to regulate functions of striatal neurons such that perturbations in ENK expression levels reflect alterations in striatal neuronal activity (Steiner and Gerfen, 1999; Parelkar and Wang, 2003). It has been reported that within the striatum, the cortically derived information is transformed where representations of motor and cognitive action sequences can be integrated into more complex behavioral responses (Graybiel, 1998) emphasizing that in addition to the role of striatum in motor control, the striatum plays an essential role in the function of more complex behaviors including decision making and executive function (Graybiel, 2008). It is likely that following exposure to morphine, GPR177 and MOR interaction within the enkephalinergic striatal neurons contributes to the mechanism underlying changes in the brain that induce adverse effects on brain function including cognitive impairment, affective disturbances and addiction (Skaer, 2004).
In summary, using immunofluorescence and immunoelectron microscopy, we provide cellular evidence suggesting potential mechanisms by which GPR177 and MOR may interact via enkephalinergic neurons to affect activity of striatal neurons following morphine exposure. Interactions between GPR177 and MOR may underlie the morphological consequences accompanying chronic opiate use (eg, shrunken neurons, decreased neurogenesis, loss of dendritic spines) that may result in impaired cognition, affective disturbances and addiction. Future studies examining whether interfering with this interaction leads to abrogating morphological alterations following chronic opiate use are required to advance our understanding of the consequences of chronic opiate exposure.
4. Experimental Procedures
4.1. Animals
Six three-month old male naive C57/BL/6 wild type mice (20–25 g; Taconic, Albany, NY) were used. The animal procedures used in this study were approved by the Institutional Animal Care and Use Committee at Thomas Jefferson University and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Mice were housed 2–3 per cage on a 12-h light schedule (lights on at 0700) in a temperature-controlled (20 ºC) colony room. They were allowed ad libitum access to standard chow and water. All efforts were made to minimize animal distress and the number of animals used.
4.2. Specificity of antisera
Anti-GPR177 antibody was generated in chickens against a peptide antigen corresponding to the C-terminal 18 amino acids (HVDGPTEIYKLTRKEAQE) of human GPR177(Gene-Tel Laboratories, Madison, WI), which is identical to the rat and mouse peptide sequence. Antibodies of the IgY subtype were harvested from egg yolks and affinity purified prior to use. The characterization and specificity of the chicken antiserum against GPR177 has been previously described (Jin et al., 2010). Western blot analysis using GPR177 antibody showed expression of the GPR177 in rat brain lysates indicating that GPR177 antibody recognized endogenous GPR177 in brain tissue. GPR177 recognized single band of proteins with approximate molecular weight of about 50kDa (Jin et al., 2010). Anti-MOR antibody was generated in rabbit against a glutaraldehyde conjugate corresponding to the C-terminal 18 amino acids of rat MOR (Surratt et al., 1994; Van Bockstaele et al., 1996a; Van Bockstaele et al., 1996b). Through immunodot-blot analysis, the MOR antiserum was shown to specifically recognize amino acid sequences of MOR, but not amino acid sequences of either delta-or kappa-opioid receptors (Cheng et al., 1996).
