Abstract
Clinical islet transplantation (CIT), the infusion of allogeneic islets within the liver, has the potential to provide precise and sustainable control of blood glucose levels for the treatment of type 1 diabetes. The success and long-term outcomes of CIT, however, are limited by obstacles such as a nonoptimal transplantation site and severe inflammatory and immunological responses to the transplant. Tissue engineering strategies are poised to combat these challenges. In this review, emerging methods for engineering an optimal islet transplantation site, as well as novel approaches for improving islet cell encapsulation, are discussed.
Keywords: bioartificial pancreas, biomaterials, devices, encapsulation, islet transplantation, scaffold
Introduction
Type 1 diabetes mellitus (T1DM) is a disorder character-ized by targeted autoimmune-directed destruction of a patient's β cells within the pancreatic islets of Langerhans.1 Approximately 15,000 patients are diagnosed with T1DM annually in the United States, adding to the approximately three million existing insulin-dependent diabetes patients.2 Exogenous insulin replacement is the most common treatment, where manual insulin delivery is dictated by periodic monitoring of blood glucose levels. Mimicking the complex and nonlinear dynamics of natural insulin release from native β cells through insulin shots or even implantable pumps is a difficult task, although engineering advancements have moved the concept of a “closed-loop” glucose sensor/insulin secretion system closer to reality.3 Given this lack of precise control, T1DM patients currently face earlier mortality and a higher risk of angiopathic lesions, often resulting in neuropathy, nephropathy, and retinopathy.4
Beta cell replacement via cellular transplantation has the promise of providing a long-term cure for T1DM. At the forefront of cellular replacement therapy is clinical islet transplantation (CIT), which currently involves the intraportal infusion of allogeneic islets.4–6 To date, these trials found strong improvements in metabolic control, with 57% of patients insulin independent and ~70% with measureable C-peptide levels at five years.6–12
While CIT is promising, it has become evident that inflammatory and immunological host responses to the implant, as well as the suboptimal location of the liver, lead to significant islet dysfunction and destruction. The early loss of transplanted islets has been partially attributed to the hepatic engraftment site, where as much as 60% of the islets may be lost during engraftment.13–15 As islet emboli lodge in the hepatic microvasculature, capillary bed occlusion results in hypoxia and subsequent inflammatory cytokine release by surrounding tissue.16 Of greater significance, islets in direct contact with blood instigate a potent inflammatory response, termed instant blood-mediated inflammatory reaction (IBMIR).16,18 Additionally, islets experience non-native mechanical stress and exposure to toxins filtering through the liver.19 Finally, allograft rejection and recurrent autoimmunity persists in spite of glucocorticoid-free immunosuppression regimens.20–25
Tissue engineering approaches, which combine biomaterials and cells to fabricate three-dimensional implants, have the potential to improve CIT outcomes by providing novel platforms for improving islet survival and engraftment. In particular, there is a strong need to investigate alternative transplant sites, supporting devices and/or scaffolds, and to develop means to minimize the powerful inflammatory and immunological responses to an allogeneic, and possibly xenogeneic, transplant. This article outlines several of these promising strategies.
Engineering an Alternate Transplantation Site
Significance of the Site
The transplantation site microenvironment plays a major role in engraftment. An ideal site would be one that provides an intimate vascular supply for adequate oxygenation and real-time access to blood glucose levels, mechanical protection to the implant, minimal inflammation, and ease in access and retrievability. With IBMIR-mediated islet death now well-documented for CIT, as well as the loss of islet retrievability, intravascular intraorgan sites such as the liver, spleen, and pancreas are not ideal. The kidney capsule, the most widely used site in rodents, has been shown to be a hospitable environment to islets, with an islet equivalent load similar to the intraportal site; however, the clinical feasibility of this site is limited.26 The peritoneal site is common for the implantation of microcapsules and devices; however, low oxygen tension and reduced vascularization result in delayed glucose responsiveness and the need for higher islet loadings.27–30
Sites that are emerging as promising alternatives to the liver, with clinical relevance, are the subcutaneous, intra- muscular, and omental sites (Figure 1). The subcutaneous site is particularly appealing given its ease of access; however, low vascular access and high mechanical stress commonly results in poor engraftment of free islets.31,32 In spite of inevitable mechanical stress, the intramuscular site has the advantages of ease in monitoring, loading, and retrievability, as well as higher vascular access compared to the subcutaneous site. Intramuscular islet transplantations in the forearm of diabetic patients have shown promise.31,33,34 Nevertheless, limited publications on this site warrant additional studies to fully evaluate the potential of this location. The fabrication of a surgically engineered pouch from the omentum has the advantage of a rich vascular supply, ease of access, and the accommodation of larger volumes for the implantation of devices or encapsulated cells.35–39 It has been postulated that this site may also promote islet engraftment, given its demonstrated ability to facilitate healing and positive remodeling in clinical settings.40–43 This site has clinical relevance and is feasible in larger animal models, further justifying the need for additional investigations as to its potential.25,44
Figure 1.

Schematic of selected sites for islet transplantation: Intramuscular (left), subcutaneous (center), and omental pouch (right).
