Abstract
BACKGROUND
Lipoatrophy is prevalent on thymidine NRTIs (tNRTI). A pilot trial showed that uridine (NucleomaxX®) increased limb fat.
METHODS
A5229 was a multicenter trial in which HIV-infected individuals with lipoatrophy on tNRTI-regimens were randomized to NucleomaxX or placebo. Primary endpoint was change in limb fat from baseline to week-48. The study was powered to detect 400-gram difference between arms at week-48. A stratified Wilcoxon rank-sum test was used to assess between-arm differences.
RESULTS
The 165 subjects were 91% male, 62% white; median age 49 years, CD4 506 cells/mm3, and limb fat 3037 grams; 81% had HIV-1 RNA ≤50 copies/mL; 76% were on AZT. Baseline characteristics were similar between groups. Only 59% completed 48-weeks of treatment, however only 3 subjects (1 on uridine) discontinued due to toxicity (diarrhea). In intent-to-treat, there was no difference for changes in limb fat between treatments at week-24 or week-48. On as-treated analysis, uridine resulted in an increase in %limb fat vs. placebo (3.4% vs. −0.8%, p=0.01) at week-24 but not at week-48 (1.8% vs.3.8%, p=0.93). Similar results were seen when limiting the analysis to subjects with ≥80% adherence. The results were not related to severity of lipoatrophy or type of tNRTI. No changes were found in facial-anthropometrics, fasting lipids, trunk-fat, CD4, or HIV-RNA.
CONCLUSIONS
We found a modest transient improvement in limb fat after 24 weeks of uridine. The lack of sustained efficacy at week-48 was not due to changes in adherence or reduction in sample size. Uridine was safe and did not impair virologic control.
Introduction
Lipoatrophy of the face, arms, buttocks, and/or legs is a distressing and stigmatizing long term effect of antiretroviral therapy (ART). The pathogenesis of lipoatrophy is not fully understood, but several lines of evidence point towards an important role of nucleoside reverse transcriptase inhibitors (NRTI) in general, and the mitochondrial toxicity of thymidine NRTIs in particular (1–3). Some NRTIs, in particular thymidine NRTIs (tNRTIs, AZT and d4T) impair DNA polymerase-gamma or thymidine-kinase 2 and therefore interfere with the replication of mitochondrial DNA (mtDNA), leading to depletion in mtDNA, increased adipocyte apoptosis and ultrastructural abnormalities of adipocyte mitochondria (1–3). Lipoatrophy can be partially reversed by substituting tNRTIs with abacavir or tenofovir or by switching to NRTI-sparing regimens (4–6). Unfortunately, the reversal of lipoatrophy in these switch studies has been slow and only partial. Thus, it remains important to find a medical treatment for lipoatrophy that would prevent further development and improve the recovery of peripheral fat.
The supplementation of uridine is a new promising strategy in the treatment of lipoatrophy (7). In cultured adipocytes, uridine supplementation prevented and treated stavudine-related mtDNA-depletion, mitochondrial dysfunction, and consequent adipocyte apoptosis (8). A small placebo-controlled study (n=20) found that 3-months of NucleomaxX improved limb fat, but also increased visceral abdominal fat and decreased HDL-cholesterol (9). Another small single arm open-labeled study found improvement of subjective lipoatrophy scores but no changes in fat or peripheral blood mononuclear cells mtDNA levels in 16 lipoatrophic subjects who were treated with the uridine supplement for 4 months (10).
Doses of oral uridine or of uridine prodrugs are well tolerated in humans with mild osmotic diarrhea being dose-limiting in excessive doses (11–12). Phenotypic HIV-resistance assays and animal data do not indicate that uridine or its metabolites interfere with NRTI at HIV reverse transcriptase and thus would not affect the antiretroviral efficacy of nucleoside analogues (13–15).
In this study we hypothesized that dietary supplementation with uridine improves limb fat content in HIV infected subjects with established clinical lipoatrophy. As a source of uridine, we used NucleomaxX® (Pharma Nord, Vojens, Denmark), a dietary supplement which has been shown to increase the serum concentration of uridine in humans from an average of about 5 μM to more than 150 μM due to its high content (17%) of uridine and triacetyluridine (16) (www.nucleomaxX.com). A further goal of this study was to gather safety data of uridine supplementation in HIV-infected patients, including on HIV disease activity, and to determine if uridine supplementation is associated with changes in the amount of trunk and facial fat and with improvement in fasting lipid and lactate levels. We elected to study the effects of uridine supplementation among subjects who were currently receiving tNRTI containing therapy, because the efficacy of uridine in reversing mitochondrial toxicity in cell culture was more evident in cells treated with tNRTIs versus other NRTIs, such as purines analogues.
METHODS
Study Design and Population
A5229 was a phase II/III, randomized, double-blind, placebo-controlled study of uridine supplementation in the form of NucleomaxX for the treatment of HIV-associated lipoatrophy. The study was approved by the Institutional Review Board at all participating sites, and a FDA investigational new drug application for the use of NucleomaxX® in HIV lipoatrophy was obtained. All patients provided written informed consent for the DEXA scanning, the collection of samples and subsequent analysis. Eligibility criteria included HIV-infected men and women of at least 18 years of age with established lipoatrophy. Subjects were required to have a plasma HIV-1 RNA level of ≤5000 copies/mL, and be receiving stable thymidine analogue-containing antiretroviral regimen for a minimum of 12 consecutive weeks prior to study entry, with no plan at study entry to significantly alter ART for the duration of the study. For this study, lipoatrophy was defined as patient self-reported and investigator-confirmed fat wasting in at least two of the following areas: face, arms, legs, or buttocks. Exclusion criteria included pregnancy, breastfeeding, liver failure, severe lactose intolerance, diabetes requiring hypoglycemic agents, as well as the use of didanosine, hormonal or immunomodulating therapies. Exclusionary laboratory results were creatinine clearance < 50 mL/min, AST and/or ALT >5 x upper limit of normal (ULN), lipase > 2.5 ULN, hemoglobin <9.0 g/dL, platelet count <50,000/mm3, or absolute neutrophil count <750/mm3.