4.3. Immunofluorescence
Three adult mice were deeply anesthetized with sodium pentobarbital (80 mg/kg) and each was transcardially perfused through the ascending aorta with 100 ml of 4% formaldehyde in 0.1 M phosphate buffer (PB; pH 7.4). Brains were then removed and post fixed in 4% formaldehyde overnight at 4°C. Sections (40 μm) through the rostrocaudal extent of striatum (Paxinos and Watson, 1997) were cut using a Vibratome (Technical Product International, St Louis, MO, USA) and rinsed extensively in 0.1 M PB and 0.1 M tris-buffered saline (TBS; pH 7.6). Sections were placed for 30 min in 1% sodium borohydride in 0.1 M PB to reduce amine-aldehyde compounds. Tissue sections were rinsed in 0.1 M PB and 0.1 M TBS. Sections were then incubated in 0.5% bovine serum albumin (BSA) and 0.25% Triton X-100 in 0.1M TBS for 30 min and rinsed thoroughly in 0.1 M TBS. Following rinses, tissue sections were incubated in a cocktail of chicken anti-GPR177 antiserum (1:1000), rabbit anti-MOR (1:2000; Chemicon, Temecula, CA) and mouse anti-leucine5-enkephalin (ENK; 1:100; Fitzgerald Laboratories, Concord, MA) in 0.5% BSA and 0.25% Triton X-100 in 0.1M TBS. Sections were incubated at room temperature for 15–18 h in a rotary shaker. Following primary antibody incubation, sections were washed in 0.1 M TBS and incubated in a secondary antibody cocktail containing tetramethyl rhodamine isothiocyanate (TRITC) donkey anti-chicken (1:200; Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA), Cy5 donkey anti-rabbit (1:200; Jackson ImmunoResearch) and fluorescein isothiocyanate (FITC) donkey-anti-mouse (1:200; Jackson ImmunoResearch) antibodies prepared in 0.1 % BSA and 0.25% Triton X-100 in 0.1 M TBS for 2 h in the dark on a rotary shaker. The tissue sections were washed thoroughly in 0.1 M TBS and 0.1 M PB then mounted on slides, allowed to dry in the dark. The slides were dehydrated in a series of alcohols, soaked in xylene and coverslipped using DPX (Sigma-Aldrich Inc., St. Louis, MO, USA). Immunofluorescence labeling on tissue sections was visualized using a Zeiss LSM 510 Meta confocal microscope (Carl Zeiss Inc., Thornwood, NY, USA). Digital images of immunofluorescent labeling were captured and imported using the LSM 5 image browser (Carl Zeiss Inc.). Figures were assembled and adjusted for brightness and contrast in Adobe Photoshop.
4.4. Immunoelectron microscopy
Three adult mice were used for immunoelectron microscopy. Methods for perfusion, sectioning, and immunohistochemical labeling of tissue sections intended for electron microscopy have been described previously (Jin et al., 2010; Van Bockstaele et al., 1996a; Van Bockstaele et al., 1996b). Briefly, mice were deeply anesthetized with sodium pentobarbital (80 mg/kg) and perfused transcardially with 10 ml heparinized saline followed with 25 ml of 3.75% acrolein (Electron Microscopy Sciences, Fort Washington, PA, USA), and 50 ml of 2% formaldehyde in 0.1 M PB (pH 7.4). The brains were removed immediately, sectioned into 1–3 mm coronal slices and postfixed in the same fixative overnight at 4ºC. Sections (40 μm) were processed for electron microscopic analysis of GPR177 and MOR in the same section. Sections containing the striatum were processed following the protocol described earlier for immunofluorescence except that Triton X-100 was not added to the solution for antibody incubation. Tissue sections were incubated in a cocktail of chicken anti-GPR177 antiserum (1:1000) and rabbit anti-MOR (1:2000; Chemicon). The following day, tissue sections were extensively rinsed three times in 0.1 M TBS and incubated in biotinylated donkey anti-rabbit (1:400; Jackson ImmunoResearch, West Grove, PA). Subsequently, a 30-minute incubation of avidin-biotin complex (Vector Laboratories, Burlingame, CA) followed. For all incubations and washes, sections were continuously agitated with a rotary shaker. MOR was visualized by a 4-min reaction in 22 mg of 3,3’-diaminobenzidine (DAB; Sigma-Aldrich Inc., St. Louis, MO) and 10 μl of 30% hydrogen peroxide in 100 ml TBS. Sections were then rinsed three times with 0.1 M TBS, followed by rinses with 0.1 M PB and 0.01M phosphate buffered saline (PBS; pH 7.4). Sections were incubated in a 0.2% gelatin-PBS and 0.8% BSA buffer for 10 min and followed by incubation in ultrasmall (< 1 nm) gold-coupled goat anti-chicken (1:100 dilution; Electron Microscopy Sciences) at room temperature for 2 h. Sections were then rinsed in buffer containing the same concentration of gelatin and BSA as above. Following rinses