Bioengineering Approaches to Enhance Islet Microenvironment
While the inherent environment of the transplantation site is strongly correlated to islet engraftment outcomes, bioengineering methods can be applied to further enhance the supporting environment or to convert an inhospitable environment to a suitable site. Given the high nutrient demand of islets, a common challenge with most sites is adequate vascular access. In order to overcome nutrient mismatch, investigators have examined the feasibility of using devices and biomaterials to accelerate angiogenesis and islet engraftment.
One strategy that has shown promise is the use of devices to promote the development of a vascular bed prior to islet transplantation. For instance, Pileggi and colleagues45 have demonstrated that prevascularization of a subcutaneous site using a cylindrical stainless steel mesh device can enhance islet function, with efficacy shown in rodent syngeneic models.45 Similar methods have been utilized by other groups, where vascularization techniques have resulted in improved islet function.46–49 In these cases, the use of hollow devices provides a means to reengineer a highly undesirable site to one that is comparable to both the liver and renal sites.
The use of highly porous, three-dimensional, macroporous scaffolds may also provide a means to reengineer the transplant site. These scaffolds have been studied extensively for bone regeneration, where they provide a mechanically stable, three-dimensional platform for uniform cell loading.50,51 The large void spaces within these scaffolds, generally greater than 70%, permit full integration into the host and infiltration of nascent blood vessels. In addition, the surface can be modified with extracellular matrix (ECM) ligands, thus promoting infiltration of vascular precursor cells and/or enhancing islet–ECM interactions, an essential factor in maintaining islet function.52 Scaffolds can be made from biodegradable materials, which can result in complete integration of the implant, or nondegradable biocompatible materials, which allow for retrieval of the implant. Studies in syngeneic murine models found the implantation of macroporous poly(lactide-co-glycolide) scaffolds within the epididymal fat pad site resulted in maintenance of the native islet architecture, more efficient conversion to euglycemia, greater weight gain, and comparable function to transplants lacking the scaffold.53 Furthermore, an ECM protein coating led to a reduction in the time required to achieve diabetes reversal in mice, postulated to be due to the promotion of endothelial cell infiltration.54 Berman and associates43 have also reported using scaffolds for implantation of islets in their evaluation of the omentum as an alternative transplantation site using a nonhuman primate model.44 Although these scaffolds do not provide immunoisolation from the host, they provide a vehicle for islet transplantation that promotes engraftment, revascularization, and integration of the implant with the host.
Additional strategies to promote islet revascularization have explored the incorporation of growth factors, vascular precursor cells, or even microvessel fragments into biomaterials.55–58 One example is the prevascularized pancreatic encapsulating device, an islet-loaded collagen disk sandwiched between two disks containing isolated microvessel fragments, where subcutaneous implants exhibited enhanced islet function and viability compared to free-islet controls.59 Alternatively, other groups have attempted to provide oxygen to the graft through oxygen generators in a transient fashion. Although not tested in vivo, co-encapsulation of islets with algae, which produce oxygen when exposed to light, and devices using electrochemical generators to decompose water into oxygen and hydrogen enhanced islet function in vitro.60,61 Newer approaches entail the development of oxygen-releasing biomaterials that could be included in specialized devices. These generate oxygen via water degradation of peroxide, which decomposes into water and oxygen under basic conditions.62
Immunoisolation through Polymer Encapsulation
While optimization of transplant sites through bio-engineering can dramatically decrease the functional islet mass for syngeneic animal models, immunological response to the transplant will still necessitate potent immunosuppressive drugs. To alleviate this issue, strategies that can significantly reduce, or even eliminate, immune attack of the transplanted islets would prove highly beneficial. Following allogeneic transplantation, the immune system relies on two pathways for the recognition of foreign antigens: direct and indirect antigen presentation (Figure 2). In the direct presentation pathway, donor antigen-presenting cells (APCs), in this case the transplanted graft itself or passenger APCs, activate T cells through the major histocompatibility complex via direct cell-to-cell contact. In the indirect presentation pathway, host APCs pick up donor cell antigen fragments and present them to T cells, inducing activation.63
Figure 2.
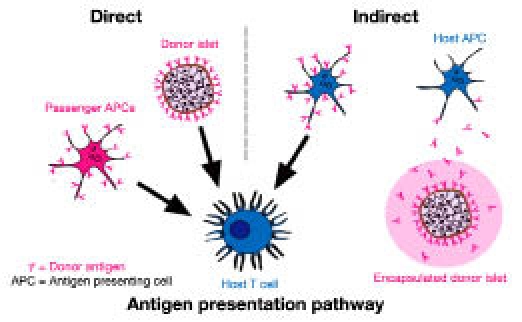
Examples of activation routes for both direct and indirect antigen presentation pathways. Direct antigen pathway activation (left schematic) is mediated by antigens presented directly by the transplanted tissue, e.g. by islets or inadvertently transplanted passenger antigen presenting cells (APCs). Indirect antigen pathway activation (right schematic) is mediated by host APC presentation of donor antigens, which are shed by the donor tissue, e.g. shed antigens diffusing through an encapsulation barrier.
Since the 1980s, researchers have tested a variety of designs for a bioartificial pancreas capable of replacing the endocrine function of the pancreas while preventing graft rejection due to immune response (Figure 3). In principle, a stable biocompatible semipermeable barrier made from a variety of natural and/or synthetic materials should separate the tissue graft from the host's immune effectors, both cellular and humoral, while allowing for proper diffusion of nutrients such as oxygen and glucose, as well as metabolic waste and therapeutic cell products, such as insulin.4,64
Figure 3.