Intervention
The subjects were centrally randomized in a double-blinded fashion to receive either NucleomaxX® or calorie, consistency, color and taste matched placebo for the 48 weeks of the study. NucleomaxX® and placebo were bought in sachets of 36 grams each from Pharma Trade Healthcare AB, Spånga, Sweden and were provided to study participants. Study participants were instructed to drink the entire contents of one sachet after thoroughly mixing it with milk, juice, or water. Since the dosing was on an every other day schedule, instructions were given that if all three sachets were not taken in one day (first 24 hour period), the unused sachet(s) could be taken the next day (second 24 hour period), so that subjects would receive a total of 3 sachets (108 grams total) in a 48-hour period. There were no food restrictions. Antiretroviral drugs were not provided by the study. The subjects were informed to maintain their current antiretroviral therapy, and not modify their exercise or dietary habits during the course of the study period.
Outcome and Follow-up
Study evaluations included physical examination, fasting blood assessments, dietary questionnaires, facial anthropometrics, and whole body dual-energy x-ray absorptiometry (DEXA) scanning at study entry, weeks 24 and 48. Additional safety visits were carried out at weeks 4, 8, 12, 18, and 36 for the purpose of clinical and laboratory monitoring for toxicities, intolerance, and adherence.
Total body DEXA scans were performed at each of the sites, after standardization using a phantom. Central reading occurred at Tufts University, and the readers were blinded to patients’ characteristics and treatment allocation. Analysis of overall and body site-specific fat were performed on the basis of the standard protocol for body composition examination.
The metabolic assessments were done in a fasting state of at least 8 hours. They included glucose, insulin, and lipoprotein serum levels. Serum electrolytes, liver enzymes, CD4 cell count, HIV-1 RNA, glucose, lipid panel, and blood lactate were measured per ACTG standards.
All visits included assessment for clinical adverse events, use of concomitant medications, and adherence. Adherence to study medication was determined by available data on sachet count of dispensed versus returned sachets at each study visit, and self-reported missed doses if returned sachet data were not available. Based on these data, an adherence score at each visit was computed at each visit, and scores were averaged over study visits for each treatment group. The adherence score for each visit was computed using: adherence % = [(number of sachets dispensed − number of sachets returned)/number of sachets expected to be taken] ×100. Permanent cessation of the drug was mandatory for grade 4 adverse events, development of diabetes, or pregnancy.
Statistical Analysis
The primary outcome measure was the change in limb fat from baseline to week 48. A sample size of 82 subjects per arm (total of 164) was required to ensure an 85% power to significantly (p<0.05) detect a difference of 400 grams in limb fat from baseline to week 48 between the two treatment arms, assuming a standard deviation of 700 grams within groups and allowing for a dropout rate of 20%. Secondary outcome measures included changes in metabolic parameters. The changes from baseline to week 48 were compared between groups using a stratified Wilcoxon rank-sum test, whereas within group changes were tested using a Wilcoxon signed rank test. Four patient populations were considered based on outcome of analysis. (1) An intention-to-treat (ITT) approach with last observation carry forward (LOCF) method was used for primary endpoint analysis along with other secondary endpoints of limb fat change and trunk fat change. (2) As-treated (AT) analysis for limb fat change was performed with completers for DEXA measurements. (3) Study treatment adherence was assessed on ITT with LOCF population, i.e. based on population of primary endpoint analysis. Therefore, limb fat change analysis based on ≥80% adherence was on LOCF population, not completers. (4) Other outcomes such as CD4 change and change in lipid measures are based on ITT principle and completers for the outcomes. Details are presented in Table 2.
Table 2.
Summary of outcome variables by treatment group; N, median(Q1, Q3)
| Outcome | NucleomaxX | Placebo | P-value |
|---|---|---|---|
| ITT, LOCF results: | |||
| Limb fat (g) change from baseline to week 48 | 75, 74(−206, 344) | 69, 110(−202, 393) | 0.64 |
| Limb fat (g) change from baseline to week 24 | 75, 54(−117, 315) | 66, 7(−202, 238) | 0.25 |
| Limb fat % change from baseline to week 48 | 75, 1.8(−6, 15) | 69, 4.1(−7.8, 13.4) | 0.99 |
| Limb fat % change from baseline to week 24 | 75, 2.7(−3.4, 12.4) | 66, 0.2(−7.3, 8.8) | 0.09 |
| Trunk fat (g) change from baseline to week 48 | 75, 274(−614,1065) | 69,550(−248,1296) | 0.09 |
| Trunk fat (g) change from baseline to week 24 | 75, 299(−379, 787) | 66, 330(−463, 923) | 0.62 |
| As-treated, completer results: | |||
| Limb fat (g) change from baseline to week 48 | 45, 74(−217, 231) | 46, 107(−247, 370) | 0.71 |
| Limb fat (g) change from baseline to week 24 | 48, 89(−65, 325) | 46, −36(−203, 144) | 0.01 |