with 0.01 M PBS, sections were incubated in 2% glutaraldehyde (Electron Microscopy Sciences) in 0.01 M PBS for 10 min. This procedure was followed by washes in 0.01 M PBS and 0.2 M sodium citrate buffer (pH 7.4). A silver enhancement kit (Amersham Bioscience Corp., Piscataway, NJ) was used for silver intensification of the gold particles. The optimal times for silver enhancement were determined by empirical observation for each experiment and ranged between 8 and 10 min. To avoid bias in labeling, some tissue sections incubated for GPR177+MOR were reversed labeled such that GPR177 was labeled for immunoperoxidase and MOR was labeled with immunogold-silver. Following intensification, tissue sections were rinsed in 0.2 M citrate buffer and 0.1 M PB, and incubated in 2% osmium tetroxide (Electron Microscopy Sciences) in 0.1 M PB for 1 h, washed in 0.1 M PB, dehydrated in an ascending series of ethanol followed by propylene oxide, and flat embedded in Epon 812 (Electron Microscopy Sciences; Leranth and Pickel, 1989). Thin sections (50–100 nm thick) were then cut with a diamond knife (Diatome-US, Fort Washington, PA, USA) using a Leica Ultracut (Leica Microsystems, Wetzlar, Germany). Captured images of selected sections were compared with captured light microscopic images of the block face before sectioning. Sections were collected on copper mesh grids and examined with an electron microscope (Morgagni, Fei Company, Hillsboro, OR). Digital images were taken with an AMT advantage HR/HR-B CCD camera system (Advance Microscopy Techniques, Danvers, MA). Figures were assembled and adjusted for brightness and contrast in Adobe Photoshop.
4.5. Identification of immunogold-silver labeling in profiles
Profiles exhibiting immunogold-silver labeling were identified by the presence, in single thin sections, of at least three immunogold-silver particles within a cellular compartment. A profile containing a small number of immunogold particles (e.g. two immunogold particles) in adjacent thin sections was designated as lacking detectable immunoreactivity. As observed in low magnification electron micrographs, background labeling in the neuropil, deemed spurious, was not commonly encountered. The criterion of three immunogold particles as indicative of GPR177 labeling is conservative and may have led to an underestimation of the number of GPR177-labeled profiles. We have previously reported GPR177-immunoreactivity in somata and dendritic processes in the striatum exhibiting at least two to three immunogold particles (Jin et al., 2010). Another factor that may have led to the underestimation of labeled profiles is the limitation of immunocytochemical methods to detect trace amounts of GPR177. Moreover, unbiased stereological methods were not used for counting labeled profiles, and the results of the numerical analysis can only be considered to be an estimate of the numbers of synapses and labeled profiles.
4.6. Control and data analysis
The quantitative approach used in this study has been described previously (Van Bockstaele et al., 1996a; Van Bockstaele et al., 1996b). Some sections were processed in parallel with the rest of the procedures identical, but one of the primary antisera was omitted. Sections processed in the absence of primary antibody did not exhibit immunoreactivity. To evaluate cross-reactivity of labeling of the primary antiserum by secondary antisera, some sections were processed for dual labeling with omission of one of the primary antisera. Semiquantitative analysis was carried out in tissue sections with optimal immunohistochemical labeling and preservation of ultrastructural morphology. At least 9 grids containing 5 to 7 thin sections each were collected from at least three plastic-embedded striatal sections from each animal. Cellular elements were identified based on previous descriptions (Peters et al., 1991). Dendrites were identified by the presence of endoplasmic reticulum and a diameter of at least 0.4 μm, while axon terminals were identified by the presence of synaptic vesicles and a diameter of at least 0.3 μm. Asymmetric and symmetric synapses were also identified based on previous descriptions (Gray, 1959). Asymmetric synapses were documented by the presence of thick postsynaptic densities, while symmetric synapses by the presence of thin densities both pre- and postsynaptic. Non-synaptic contacts, or appositions, were defined by closely spaced plasma membranes of axons, other axon terminals, or dendrites; these lacked recognizable specializations.