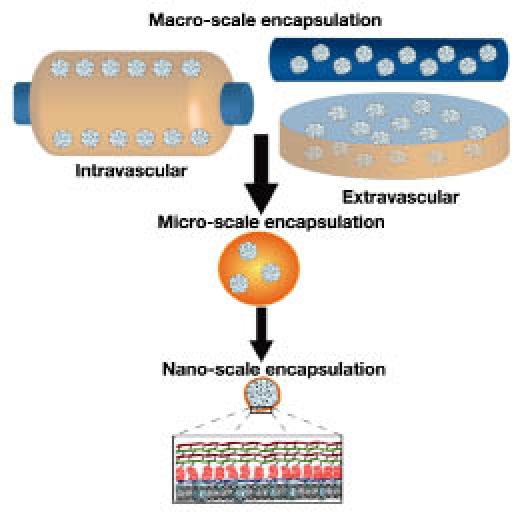
Summary of encapsulation devices from the macro- to nanoscale. Macro-scale encapsulation devices include intravascular, which are perfused with blood, or extravascular devices. Micro-scale devices are typically microcapsules (as illustrated). Nano-scale encapsulation commonly employs the coating of the islet spheroid with polymeric layers, as illustrated in expanded view.
Macroscale Encapsulation
Bioartificial pancreas devices are generally classified in two categories according to their implantation strategies: intravascular or extravascular. Several groups investigated arteriovenous shunts anastomosed directly into the circulatory system. These early intravascular devices generally consist of a synthetic hollow fiber semipermeable membrane that passes through a compartment seeded with pancreatic islets.65–68 It was reasoned that close proximity to circulation would facilitate proper insulin secretion kinetics in response to blood glucose levels; however, in vivo studies were plagued with problems of membrane collapse, thrombosis, and limitations in transport properties.65,69
Extravascular devices refer to macroencapsulated cells that are implanted outside of the vasculature, e.g., subcutaneously, intraperitoneally, or in the omentum. Although these devices, such as hollow fibers, diffusion chambers, and polymeric sheets, yielded encouraging results in rodents70–77 and canines,78–80 their large size and exclusive reliance on diffusive transport resulted in islet dysfunction and device failure in the long term. Mathematical modeling predicts inadequate transport profiles, indicating scalability of these devices to larger animal models will be problematic after islet density optimization, thereby rendering such implants bulky or requiring multiple devices.71,78,81–84 More recently, Gimi and coworkers85 reported on a microcontainer made from an epoxy-based polymer with 50 μm thick walls and a nanoporous lid assembled through adhesion layering techniques. Advantages of these microcontainers include reproducibility and precision due to the automated nature of the manufacturing process, increased mechanical strength, small size resulting in proper transport properties, and the ability to monitor in vivo islet function noninvasively through functional magnetic resonance imaging. While encapsulation of mouse islets in these microcontainers did not hamper their function, further studies need to be performed to optimize automation of islet loading and lid placement and evaluate in vivo function.
Microscale Encapsulation and Conformal Coating
Encapsulation of small groups or individual islets within micron scale capsules evolved as an alternative strategy to macroscale devices, where the increased surface-area-to-volume ratio results in enhanced transport properties. Traditional islet microencapsulation involves enclosing islets in a semipermeable alginate/poly-lysine (PLL) capsule held together by ionic interactions where porosity, and thus diffusive properties, is commonly controlled by altering the quantity and molecular weight of PLL used during processing.86–88 Agarose has also been extensively studied as an encapsulation material, where beads are generated by cooling cell/agarose-oil emulsions to induce gelation.89,90
Although the surface-to-volume ratio is improved, these capsules have drawbacks. Despite their reduced size, a large void space remains between the islet and its surrounding environment, imposing significant increases in implant size and longer diffusion distances for nutrients and insulin.91 Depending on the implant site and state of vascularization, this large void space could lead to graft dysfunction and apoptosis due to hypoxia92 and a lag in glucose-stimulated insulin release into the bloodstream.93,94 In addition, the instability of the ionic interactions lead to decomposition of the capsule under physiologic conditions over time.95
While reduction of the alginate capsule size has been achieved via air-driven droplet generators96,97 or high voltage pulses,98–100 these methods resulted in an increased incidence of inadequate or incomplete coating of the islets and thus graft rejection by immune attack.87 To avoid these issues and precisely control membrane properties, several groups developed methods to conformally coat islets in polymeric layers in the range of 10–100 μm thick. Approaches include entrainment through traversing liquid–liquid interfaces via a variety of methods such as centrifugation,101,102 selective withdrawal,103 emulsions,104 and interfacial photopolymerization.105,106 All of these methods establish the feasibility of conformal coating for islet encapsulation, but further in vivo studies need to be performed to evaluate the potential of these coatings to prevent rejection.