| Limb fat % change from baseline to week 48 | 45, 1.8(−7.2, 11.5) | 46, 3.8(−7.8, 14.3) | 0.93 |
| Limb fat % change from baseline to week 24 | 48, 3.4(−2.4, 12.5) | 46, −0.8(−7.5, 6.5) | 0.01 |
| For at least 80% adherent subjects, LOCF results: | |||
| Limb fat % change from baseline to week 48 | 48, 3.9(−6.6, 13.7) | 45, 3.8(−7.8, 13.4) | 0.92 |
| Limb fat % change from baseline to week 24 | 48, 3.4(−2.4, 12.4) | 44, −0.8(−8.2, 7.8) | 0.01 |
| ITT, completer results: | |||
| HIV-1 RNA ≤50 cp/mL at week 48 | 59/67 (88%) | 54/69 (78%) | 0.17λ |
| CD4 cell count change from baseline to week 48 | 80, 33(−44, 90) | 80, 28(−43, 108) | 0.88 |
| Fasting lactate (mmol/L) change from baseline to week 48 | 67, 0.2(−0.2, 0.6) | 62, 0.1(−0.2, 0.4) | 0.60 |
| Fasting glucose (mg/dL) change from baseline to week 48 | 78, 2(−6, 9) | 78, 4(−5, 11) | 0.13 |
| Fasting total cholesterol (mg/dL) change from baseline to week 48 | 77, 2(−15, 22) | 75, −6(−22, 18) | 0.17 |
| Fasting triglycerides (mg/dL) change from baseline to week 48 | 77, −8(−29, 49) | 75, 13(−28, 62) | 0.43 |
| Fasting HDL (mg/dL) change from baseline to week 48 | 77, 1(−3, 4) | 73, −1(−6, 2) | 0.03 |
| Fasting LDL (mg/dL) change from baseline to week 48 | 67, 1(−12, 23) | 61, −3(−21, 15) | 0.10 |
| Fasting non-HDL (mg/dL) change from baseline to week 48 | 66, 2 (−13, 18) | 61, 2 (−18, 17) | 0.76 |
| Infraorbital fat- fold (mm) change from baseline to week 48 | 77, 0.3(−1, 2) | 74, 0.3(−0.7, 1) | 0.88 |
| Buccal fat-fold (mm) change from baseline to week 48 | 77, 0.3(−1, 1.3) | 74, 0(−0.7, 1.7) | 0.94 |
| Submandible fat-fold (mm) change from baseline to week 48 | 77, 0(−1, 1) | 74, 0.3(0, 1.3) | 0.06 |
P-value from stratified Wilcoxon rank sum test,
P-value from Fisher’s exact test
RESULTS
Patient Characteristics
A total of 167 subjects were enrolled from October 5, 2006 to January 7, 2008 from 31 ACTG sites. Two subjects never started study drug. Of the 165 subjects who started study treatment, 83 were randomized to receive NucleomaxX. Table 1 shows the baseline characteristics of the study groups. Overall 150 individuals (91%) were male; 102 (62%) were White/Non-Hispanic, and 26 (16%) were previous or current IV drug users. The overall median age at enrollment was 49 years. The baseline median limb fat was 3037 grams, trunk fat 8114 grams, and CD4 count 506 cells/mm3. At entry, 81% had HIV-1 RNA ≤ 50 copies/mL. The randomization was stratified by type of tNRTI that subjects were receiving at study entry: 126 (76%) were on an AZT-containing regimen, and 39 (24%) were on a d4T-containing regimen. Demographics, clinical and HIV disease parameters were similar between the groups. In particular, there were no statistically significant differences in body mass index (BMI), limb fat, trunk fat, lipids, lactate, or glucose parameters at study entry.
Table 1.
Baseline characteristics by treatment group
| All Subjects (N=165) | NucleomaxX (N=83) | Placebo (N=82) | |||
|---|---|---|---|---|---|
| Gender | Male | 150 (91%) | 75 (90%) | 75 (91%) | |
| Female | 15 (9%) | 8 (10%) | 7 (9%) | ||
| Race/Ethnicity | White | 102 (62%) | 50 (60%) | 52 (63%) | |
| Black | 25 (15%) | 11 (13%) | 14 (17%) | ||
| Hispanic | 27 (16%) | 15 (18%) | 12 (15%) | ||
| Asian | 4 (2%) | 3 (4%) | 1 (1%) | ||
| American Indian | 5 (3%) | 3 (4%) | 2 (2%) | ||
| Other/Invalid | 1 (1%) | 1 (1%) | 0 (0%) | ||
| Missing/Unknown | 1 (1%) | 0 (0%) | 1 (1%) | ||
| IV Drug Use at Baseline | Never | 139 (84%) | 72 (87%) | 67 (82%) | |
| Currently | 1 (1%) | 1 (1%) | 0 (0%) | ||
| Previously | 25 (15%) | 10 (12%) | 15 (18%) | ||
| Age at Baseline (Yrs) | Median (Q1, Q3) | 49 (44, 53) | 47 (43, 54) | 49 (44, 53) | |
| 30–39 | 19 (12%) | 9 (11%) | 10 (12%) | ||
| 40–49 | 70 (42%) | 38 (46%) | 32 (39%) | ||
| 50–59 | 66 (40%) | 28 (34%) | 38 (46%) | ||
| Over 60 | 10 (6%) | 8 (10%) | 2 (2%) | ||
| Stratification Factor | AZT-containing | 126 (76%) | 63 (76%) | 63 (77%) | |
| D4T-containing | 39 (24%) | 20 (24%) | 19 (23%) | ||
| CD4 Count | Median (Q1, Q3) | 506 (353, 724) | 506 (353, 724) | 504 (376, 709) | |
| < 100 | 1 (1%) | 0 (0%) | 1 (1%) | ||
| 100–199 | 8 (5%) | 6 (7%) | 2 (2%) | ||
| 200–299 | 14 (8%) | 7 (8%) | 7 (9%) | ||
| 300–500 | 55 (33%) | 26 (31%) | 29 (35%) | ||
| > 500 | 82 (50%) | 41 (49%) | 41 (50%) | ||
| Missing/Unknown | 5 (3%) | 3 (4%) | 2 (2%) | ||
| HIV-1 R N A ≤50 cp/mL | 134 (81%) | 70 (84%) | 64 (78%) | ||
| BMI (kg/m2) | N, Median (Q1, Q3) | 161, 23.9 (21.9, 26.0) | 81, 23.6 (21.4, 25.9) | 80, 24.3 (22.2, 26.5) | |
| Limb fat (grams) | N, Median (Q1, Q3) | 165, 3037(2002, 4403) | 83, 3149 (1833, 4210) | 82, 2995 (2082, 4847) | |
Note: No statistically significant difference for baseline characteristics between the study arms; Q1=first quartile, Q3=third quartile
Subject Disposition
Of the 165 subjects who received study treatment, 97 (59%) completed 48 weeks of study treatment. Of these, 49 were randomized to receive NucleomaxX and 48 to placebo. The median (quartile Q1, Q3) follow-up on study treatment was 47 (23, 48) and 47 (18, 48) weeks for the NucleomaxX arm and the placebo arm, respectively. The major reason for discontinuation was bad taste [13(16%) NucleomaxX and 8(10%) placebo; p=0.35]. However, only 3 subjects (1 on NucleomaxX and 2 on placebo) discontinued treatment due to protocol-defined toxicity (diarrhea).