GPR177 and MOR immunolabeling was identified as either cytoplasmic or plasmalemmal. Immunogold-silver grains were classified as plasmalemmal if they were associated with the plasma membrane, and cytoplasmic if they were not in contact with the plasma membrane. Dendritic profiles were classified as “small” or “large” based on their perimeter. Small dendrites were dendritic profiles with a perimeter smaller than 5μm and all other profiles more than 5μm as large dendrites. The density of GPR177 in the cytoplasm was calculated as the number of cytoplasmic immunogold-silver particles per unit area (μm2). Likewise, the density of GPR177 on the plasma membrane was calculated as the number of plasmalemmal immunogold-silver particles per unit perimeter (μm). All values were obtained per animal and the average of the three animals was calculated. The perimeter and area of each dendritic profile was obtained using Image J software (NIH).
Research Highlights.
GPR177 and MOR are co-localized in striatal somata and dendritic processes.
GPR177 was localized along the plasma membrane and within the cytoplasm of MOR-labeled dendrites.
Co-localized GPR177 and MOR more often received symmetric synapses.
GPR177 and MOR are localized in neurons containing enkephalin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Bailey CP, Connor M. Opioids: cellular mechanisms of tolerance and physical dependence. Curr Opin Pharmacol. 2005;5:60–68. doi: 10.1016/j.coph.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Beckstead RM, Kersey KS. Immunohistochemical demonstration of differential substance P-, met-enkephalin-, and glutamic-acid-decarboxylase-containing cell body and axon distributions in the corpus striatum of the cat. J Comp Neurol. 1985;232:481–498. doi: 10.1002/cne.902320406. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. The corticostriatal projection: from synaptic plasticity to dysfunctions of the basal ganglia. Trends Neurosci. 1996;19:19–24. doi: 10.1016/0166-2236(96)81862-5. [DOI] [PubMed] [Google Scholar]
- Chen TC, Cheng YY, Sun WZ, Shyu BC. Differential regulation of morphine antinociceptive effects by endogenous enkephalinergic system in the forebrain of mice. Mol Pain. 2008;4:41. doi: 10.1186/1744-8069-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng PY, Moriwaki A, Wang JB, Uhl GR, Pickel VM. Ultrastructural localization of mu-opioid receptors in the superficial layers of the rat cervical spinal cord: extrasynaptic localization and proximity to Leu5-enkephalin. Brain Res. 1996;731:141–154. doi: 10.1016/0006-8993(96)00492-1. [DOI] [PubMed] [Google Scholar]
- Ciani L, Salinas PC. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat Rev Neurosci. 2005;6:351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- Contet C, Kieffer BL, Befort K. Mu opioid receptor: a gateway to drug addiction. Curr Opin Neurobiol. 2004;14:370–378. doi: 10.1016/j.conb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci USA. 2000;97:7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F, Walaas I, Iversen E. Localization of GABAergic, cholinergic and aminergic structures in the mesolimbic system. J Neurochem. 1977;29:221–230. doi: 10.1111/j.1471-4159.1977.tb09612.x. [DOI] [PubMed] [Google Scholar]
- Franch-Marro X, Wendler F, Guidato S, Griffith J, Baena-Lopez A, Itasaki N, Maurice MM, Vincent JP. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat Cell Biol. 2008;10:170–177. doi: 10.1038/ncb1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Jiang M, Mirando AJ, Yu HM, Hsu W. Reciprocal regulation of Wnt and Gpr177/mouse Wntless is required for embryonic axis formation. Proc Natl Acad Sci USA. 2009;106:18598–18603. doi: 10.1073/pnas.0904894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Wilson CJ. The basal ganglia. In: Swanson LWBA, Hokfelt T, editors. Handbook of chemical neuroanatomy. 12. Amsterdam: Elsevier Science; 1996. pp. 371–468. [Google Scholar]