Nanoscale Encapsulation
While research in conformal coating was progressing, researchers in the field of blood transfusion were developing alternative cell-coating strategies for a universal blood substitute. Sparked by the findings that covalent attachment of methoxy-polyethylene glycol (PEG) to exogenous proteins increased half-life and reduced immunogenicity without affecting function,107,108 researchers attempted to immune-camouflage red blood cells with a biocompatible steric barrier by cross linking the cell surface proteins with methoxy- PEG. Indeed, PEGylation of red blood cells via a cyanuric chloride cross linker resulted in reduced antigenicity in vitro and in vivo and maintained normal cell function. This opened the doors for PEGylation of a wide variety of tissues used for transplantation, including pancreatic islets, and gave rise to the concept of nanoscale encapsulation.109
Several groups have carried out PEGylation on the surface of islets through varying approaches, which include linking islet surface amine groups with isocyanate and N-hydroxysuccinimide functionalized PEG polymers110–114 or inserting lipid moieties linked to a PEG chain within islet cell membranes.115 Not only did PEGylation have no adverse effects on islet viability or function,110,111 but it was also found to reduce islet recognition and activation of immune cells in vitro,112,113 prolong survival of the allograft in the absence of immunosuppression,116 and reverse diabetes when combined with mild immunosuppression in rodent models.117
Covalent modification of amine groups on islet surface proteins presents a problem due to periodic turnover of membrane components110 and possible interference with cell surface protein activity.118 To avoid these issues, Wilson and colleagues119 have used a noncovalent approach of coating islets via electrostatic interactions with modified PLL. Exposure to PLL alone, as with other polycations, results in high levels of cytotoxicity; however, if modified to the appropriate degree with PEG, the PLL, referred to as PP-OCH3, can interact with the islet surface without inducing apoptosis. In addition, the chemoselective reactive groups hydrazide, azide, and biotin were introduced by functionalization of the PEG macromers prior to PLL modification.119–121
PEGylation and noncovalent coating of islet surfaces have also opened the door to the fabrication of complex coatings though layer-by-layer assembly. These layers are stabilized by ionic interactions between oppositely charged polymers122 or by complimentary chemoselective reactive groups tethered to adjacent layers.118 While still in the preliminary stages, nanoscale encapsulation has the potential to allow for the reengineering of the islet surface with polymers in a manner that is precisely controlled.
“Bioactive” Polymers to Optimize Microenvironment
There has been growing interest in modifying encap-sulation materials to confer biological functionality, thus controlling the in vivo microenvironment to enhance islet function and modulate immune response. For example, after isolation, islets exhibit a progressive decline in function as measured by insulin expression, insulin content, and glucose-stimulated secretion.119 This can be circumvented by reestablishing islet–ECM interactions using ECM protein coatings or adhesive peptide sequences.123 Weber and associates124 exploited this and demonstrated enhanced glucose-stimulated insulin secretion of murine islets for up to a month in vitro following encapsulation in PEG hydrogels containing collagen IV, laminin, and the adhesive peptide RGD (arginine-glycine-aspartic acid). Lin and Anseth125 described a PEG-diacrylate-derived hydrogel cofunctionalized with the laminin adhesive sequence IKVAV and a glucagon-like peptide-1 analog modified with a carboxyl terminal cysteine group to allow for covalent thiol-acrylate photo cross linking. Glucagon-like peptide-1 has been previously described to protect islets from cytokine-induced apoptosis and enhance insulin secretion. Murine islets encapsulated in these gels exhibited enhanced viability and function compared to controls, although overall viability was low due to free radical generation during the polymerization process.125
Surface modifications of the polymeric coatings with anti-inflammatory agents can serve to mask IBMIR-associated responses and generalized inflammatory processes. For example, reactive groups on functionalized encapsulating polymers can be used for ligation of different bioactive effectors such as thrombomodulin, with the idea of generating a localized anti-inflammatory microenvironment.120,121 Other examples include tethering of urokinase and heparinization.116,126,127
While encapsulation has the capacity to prevent immune activation via the direct antigen presentation pathway, antigen shedding from the transplanted cells and subsequent indirect pathway activation is difficult to prevent due to permeability requirements that must be satisfied to allow nutrient influx and insulin outflux (see Figure 2). Furthermore, proinflammatory cytokines freely diffuse through the polymers, instigating graft cellular apoptosis.88 To combat these responses, another avenue for polymer functionalization seeks to confer additional immune-protective effects in vivo via immuno-modulation of the host environment. Su and coworkers128 described a four-arm PEG-derived hydrogel network generated through amine-thioester native chemical ligation cofunctionalized with an interleukin (IL)-1β antagonist peptide sequence and an adhesive peptide sequence via maleimide-thiol cross linking. The scheme allows for efficient control of gelation and functionalization due to the chemoselectivity of the reactions. Despite debatable results of cytotoxic T cell coculture experiments and the preliminary nature of the publication, this study showed enhanced viability and function as measured by glucose-stimulated insulin secretion in MIN6 cell clusters as a result of IL-1 receptor inhibition after exposure to multiple cytokines. Lin and collegues129 described the cofunctionalization of PEG-diacrylate hydrogels with an RGD adhesive peptide and a tumor necrosis factor (TNF)-α-sequestering peptide sequence, resulting in inhibition of TNF receptor 1 activation. Upon encapsulation within these gels, TNF-α challenged murine islets exhibited decreased caspase 3/7 activity, indicative of inhibition of apoptotic pathways, along with metabolic activity and insulin secretion comparable to that of encapsulated, unchallenged islets. Modifications to encapsulation materials such as these show promise in enhancing islet function and prolonging graft survival once implanted in the recipient.