Adherence Assessment
The median adherence score was 97.6% for NucleomaxX compared to 98.8% for placebo for a follow-up period of 48 weeks. There was no significant difference in adherence between the study treatments for either the first 24 weeks of the study (P=0.28) or the entire study (P=0.39).
Primary Endpoint: Changes in Limb Fat
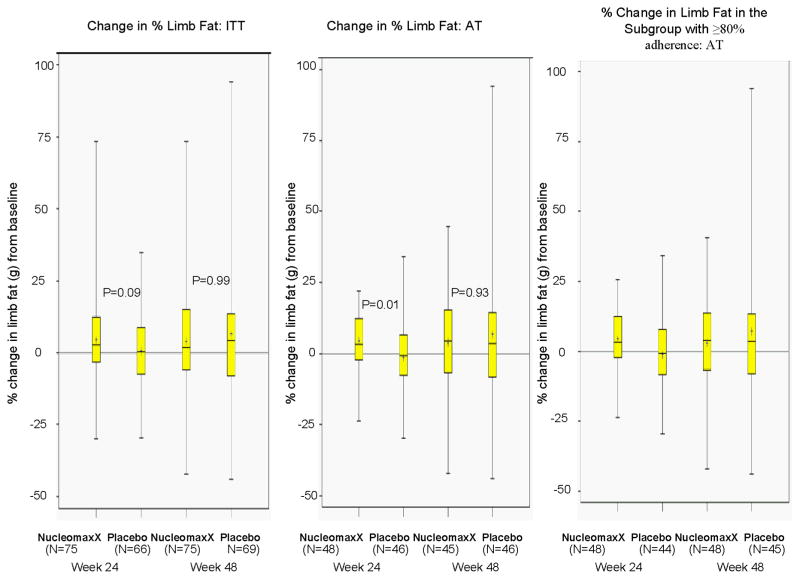
The primary endpoint, change in DEXA-measured limb fat from baseline to week 48, is depicted in Figure 1 and Table 2. By ITT and LOCF, NucleomaxX (N=75) and placebo (N=69) groups had a median (Q1, Q3) change of 74 grams (−206, 344) and 110 grams (−202, 393) in limb fat, respectively from baseline to week 48 (p=0.64), and a median percent change of 1.8% (−6.0, 15.0) and 4.1% (−7.8, 13.4), respectively (p=0.99). The median change in limb fat from baseline to week 24 in the NucleomaxX arm (N=75) was 54g compared with 7g in placebo (N=66) group (p=0.25). The treatment comparison was not statistically significant for percent limb fat change from baseline to week 24 either (2.7% vs. 0.2%; p=0.09).
Figure 1.
Changes in limb fat by intent to treat analysis, as treated analysis, and when the analysis restricted to the subgroup with at least 80% adherence
In the as-treated analyses with DEXA completers, the median percent change in limb fat was greater in the NucleomaxX arm when compared to Placebo (3.4% vs. −0.8%; p=0.01) at week 24 but not at week 48 (1.8% vs. 3.8%; p=0.93). Similar results were obtained from as-treated analysis of absolute change in limb fat, i.e. the median change in limb fat was greater in the NucleomaxX group compared to placebo (89 vs. −36 grams; p=0.012) at week 24, but not at week 48 (74 vs. 107 grams; p=0.71).
The results were similar when the AZT-containing stratum and the d4T-containing stratum were analyzed separately. During the study period, seven subjects in the NucleomaxX arm and six subjects in the placebo arm had discontinued AZT/ZDV or d4T prior to week 48 with a similar duration in the time to discontinuation of tNRTIs in both arms. The results were unchanged when these subjects were excluded from the analysis. The median change in daily caloric consumption from baseline to week 48 was slightly greater for the NucleomaxX arm (12 kcal/day) than for the Placebo arm (−156 kcal/day), but the difference was not statistically significant (P=0.11).
Further analysis of limb fat change from baseline to week 48 and to week 24 based on within group comparisons (using Wilcoxon signed rank test) showed very similar results to between group comparisons. For absolute change, there was some evidence of limb fat gain in NucleomaxX group (P=0.05) but not in placebo group (P=0.88) for week 24, but there was no evidence of significant change from baseline to week 48 in either group (NucleomaxX p=0.21, placebo p=0.13). Results based on percent change limb fat from within group analysis were also in agreement with those of absolute change, i.e. NucleomaxX p=0.008 and placebo p=0.90 at week 24, and NucleomaxX p=0.08 and placebo p=0.132 at week 48.