- Goodman RM, Thombre S, Firtina Z, Gray D, Betts D, Roebuck J, Spana EP, Selva EM. Sprinter: a novel transmembrane protein required for Wg secretion and signaling. Development. 2006;133:4901–4911. doi: 10.1242/dev.02674. [DOI] [PubMed] [Google Scholar]
- Graveland GA, DiFiglia M. The frequency and distribution of medium-sized neurons with indented nuclei in the primate and rodent neostriatum. Brain Res. 1985;327:307–311. doi: 10.1016/0006-8993(85)91524-0. [DOI] [PubMed] [Google Scholar]
- Gray EG. Axosomatic and axo-dendritic synapses of the cerebral cortex: an electron microscopic study. J Anat. 1959;93:420–433. [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiol Learn Mem. 1998;70:119–136. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Harburg GC, Hall FS, Harrist AV, Sora I, Uhl GR, Eisch AJ. Knockout of the mu opioid receptor enhances the survival of adult-generated hippocampal granule cell neurons. Neuroscience. 2007;144:77–87. doi: 10.1016/j.neuroscience.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Kittanakom S, Wong V, Reyes BAS, Van Bockstaele EJ, Stagljar I, Berrettini W, Levenson R. Interaction of the mu-opioid receptor with GPR177 (Wntless) inhibits Wnt secretion: potential implications for opioid dependence. BMC Neurosci. 11:33. doi: 10.1186/1471-2202-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn L, Alonso G, Normand E, Manzoni OJ. Repeated morphine treatment alters polysialylated neural cell adhesion molecule, glutamate decarboxylase-67 expression and cell proliferation in the adult rat hippocampus. Eur J Neurosci. 2005;21:493–500. doi: 10.1111/j.1460-9568.2005.03883.x. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Murray GK, Robbins TW. Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Curr Opin Neurobiol. doi: 10.1016/j.conb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Kieffer BL. Opioids: first lessons from knockout mice. Trends Pharmacol Sci. 1999;20:19–26. doi: 10.1016/s0165-6147(98)01279-6. [DOI] [PubMed] [Google Scholar]
- Koch T, Schulz S, Pfeiffer M, Klutzny M, Schroder H, Kahl E, Hollt V. C-terminal splice variants of the mouse mu-opioid receptor differ in morphine-induced internalization and receptor resensitization. J Biol Chem. 2001;276:31408–31414. doi: 10.1074/jbc.M100305200. [DOI] [PubMed] [Google Scholar]
- Koch T, Widera A, Bartzsch K, Schulz S, Brandenburg LO, Wundrack N, Beyer A, Grecksch G, Hollt V. Receptor endocytosis counteracts the development of opioid tolerance. Mol Pharmacol. 2005;67:280–287. doi: 10.1124/mol.104.004994. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM, Wilson CJ. Comparison of IPSCs evoked by spiny and fast-spiking neurons in the neostriatum. J Neurosci. 2004;24:7916–7922. doi: 10.1523/JNEUROSCI.2163-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, Pickel VM. Electron microscopic preembedding double-labeling methods. In: Heimer L, Zaborszky L, editors. Neuroanatomical tracing methods 2. 1. Plenum Press; New York: 1989. pp. 129–172. [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol. 1994a;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Thompson RC, Akil H, Watson SJ. mu-Opioid receptor mRNA expression in the rat CNS: comparison to mu-receptor binding. Brain Res. 1994b;643:245–265. doi: 10.1016/0006-8993(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Milligan G. Opioid receptors and their interacting proteins. Neuromolecular Med. 2005;7:51–59. doi: 10.1385/NMM:7:1-2:051. [DOI] [PubMed] [Google Scholar]
- Parelkar NK, Wang JQ. Preproenkephalin mRNA expression in rat dorsal striatum induced by selective activation of metabotropic glutamate receptor subtype-5. Synapse. 2003;47:255–261. doi: 10.1002/syn.10174. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1997. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster Hd. The Fine Structure of the Nervous System. Oxford University Press; New York: 1991. [Google Scholar]