Conclusions
The potential of CIT to provide a life-long cure for T1DM is a lure too powerful to ignore. While current results from CIT trials do not quite fulfill the promise of this therapy, lessons learned from these studies have proven invaluable. By identifying key challenges in maintaining the survival and function of transplanted islets, both in the short and long term, new strategies are evolving in the research pipeline. As outlined in this article, researchers are working to engineer an optimal islet transplantation site. In addition, novel platforms are being developed that seek to combat the significant inflammatory and immunological responses facing allogeneic islet transplantation. For example, the fabrication of novel biomaterials capable of harnessing nature's own agents for combating inflammation could generate a localized anti-inflammatory environment potent enough to mask the transplant from inflammatory-mediated damage. Undoubtedly, the future of diabetes therapies will entail agents emerging from these bioengineering platforms.
Abbreviations
- APC
antigen-presenting cell
- CIT
clinical islet transplantation
- ECM
extracellular matrix
- IBMIR
instant blood-mediated inflammatory reaction
- IL
interleukin
- PEG
polyethylene glycol
- PLL
poly-lysine
- T1DM
type 1 diabetes mellitus
- TNF
tumor necrosis factor
References
- 1.Mathis D, Vence L, Benoist C. Beta-cell death during progression to diabetes. Nature. 2001;414(6865):792–798. doi: 10.1038/414792a. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf. [Google Scholar]
- 3.Klonoff DC, Reyes JS. Insulin pump safety meeting: summary report. J Diabetes Sci Technol. 2009;3(2):396–402. doi: 10.1177/193229680900300224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silva AI, de Matos AN, Brons IG, Mateus M. An overview on the development of a bio-artificial pancreas as a treatment of insulindependent diabetes mellitus. Med Res Rev. 2006;26(2):181–222. doi: 10.1002/med.20047. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro AM, Lakey JR. Future trends in islet cell transplantation. Diabetes Technol Ther. 2000;2(3):449–452. doi: 10.1089/15209150050194314. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 7.Pileggi A, Ricordi C, Kenyon NS, Froud T, Baidal DA, Kahn A, Selvaggi G, Alejandro R. Twenty years of clinical islet transplantation at the Diabetes Research Institute–University of Miami. Clin Transpl. 2004:177–204. [PubMed] [Google Scholar]
- 8.Faradji RN, Froud T, Messinger S, Monroy K, Pileggi A, Mineo D, Tharavanij T, Mendez AJ, Ricordi C, Alejandro R. Long-term metabolic and hormonal effects of exenatide on islet transplant recipients with allograft dysfunction. Cell Transplant. 2009;18(10):1247–1259. doi: 10.3727/096368909X474456. [DOI] [PubMed] [Google Scholar]
- 9.Froud T, Faradji RN, Pileggi A, Messinger S, Baidal DA, Ponte GM, Cure PE, Monroy K, Mendez A, Selvaggi G, Ricordi C, Alejandro R. The use of exenatide in islet transplant recipients with chronic allograft dysfunction: safety, efficacy, and metabolic effects. Transplantation. 2008;86(1):36–45. doi: 10.1097/TP.0b013e31817c4ab3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faradji RN, Tharavanij T, Messinger S, Froud T, Pileggi A, Monroy K, Mineo D, Baidal DA, Cure P, Ponte G, Mendez AJ, Selvaggi G, Ricordi C, Alejandro R. Long-term insulin independence and improvement in insulin secretion after supplemental islet infusion under exenatide and etanercept. Transplantation. 2008;86(12):1658–1665. doi: 10.1097/TP.0b013e31818fe448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tharavanij T, Betancourt A, Messinger S, Cure P, Leitao CB, Baidal DA, Froud T, Ricordi C, Alejandro R. Improved long-term health-related quality of life after islet transplantation. Transplantation. 2008;86(9):1161–1167. doi: 10.1097/TP.0b013e31818a7f45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cure P, Pileggi A, Froud T, Messinger S, Faradji RN, Baidal DA, Cardani R, Curry A, Poggioli R, Pugliese A, Betancourt A, Esquenazi V, Ciancio G, Selvaggi G, Burke GW, 3rd, Ricordi C, Alejandro R. Improved metabolic control and quality of life in seven patients with type 1 diabetes following islet after kidney transplantation. Transplantation. 2008;85(6):801–812. doi: 10.1097/TP.0b013e318166a27b. [DOI] [PubMed] [Google Scholar]
- 13.Biarnés M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E. Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes. 2002;51(1):66–72. doi: 10.2337/diabetes.51.1.66. [DOI] [PubMed] [Google Scholar]