Subgroup analyses were undertaken to evaluate whether there was any evidence of variation in change of limb fat according to gender, race/ethnicity, and age by randomized treatment arms. No significant interaction effect was found (p>0.05). In addition, change in limb fat was not found to significantly correlate with baseline values of BMI and limb fat (both p>0.05).
After adjustment for adherence, the regression analysis showed that there was no significant difference (p=0.88) between NucleomaxX and placebo for absolute or percent change in limb fat from baseline to week 48. However there was a suggestive relationship (p=0.06) between percent limb fat change from baseline to week 24 and treatment group after adjustment for adherence during the same period. When limiting the analysis to subjects with at least 80% adherence, there was a significant difference in % change in limb fat at week 24 favoring NucleomaxX (3.4% vs. −0.8%, respectively; p=0.01), but not at week 48 (3.9% vs. 3.8%; p=0.92), and similarly a significant difference in absolute change in limb fat (88.5 vs. −35.5 grams; p=0.02) at week 24 but not at week 48 (105.5 vs. 103 grams; p=0.84).
Changes in Trunk Fat
At week 48, the median change in trunk fat was 274 g for the NucleomaxX arm, and was 550g for the placebo arm (p=0.10). In the as-treated analysis, the median change was 225g for the NucleomaxX arm and 563g for the placebo arm, respectively (p=0.09). No significant differences were found in the response to treatment by gender, race/ethnicity, or age. At week 24, the median change in trunk fat was 299g for the NucleomaxX arm and 330g for the placebo arm (p=0.62). As-treated analysis yielded similar results.
Other Mitochondrial and Metabolic Endpoints
No statistically significant between-arm difference was seen in changes in body mass index. Also no changes were seen in any of the facial anthropometrics measurements (infraorbital, buccal, submandible fat-folds) between groups. No differences in changes from baseline to week 48 were seen in hemoglobin, leukocytes, creatinine kinase, fasting glucose, lactate, or triglycerides between the NucleomaxX and placebo arm. However, notable although modest effects on the cholesterol concentrations were seen. The median change in fasting total cholesterol was significantly greater for the NucleomaxX arm than for the Placebo arm (1 vs. −2 mg/dL, P=0.031) at week 24, but not at week 48 (2 vs. −6 mg/dL; P=0.17). The median change in fasting LDL was marginally smaller for the NucleomaxX arm (−1 vs. −2 mg/dL in placebo; P=0.078) at week 24, and at week 48 (1 vs. −3 mg/dL, P=0.10). The median change in fasting HDL tended to be higher in NucleomaxX compared to placebo (1 vs. −1 mg/dL; p=0.076) at week 24, and was significantly greater (1 vs. −1 mg/dL; P=0.034) at week 48.
Adverse Events/Effect on HIV Disease Activity
Overall, 46/165 (28%) subjects reported grade 3 or above toxicities during the study (31% in the NucleomaxX arm vs. 24% in the placebo arm; p=0.39). The most common (>5 events) Grade ≥3 toxicities were: ache/pain/discomfort (6% in the NucleomaxX arm and 4% in the placebo arm), diarrhea/loose stools (5% in the NucleomaxX arm and 4% in the placebo arm), and abnormal creatine kinase (4% in the NucleomaxX arm and 6% in the placebo arm). There was no difference between the groups in the rate of any of these adverse events.
The protocol defined toxicities were diarrhea and hyperglycemia. Grade 2 or above diarrhea/loose stools was reported in 9 (11%) subjects in the NucleomaxX arm and 6 (7%) subjects in the Placebo arm (p=0.59). Grade 2 or above elevated fasting blood sugar was reported in 5 (6%) subjects in the NucleomaxX arm and 6 (7%) subjects in placebo. Two cases of diabetes occurred during the study, both in the placebo arm, and both had a history of impaired glucose tolerance prior to study enrollment. In addition, NucleomaxX treatment was not found to be associated with higher risk of any diagnosis compared with Placebo.
At entry, 70 (84%) subjects in the NucleomaxX arm and 64 (78%) subjects in the Placebo arm had HIV-1 RNA ≤50 copies/mL. Of the subjects with data available, 59/67 (88%) subjects in the NucleomaxX arm and 54/69 (78%) subjects in the Placebo arm had HIV-1 RNA ≤50 copies/mL at week 48 (p=0.17). The median change in CD4 count from baseline to week 48 was 33 and 28 cells/mm3 for the NucleomaxX and Placebo arms, respectively (P=0.88).
DISCUSSION
The aim of this study was to determine if the pyrimidine precursor uridine increased limb fat in patients with HIV-lipoatrophy. This study was initiated after several cell culture studies showed that uridine supplementation of hepatocyes and adipocytes abrogated mitochondrial toxicities of tNRTIs (8, 17–18)
It has been demonstrated in cultured hepatocytes that uridine or its metabolites compete with tNRTIs at steps of mitochondrial pyrimidine import, degradation or phosphorylation thus countering mtDNA depletion due to tNRTIs and secondary impairment of the electron transport trough the respiratory chain (18). Because the latter is also crucial for the function of dihydroorotate dehydrogenase (DHODH), a key enzyme in the de novo synthesis of all intracellular pyrimidines, tNRTI-related mitochondrial toxicity may lead to depletion in building blocks for mtDNA synthesis and thus increase the relative amounts of pyrimidine NRTIs. Through this mechanism, pyrimidine NRTIs may then compete more efficiently at DNA polymerase-gamma. A vicious circle is closed and may contribute to a further loss of mtDNA and oxidative capacity. By providing exogenous uridine from which all intracellular pyrimidines can be salvaged, this vicious circle may be disrupted and mitochondrial DNA and functions restored (18).