- Pickel VM, Chan J, Sesack SR. Cellular basis for interactions between catecholaminergic afferents and neurons containing Leu-enkephalin-like immunoreactivity in rat caudate-putamen nuclei. J Neurosci Res. 1992;31:212–230. doi: 10.1002/jnr.490310203. [DOI] [PubMed] [Google Scholar]
- Raehal KM, Bohn LM. Mu opioid receptor regulation and opiate responsiveness. Aaps J. 2005;7:E587–591. doi: 10.1208/aapsj070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse. 1999;33:160–162. doi: 10.1002/(SICI)1098-2396(199908)33:2<160::AID-SYN6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Salinas PC, Zou Y. Wnt signaling in neural circuit assembly. Annu Rev Neurosci. 2008;31:339–358. doi: 10.1146/annurev.neuro.31.060407.125649. [DOI] [PubMed] [Google Scholar]
- Skaer TL. Practice guidelines for transdermal opioids in malignant pain. Drugs. 2004;64:2629–2638. doi: 10.2165/00003495-200464230-00002. [DOI] [PubMed] [Google Scholar]
- Sklair-Tavron L, Shi WX, Lane SB, Harris HW, Bunney BS, Nestler EJ. Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci USA. 1996;93:11202–11207. doi: 10.1073/pnas.93.20.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Enkephalin regulates acute D2 dopamine receptor antagonist-induced immediate-early gene expression in striatal neurons. Neuroscience. 1999;88:795–810. doi: 10.1016/s0306-4522(98)00241-3. [DOI] [PubMed] [Google Scholar]
- Surratt CK, Johnson PS, Moriwaki A, Seidleck BK, Blaschak CJ, Wang JB, Uhl GR. -mu opiate receptor. Charged transmembrane domain amino acids are critical for agonist recognition and intrinsic activity. J Biol Chem. 1994;269:20548–20553. [PubMed] [Google Scholar]
- Taverna S, Canciani B, Pennartz CM. Membrane properties and synaptic connectivity of fast-spiking interneurons in rat ventral striatum. Brain Res. 2007;1152:49–56. doi: 10.1016/j.brainres.2007.03.053. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EE, Cheng P, Moriwaki A, Uhl GR, Pickel VM. Ultrastructural evidence for prominent distribution of the mu-opioid receptor at extrasynaptic sites on noradrenergic dendrites in the rat nucleus locus coeruleus. J Neurosci. 1996a;16:5037–5048. doi: 10.1523/JNEUROSCI.16-16-05037.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EE, Moriwaki A, Uhl GR. Mu-opioid receptor is located on the plasma membrane of dendrites that receive asymmetric synapses from axon terminals containing leucine-enkephalin in the rat nucleus locus coeruleus. J Comp Neurol. 1996b;376:65–74. doi: 10.1002/(SICI)1096-9861(19961202)376:1<65::AID-CNE4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- von Zastrow M, Svingos A, Haberstock-Debic H, Evans C. Regulated endocytosis of opioid receptors: cellular mechanisms and proposed roles in physiological adaptation to opiate drugs. Curr Opin Neurobiol. 2003;13:348–353. doi: 10.1016/s0959-4388(03)00069-2. [DOI] [PubMed] [Google Scholar]
- Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annu Rev Biochem. 2004;73:953–990. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- Wang H, Gracy KN, Pickel VM. Mu-opioid and NMDA-type glutamate receptors are often colocalized in spiny neurons within patches of the caudate-putamen nucleus. J Comp Neurol. 1999;412:132–146. doi: 10.1002/(sici)1096-9861(19990913)412:1<132::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Wang H, Moriwaki A, Wang JB, Uhl GR, Pickel VM. Ultrastructural immunocytochemical localization of mu opioid receptors and Leu5-enkephalin in the patch compartment of the rat caudate-putamen nucleus. J Comp Neurol. 1996;375:659–674. doi: 10.1002/(SICI)1096-9861(19961125)375:4<659::AID-CNE7>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Watson DJ, Stanton ME. Spatial discrimination reversal learning in weanling rats is impaired by striatal administration of an NMDA-receptor antagonist. Learn Mem. 2009;16:564–572. doi: 10.1101/lm.1448009. [DOI] [PMC free article] [PubMed] [Google Scholar]