- 14.Davalli AM, Scaglia L, Zangen DH, Hollister J, Bonner-Weir S, Weir GC. Vulnerability of islets in the immediate posttransplantation period. Dynamic changes in structure and function. Diabetes. 1996;45(9):1161–1167. doi: 10.2337/diab.45.9.1161. [DOI] [PubMed] [Google Scholar]
- 15.Mattsson G, Jansson L, Nordin A, Andersson A, Carlsson PO. Evidence of functional impairment of syngeneically transplanted mouse pancreatic islets retrieved from the liver. Diabetes. 2004;53(4):948–954. doi: 10.2337/diabetes.53.4.948. [DOI] [PubMed] [Google Scholar]
- 16.Yin D, Ding JW, Shen J, Ma L, Hara M, Chong AS. Liver ischemia contributes to early islet failure following intraportal transplantation: benefits of liver ischemic-preconditioning. Am J Transplant. 2006;6(1):60–68. doi: 10.1111/j.1600-6143.2005.01157.x. [DOI] [PubMed] [Google Scholar]
- 17.Ozmen L, Ekdahl KN, Elgue G, Larsson R, Korsgren O, Nilsson B. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes. 2002;51(6):1779–1784. doi: 10.2337/diabetes.51.6.1779. [DOI] [PubMed] [Google Scholar]
- 18.Wilson JT, Chaikof EL. Thrombosis and inflammation in intraportal islet transplantation: a review of pathophysiology and emerging therapeutics. J Diabetes Sci Technol. 2008;2(5):746–759. doi: 10.1177/193229680800200502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witkowski P, Sondermeijer H, Hardy MA, Woodland DC, Lee K, Bhagat G, Witkowski K, See F, Rana A, Maffei A, Itescu S, Harris PE. Islet grafting and imaging in a bioengineered intramuscular space. Transplantation. 2009;88(9):1065–1074. doi: 10.1097/TP.0b013e3181ba2e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardani R, Pileggi A, Ricordi C, Gomez C, Baidal DA, Ponte GG, Mineo D, Faradji RN, Froud T, Ciancio G, Esquenazi V, Burke GW, 3rd, Selvaggi G, Miller J, Kenyon NS, Alejandro R. Allosensitization of islet allograft recipients. Transplantation. 2007;84(11):1413–1427. doi: 10.1097/01.tp.0000290388.70019.6e. [DOI] [PubMed] [Google Scholar]
- 21.Huurman VA, Velthuis JH, Hilbrands R, Tree TI, Gillard P, van der Meer-Prins PM, Duinkerken G, Pinkse GG, Keymeulen B, Roelen DL, Claas FH, Pipeleers DG, Roep BO. Allograft-specific cytokine profiles associate with clinical outcome after islet cell transplantation. Am J Transplant. 2009;9(2):382–388. doi: 10.1111/j.1600-6143.2008.02479.x. [DOI] [PubMed] [Google Scholar]
- 22.Huurman VA, Hilbrands R, Pinkse GG, Gillard P, Duinkerken G, van de Linde P, van der Meer-Prins PM, Versteeg-van der Voort Maarschalk MF, Verbeeck K, Alizadeh BZ, Mathieu C, Gorus FK, Roelen DL, Claas FH, Keymeulen B, Pipeleers DG, Roep BO. Cellular islet autoimmunity associates with clinical outcome of islet cell transplantation. PLoS One. 2008;3(6):e2435. doi: 10.1371/journal.pone.0002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lunsford KE, Jayanshankar K, Eiring AM, Horne PH, Koester MA, Gao D, Bumgardner GL. Alloreactive (CD4-Independent) CD8+ T cells jeopardize long-term survival of intrahepatic islet allografts. Am J Transplant. 2008;8(6):1113–1128. doi: 10.1111/j.1600-6143.2008.02219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rickels MR, Kamoun M, Kearns J, Markmann JF, Naji A. Evidence for allograft rejection in an islet transplant recipient and effect on beta-cell secretory capacity. J Clin Endocrinol Metab. 2007;92(7):2410–2414. doi: 10.1210/jc.2007-0172. [DOI] [PubMed] [Google Scholar]
- 25.Kin T, Korbutt GS, Rajotte RV. Survival and metabolic function of syngeneic rat islet grafts transplanted in the omental pouch. Am J Transplant. 2003;3(3):281–285. doi: 10.1034/j.1600-6143.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 26.Merani S, Toso C, Emamaullee J, Shapiro AM. Optimal implantation site for pancreatic islet transplantation. Br J Surg. 2008;95(12):1449–1461. doi: 10.1002/bjs.6391. [DOI] [PubMed] [Google Scholar]
- 27.Wahoff DC, Sutherland DE, Hower CD, Lloveras JK, Gores PF. Free intraperitoneal islet autografts in pancreatectomized dogs–impact of islet purity and posttransplantation exogenous insulin. Surgery. 1994;116(4):742–750. [PubMed] [Google Scholar]
- 28.Siebers U, Horcher A, Bretzel RG, Klöck G, Zimmermann U, Federlin K, Zekorn T. Transplantation of free and microencapsulated islets in rats: evidence for the requirement of an increased islet mass for transplantation into the peritoneal site. Int J Artif Organs. 1993;16(2):96–99. [PubMed] [Google Scholar]