In our study, uridine supplementation did not improve limb fat after 48 weeks of treatment, despite a suggestion of a small but statistically significant effect at week 24 in the most adherent subjects. In a previous pilot study of NucleomaxX in HIV lipoatrophy, 20 subjects on AZT or d4T showed a remarkable increase (mean ± SD change of 880 ± 140 grams) in limb fat after only 3 months of NucleomaxX (9). A few differences between this study and the pilot study may explain the discrepancies in the findings. First, although the Finnish pilot study used the same product (NucleomaxX), the dose used was different: 36 grams three times daily for ten days each month, rather than every other day as used in our study. It is possible that more intensive, intermittent courses of uridine supplementation may be more effective. Second, participants in the Finnish study had been receiving ART for a median of 18 months prior to study drug, whereas in our study the cumulative duration of NRTI was unknown. It is possible that our patients had significantly longer exposure to tNRTIs and that like with other lipoatrophy interventions (such as AZT-to-TDF switch), prolonged use of tNRTIs before the lipoatrophy intervention jeopardizes the response of limb fat (19).
Although the safety profile of oral uridine or its prodrugs was reported to be excellent in HIV-negative subjects (20–21), it is important to rule out a negative effect of uridine or its metabolites on the ability of antiretroviral nucleoside analogues to inhibit HIV reverse transcriptase. While such an effect was previously excluded for a number of NRTIs and their combinations in phenotypic HIV-resistance assays and for zidovudine in an animal model (13–15), only small studies have been conducted in HIV-infected subjects (9–10). Consistent with the prior preclinical data, our study failed to detect a significant interaction between uridine and the antiretroviral or immunologic efficacy.
Despite lipoatrophy’s frequent association with insulin resistance, the sugar cane derived dietary supplement did not have deleterious effects on glucose metabolism. However, glucose metabolism was only measured by fasting glucose in this study. Therefore, we could have failed to detect pre-diabetes or worsening insulin resistance. Similarly, we found no clinically meaningful changes in fasting lipid levels, despite earlier suggestion of decreased HDL-cholesterol after NucleomaxX treatment (9).
We recognize the limitations of this study, mainly the large number of discontinuations due to low grade toxicities and intolerance to the study drug. In addition, our measure of adherence may be arguably suboptimal, however sachet/pill count has been validated for use in other ACTG studies. Additionally, 13 subjects (7 on NucleomaxX) changed tNRTI during the study which could significantly confound the results, although the results were unchanged when these subjects were excluded.
In summary, NucleomaxX was safe but not well tolerated by some subjects, mainly due to the bitter taste of pyrimidines. However, even in the most adherent subjects, limb fat did not improve by week 48 despite a suggestion of a modest effect at 24 weeks. Despite the statistical significance, this effect size was very modest and unlikely to be appreciated by the participants. Finally, this study cannot comment on the effect of uridine supplementation in subjects receiving tNRTI-sparing regimen, however the proposed mechanism of action suggests that this strategy should not be effective in these subjects.
Acknowledgments
This work was supported by NCCAM AT003111 (GM), and the AIDS Clinical Trials Group (see Appendix).
Acknowledgement Appendix for A5229
Michael Morgan and Brenda R. Jackson, RN- Vanderbilt Therapeutics Clinical Research Site (Site 3652) CTU Grant # U01-AI069439; RR00095
Kerriann Herbert, RD, CDN and Megan Mendillo, MPH- New York University/NYC HHC at Bellevue Hospital (Site 0401) CTU Grant # AI27665; AI069532; M01RR00096
Gary Matthew Cox, MD and Martha Silberman, RN- Duke University Medical Center (Site 1601) CTU Grant # 5U01 AI069 484-02
Christine Hurley, RN and Roberto Corales, DO- AIDS Community health Center (Site 1108) CTU Grant # U01AI069511-02; CRC: 5-MO1 RR00044
Valery Hughes, NP and Todd Stroberg, RN- Cornell CRS (Site 7804) CTU Grant # AI69419; RR024996
Judith Silverstein Currier, MD, MSc and Ardis Moe, MD- UCLA CARE Center (Site 0601) CTU Grant # U01 A106942;M01-RR00865
David A. Wininger, MD and Laura J. Laughlin, RN- The Ohio State University (Site 2301) CTU Grant # AI069474; GCRC Grant # M01-RR00034
Melinda Robinson, RN and Rebecca Creamer- Alabama Therapeutics (Site 5801) CTU Grant # U01 AI069452; M01 RR-00032
Ann C. Collier, MD, Shelia Dunaway, MD- University of Washington ACTU (Site 1401) CTU Grant # AI069434; RR-00037; UL1 RR-025014
Benigno Rodriguez, MD and Trisha Walton BSN, RN- Case Clinical Research (Site 2501) CTU Grant # AI69501
Fred R. Sattler, MD and Hannah Edmondson, RN, MPH- University of Southern California (Site 1201) CTU Grant # AI069428
Ighovwerha Ofotokun, MD and Shannon Hebert, RN- Emory University HIV/AIDS Clinical Trials Unit (Site 5802) CTU Grant # U01AI69418-01; CFAR Grant #P30 AI050409
Carl J. Fichtenbaum, MD and Fran Hyc SC RN ACRN MHA-University of Cincinnati CRS (Site 2401) CTU Grant # AI069513
Kim Whitely, RN and Ann Marie Anderson, RN- MetroHealth Medical Center (Site 2503) CTU Grant # AI69501
Eric S Daar, MD and Mario Guerrero, MD- Harbor-UCLA Medical Center (Site 0603) CTU Grant # AI069424
Julie Hoffman, RN and Jack Degna, MPH- UCSD (Site 0701) CTU Grant # AI69432
Karen T. Tashima MD and Pamela Poethke RN-The Miriam Hospital CRS 2951 (Site 2951) CTU Grant # AI069472
David Currin, RN and Susan Pedersen, BS, BSN- (Site 3201) CTU Grant # AI069423-03; M01 RR000046-48; AI050410(-11)
Beverly Putnam, RN, ANP and John R. Koeppe, MD- Colorado ACTU (Site 6101) CTU Grant # AI69450; AI054907; RR025780
Jane Norris, PA-C and Deborah Slamowitz, RN- Stanford University (Site 0501) CTU Grant # AI69556
Allan R. Tenorio, MD and Beverly E. Sha, MD- Rush University Medical Center (Site 2702) CTU Grant # U01 AI069471
Deborah McMahon, MD and Carol Oriss, BSN, RN- University of Pittsburgh (Site 1001) CTU Grant # 1 UO1 AI 069494-01
Amneris Luque MD; Mary Adams, RN- University of Rochester (Site 1101) CTU Grant # U01AI069511-02; CRC: 5-MO1 RR00044
Jorge L. Santana Bagur, MD and Olga Mendez, MD- Puerto Rico-AIDS Clinical Trials Unit (Site 5401) CTU Grant # 5 U0I AI069415-03
Ilene Wiggins, RN and Andrea Weiss, RPh- Johns Hopkins University CTU (Site 0201) CTU Grant # AI69465; GCRC grant number U54-RR023561
Henry H. Balfour, Jr. MD and Heather E. Vezina, PharmD- University of Minnesota (Site 1501) CTU Grant # AI27661
Manuel Revuelta, MD and Donna Mildvan, MD- Beth Israel Medical Center (Site 2851) CTU Grant # AI46370
Lorna Nagamine, RN and Scott Souza, PharmD- University of Hawaii ACTU (Site 5201) CTU Grant # AI34853
Michael Yin, MD and Jolene Noel-Connor, RN Columbia University HIV Prevention and Treatment (Site 30329). CTU Grant # AI069470
Footnotes
This study was presented at the 17 th Conference on Retroviruses and Opportunistic Infections, February 2010, San Francisco, CA
Description of the role of authors:
Grace A McComsey: designed the study, wrote the protocol, enrolled patients, supervised the conduct of the study at the sites, wrote the manuscript which was edited by the coauthors
Ulrich A Walker: Assisted in study design, interpretation of the data, edited manuscript Chakra B Budhathoki: Statistical analysis and interoretation of the data, edited manuscript
Zhaohui Su: Statistical analysis and interoretation of the data, edited manuscript Judith S Currier: Assisted in study design, interpretation of the data, enrolled patients, edited manuscript
Lisa Kosmiski: Assisted in study design, interpretation of the data, enrolled patients, edited manuscrip
Linda G Naini: study management, edited manuscript
Stéphannie Charles: data management, edited manuscript
Kathy Medvik: Laboratory assistant, edited manuscript
Judith A Aberg: Study design, patient enrollment, interpretation of the data, and editing of the manuscript
Potential conflicts: Grace A McComsey has served as a scientific advisor for Bristol Myers Squibb, GlaxoSmithKline, Abbott, and Gilead Sciences, has received research grants from Bristol Myers Squibb, GlaxoSmithKline, Abbott, Merck, and Gilead Sciences, and is currently serving as the DSMB Chair for a Pfizer-sponsored study. UA Walker has applied for patents regarding to use of uridine or its precursors in lipodystrophy, serves as a consultant for the company that produces NucleomaxX®, and has received research grants from GlaxoSmithKline and Gilead Sciences. Judith Currier has received research grants from Merck and Company, Theratechnologies, Schering Plough and Tibotec. Judith Aberg has served as a scientific advisor to Abbott Laboratories, Boehringer Ingelheim Pharmaceutical, Inc, Gilead Sciences, Inc, GlaxoSmithKline, Merck & Co, Inc, Pfizer Inc, Theratechnologies Inc, Tibotec Therapeutics, and ViiV Healthcare. She has received research support from Gilead Sciences, Inc, GlaxoSmithKline, Merck & Co, Inc, Pfizer Inc, Schering-Plough Corp, Theratechnologies Inc, Tibotec Therapeutics, Virco Lab, Inc, and Wyeth. She has received honoraria from Abbott Laboratories, Bristol-Myers Squibb, and Gilead Sciences, Inc.