- 29.Zimmermann U, Nöth U, Gröhn P, Jork A, Ulrichs K, Lutz J, Haase A. Non-invasive evaluation of the location, the functional integrity and the oxygen supply of implants: 19F nuclear magnetic resonance imaging of perfluorocarbon-loaded Ba2+-alginate beads. Artif Cells Blood Substit Immobil Biotechnol. 2000;28(2):129–146. doi: 10.3109/10731190009118576. [DOI] [PubMed] [Google Scholar]
- 30.Klossner J, Kivisaari J, Niinikoski J. Oxygen and carbon dioxide tensions in the abdominal cavity and colonic wall of the rabbit. Am J Surg. 1974;127(6):711–715. doi: 10.1016/0002-9610(74)90354-7. [DOI] [PubMed] [Google Scholar]
- 31.Juang JH, Hsu BR, Kuo CH. Islet transplantation at subcutaneous and intramuscular sites. Transplant Proc. 2005;37(8):3479–3481. doi: 10.1016/j.transproceed.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 32.Juang JH, Bonner-Weir S, Ogawa Y, Vacanti JP, Weir GC. Outcome of subcutaneous islet transplantation improved by polymer device. Transplantation. 1996;61(11):1557–1561. doi: 10.1097/00007890-199606150-00001. [DOI] [PubMed] [Google Scholar]
- 33.Rafael E, Tibell A, Rydén M, Lundgren T, Sävendahl L, Borgström B, Arnelo U, Isaksson B, Nilsson B, Korsgren O, Permert J. Intramuscular autotransplantation of pancreatic islets in a 7-yearold child: a 2-year follow-up. Am J Transplant. 2008;8(2):458–462. doi: 10.1111/j.1600-6143.2007.02060.x. [DOI] [PubMed] [Google Scholar]
- 34.Stegall MD. Monitoring human islet allografts using a forearm biopsy site. Ann Transplant. 1997;2(3):8–11. [PubMed] [Google Scholar]
- 35.Kobayashi T, Aomatsu Y, Iwata H, Kin T, Kanehiro H, Hisanga M, Ko S, Nagao M, Harb G, Nakajima Y. Survival of microencapsulated islets at 400 days posttransplantation in the omental pouch of NOD mice. Cell Transplant. 2006;15(4):359–365. doi: 10.3727/000000006783981954. [DOI] [PubMed] [Google Scholar]
- 36.Ao Z, Matayoshi K, Lakey JR, Rajotte RV, Warnock GL. Survival and function of purified islets in the omental pouch site of outbred dogs. Transplantation. 1993;56(3):524–529. doi: 10.1097/00007890-199309000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Yasunami Y, Lacy PE, Finke EH. A new site for islet transplantation--a peritoneal-omental pouch. Transplantation. 1983;36(2):181–182. doi: 10.1097/00007890-198308000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Simeonovic CJ, Dhall DP, Wilson JD, Lafferty KJ. A comparative study of transplant sites for endocrine tissue transplantation in the pig. Aust J Exp Biol Med Sci. 1986;64(Pt 1):37–41. doi: 10.1038/icb.1986.4. [DOI] [PubMed] [Google Scholar]
- 39.Chaffanjon PC, Kenyon NM, Ricordi C, Kenyon NS. Omental anatomy of non-human primates. Surg Radiol Anat. 2005;27(4):287–291. doi: 10.1007/s00276-005-0329-4. [DOI] [PubMed] [Google Scholar]
- 40.Zaha H, Inamine S. Laparoscopically harvested omental flap: results for 96 patients. Surg Endosc. 2009;24(1):103–107. doi: 10.1007/s00464-009-0533-0. [DOI] [PubMed] [Google Scholar]
- 41.Ferron G, Garrido I, Martel P, Gesson-Paute A, Classe JM, Letourneur B, Querleu D. Combined laparoscopically harvested omental flap with meshed skin grafts and vacuum-assisted closure for reconstruction of complex chest wall defects. Ann Plast Surg. 2007;58(2):150–155. doi: 10.1097/01.sap.0000237644.29878.0f. [DOI] [PubMed] [Google Scholar]
- 42.Zaha H, Inamine S, Naito T, Nomura H. Laparoscopically harvested omental flap for immediate breast reconstruction. Am J Surg. 2006;192(4):556–558. doi: 10.1016/j.amjsurg.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 43.Acarturk TO, Swartz WM, Luketich J, Quinlin RF, Edington H. Laparoscopically harvested omental flap for chest wall and intrathoracic reconstruction. Ann Plast Surg. 2004;53(3):210–216. doi: 10.1097/01.sap.0000116285.98328.f7. [DOI] [PubMed] [Google Scholar]
- 44.Berman DM, O&Neil JJ, Coffey LC, Chaffanjon PC, Kenyon NM, Ruiz P, Jr, Pileggi A, Ricordi C, Kenyon NS. Long-term survival of nonhuman primate islets implanted in an omental pouch on a biodegradable scaffold. Am J Transplant. 2009;9(1):91–104. doi: 10.1111/j.1600-6143.2008.02489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pileggi A, Molano RD, Ricordi C, Zahr E, Collins J, Valdes R, Inverardi L. Reversal of diabetes by pancreatic islet transplantation into a subcutaneous, neovascularized device. Transplantation. 2006;81(9):1318–1324. doi: 10.1097/01.tp.0000203858.41105.88. [DOI] [PubMed] [Google Scholar]