References
- 1.Nolan D, Hammond E, Martin A, Taylor L, Herrmann S, McKinnon E, et al. Mitochondrial DNA depletion and morphologic changes in adipocytes associated with nucleoside reverse transcriptase inhibitor therapy. AIDS. 2003;17(9):1329–38. doi: 10.1097/00002030-200306130-00007. [DOI] [PubMed] [Google Scholar]
- 2.Walker UA, Bickel M, Lutke Volksbeck SI, Ketelsen UP, Schofer H, Setzer B, et al. Evidence of nucleoside analogue reverse transcriptase inhibitor--associated genetic and structural defects of mitochondria in adipose tissue of HIV-infected patients. J Acquir Immune Defic Syndr. 2002;29(2):117–21. doi: 10.1097/00042560-200202010-00002. [DOI] [PubMed] [Google Scholar]
- 3.McComsey GA, Walker UA. Role of mitochondria in HIV lipoatrophy: insight into pathogenesis and potential therapies. Mitochondrion. 2004;4(2–3):111–8. doi: 10.1016/j.mito.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 4.McComsey GA, Ward DJ, Hessenthaler SM, Sension MG, Shalit P, Lonergan JT, et al. Improvement in lipoatrophy associated with highly active antiretroviral therapy in human immunodeficiency virus-infected patients switched from stavudine to abacavir or zidovudine: the results of the TARHEEL study. Clin Infect Dis. 2004;38(2):263–70. doi: 10.1086/380790. [DOI] [PubMed] [Google Scholar]
- 5.Martin A, Smith DE, Carr A, Ringland C, Amin J, Emery S, et al. Reversibility of lipoatrophy in HIV-infected patients 2 years after switching from a thymidine analogue to abacavir: the MITOX Extension Study. AIDS. 2004;18(7):1029–36. doi: 10.1097/00002030-200404300-00011. [DOI] [PubMed] [Google Scholar]
- 6.Moyle GJ, Sabin CA, Cartledge J, Johnson M, Wilkins E, Churchill D, et al. A randomized comparative trial of tenofovir DF or abacavir as replacement for a thymidine analogue in persons with lipoatrophy. AIDS. 2006;20(16):2043–50. doi: 10.1097/01.aids.0000247574.33998.03. [DOI] [PubMed] [Google Scholar]
- 7.Walker UA, Venhoff N, Koch EC, Olschewski M, Schneider J, Setzer B. Uridine abrogates mitochondrial toxicity related to nucleoside analogue reverse transcriptase inhibitors in HepG2 cells. Antivir Ther. 2003;8(5):463–70. [PubMed] [Google Scholar]
- 8.Walker UA, Auclair M, Lebrecht D, Kornprobst M, Capeau J, Caron M. Uridine abrogates the adverse effects of antiretroviral pyrimidine analogues on adipose cell functions. Antivir Ther. 2006;11(1):25–34. [PubMed] [Google Scholar]
- 9.Sutinen J, Walker UA, Sevastianova K, Klinker H, Hakkinen AM, Ristola M, et al. Uridine supplementation for the treatment of antiretroviral therapy-associated lipoatrophy: a randomized, double-blind, placebo-controlled trial. Antivir Ther. 2007;12(1):97–105. [PubMed] [Google Scholar]
- 10.McComsey GA, O’Riordan M, Setzer B, Lebrecht D, Baron E, Walker UA. Uridine supplementation in HIV lipoatrophy: pilot trial on safety and effect on mitochondrial indices. Eur J Clin Nutr. 2008;62(8):1031–7. doi: 10.1038/sj.ejcn.1602793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Groeningen CJ, Leyva A, Kraal I, Peters GJ, Pinedo HM. Clinical and pharmacokinetic studies of prolonged administration of high-dose uridine intended for rescue from 5-FU toxicity. Cancer Treat Rep. 1986;70(6):745–50. [PubMed] [Google Scholar]
- 12.Leyva A, van Groeningen CJ, Kraal I, Gall H, Peters GJ, Lankelma J, et al. Phase I and pharmacokinetic studies of high-dose uridine intended for rescue from 5-fluorouracil toxicity. Cancer Res. 1984;44(12 Pt 1):5928–33. [PubMed] [Google Scholar]
- 13.Sommadossi JP, Carlisle R, Schinazi RF, Zhou Z. Uridine reverses the toxicity of 3′-azido-3′-deoxythymidine in normal human granulocyte-macrophage progenitor cells in vitro without impairment of antiretroviral activity. Antimicrob Agents Chemother. 1988;32(7):997–1001. doi: 10.1128/aac.32.7.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calabresi P, Falcone A, St Clair MH, Wiemann MC, Chu SH, Darnowski JW. Benzylacyclouridine reverses azidothymidine-induced marrow suppression without impairment of anti-human immunodeficiency virus activity. Blood. 1990;76(11):2210–5. [PubMed] [Google Scholar]
- 15.Koch EC, Schneider J, Weis R, Penning B, Walker UA. Uridine excess does not interfere with the antiretroviral efficacy of nucleoside analogue reverse transcriptase inhibitors. Antivir Ther. 2003;8(5):485–7. [PubMed] [Google Scholar]
- 16.Venhoff N, Zilly M, Lebrecht D, Schirmer D, Klinker H, Thoden J, et al. Uridine pharmacokinetics of mitocnol, a sugar cane extract. AIDS. 2005;19(7):739–40. doi: 10.1097/01.aids.0000166101.44262.52. [DOI] [PubMed] [Google Scholar]
- 17.Lebrecht D, Vargas-Infante YA, Setzer B, Kirschner J, Walker UA. Uridine supplementation antagonizes zalcitabine-induced microvesicular steatohepatitis in mice. Hepatology. 2007;45(1):72–9. doi: 10.1002/hep.21490. [DOI] [PubMed] [Google Scholar]
- 18.Setzer B, Lebrecht D, Walker UA. Pyrimidine nucleoside depletion sensitizes to the mitochondrial hepatotoxicity of the reverse transcriptase inhibitor stavudine. Am J Pathol. 2008;172(3):681–90. doi: 10.2353/ajpath.2008.070613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher M, Moyle GJ, Shahmanesh M, Orkin C, Kingston M, Wilkins E, et al. A randomized comparative trial of continued zidovudine/lamivudine or replacement with tenofovir disoproxil fumarate/emtricitabine in efavirenz-treated HIV-1-infected individuals. J Acquir Immune Defic Syndr. 2009;51(5):562–8. doi: 10.1097/QAI.0b013e3181ae2eb9. [DOI] [PubMed] [Google Scholar]
- 20.Webster DR, Simmonds HA, Potter CF, Becroft DM. Purine and pyrimidine metabolism in hereditary oroticaciduria during a 15 year follow-up study. Adv Exp Med Biol. 1979;122B:203–8. doi: 10.1007/978-1-4684-8559-2_34. [DOI] [PubMed] [Google Scholar]
- 21.Manis FR, Cohn LB, McBride-Chang C, Wolff JA, Kaufman FR. A longitudinal study of cognitive functioning in patients with classical galactosaemia, including a cohort treated with oral uridine. J Inherit Metab Dis. 1997;20(4):549–55. doi: 10.1023/a:1005357622551. [DOI] [PubMed] [Google Scholar]