- 46.Yuasa T, Rivas-Carrillo JD, Navarro-Alvarez N, Soto-Gutierrez A, Kubota Y, Tabata Y, Okitsu T, Noguchi H, Matsumoto S, Nakaji S, Tanaka N, Kobayashi N. Neovascularization induced around an artificial device implanted in the abdomen by the use of gelatinized fibroblast growth factor 2. Cell Transplant. 2009;18(5):683–688. doi: 10.1177/096368970901805-625. [DOI] [PubMed] [Google Scholar]
- 47.Halberstadt CR, Williams D, Emerich D, Goddard M, Vasconcellos AV, Curry W, Bhatia A, Gores PF. Subcutaneous transplantation of islets into streptozocin-induced diabetic rats. Cell Transplant. 2005;14(8):595–605. doi: 10.3727/000000005783982792. [DOI] [PubMed] [Google Scholar]
- 48.Sakurai T, Satake A, Nagata N, Gu Y, Hiura A, Doo-Hoon K, Hori H, Tabata Y, Sumi S, Inoue K. The development of new immunoisolatory devices possessing the ability to induce neovascularization. Cell Transplant. 2003;12(5):527–535. doi: 10.3727/000000003108746984. [DOI] [PubMed] [Google Scholar]
- 49.Sakurai T, Satake A, Sumi S, Inoue K, Nagata N, Tabata Y, Miyakoshi J. The efficient prevascularization induced by fibroblast growth factor 2 with a collagen-coated device improves the cell survival of a bioartificial pancreas. Pancreas. 2004;28(3):e70–79. doi: 10.1097/00006676-200404000-00028. [DOI] [PubMed] [Google Scholar]
- 50.Kim HW, Lee SY, Bae CJ, Noh YJ, Kim HE, Kim HM, Ko JS. Porous ZrO2 bone scaffold coated with hydroxyapatite with fluorapatite intermediate layer. Biomaterials. 2003;24(19):3277–3284. doi: 10.1016/s0142-9612(03)00162-5. [DOI] [PubMed] [Google Scholar]
- 51.Tamai N, Myoui A, Tomita T, Nakase T, Tanaka J, Ochi T, Yoshikawa H. Novel hydroxyapatite ceramics with an interconnective porous structure exhibit superior osteoconduction in vivo. J Biomed Mater Res. 2002;59(1):110–117. doi: 10.1002/jbm.1222. [DOI] [PubMed] [Google Scholar]
- 52.Weber LM, Hayda KN, Anseth KS. Cell-matrix interactions improve beta-cell survival and insulin secretion in threedimensional culture. Tissue Eng Part A. 2008;14(12):1959–1968. doi: 10.1089/ten.tea.2007.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blomeier H, Zhang X, Rives C, Brissova M, Hughes E, Baker M, Powers AC, Kaufman DB, Shea LD, Lowe WL., Jr Polymer scaffolds as synthetic microenvironments for extrahepatic islet transplantation. Transplantation. 2006;82(4):452–459. doi: 10.1097/01.tp.0000231708.19937.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salvay DM, Rives CB, Zhang X, Chen F, Kaufman DB, Lowe WL, Jr, Shea LD. Extracellular matrix protein-coated scaffolds promote the reversal of diabetes after extrahepatic islet transplantation. Transplantation. 2008;85(10):1456–1464. doi: 10.1097/TP.0b013e31816fc0ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee KY, Peters MC, Anderson KW, Mooney DJ. Controlled growth factor release from synthetic extracellular matrices. Nature. 2000;408(6815):998–1000. doi: 10.1038/35050141. [DOI] [PubMed] [Google Scholar]
- 56.Lee KW, Yoon JJ, Lee JH, Kim SY, Jung HJ, Kim SJ, Joh JW, Lee HH, Lee DS, Lee SK. Sustained release of vascular endothelial growth factor from calcium-induced alginate hydrogels reinforced by heparin and chitosan. Transplant Proc. 2004;36(8):2464–2465. doi: 10.1016/j.transproceed.2004.08.078. [DOI] [PubMed] [Google Scholar]
- 57.Linn T, Erb D, Schneider D, Kidszun A, Elçin AE, Bretzel RG, Elçin YM. Polymers for induction of revascularization in the rat fascial flap: application of vascular endothelial growth factor and pancreatic islet cells. Cell Transplant. 2003;12(7):769–778. doi: 10.3727/000000003108747244. [DOI] [PubMed] [Google Scholar]
- 58.Dziubla TD, Lowman AM. Vascularization of PEG-grafted macroporous hydrogel sponges: a three-dimensional in vitro angiogenesis model using human microvascular endothelial cells. J Biomed Mater Res A. 2004;68(4):603–614. doi: 10.1002/jbm.a.20023. [DOI] [PubMed] [Google Scholar]
- 59.Hiscox AM, Stone AL, Limesand S, Hoying JB, Williams SK. An islet-stabilizing implant constructed using a preformed vasculature. Tissue Eng Part A. 2008;14(3):433–440. doi: 10.1089/tea.2007.0099. [DOI] [PubMed] [Google Scholar]
- 60.Bloch K, Papismedov E, Yavriyants K, Vorobeychik M, Beer S, Vardi P. Photosynthetic oxygen generator for bioartificial pancreas. Tissue Eng. 2006;12(2):337–344. doi: 10.1089/ten.2006.12.337. [DOI] [PubMed] [Google Scholar]
- 61.Wu H, Avgoustiniatos ES, Swette L, Bonner-Weir S, Weir GC, Colton CK. In situ electrochemical oxygen generation with an immunoisolation device. Ann N Y Acad Sci. 1999;875:105–125. doi: 10.1111/j.1749-6632.1999.tb08497.x. [DOI] [PubMed] [Google Scholar]
- 62.Harrison BS, Eberli D, Lee SJ, Atala A, Yoo JJ. Oxygen producing biomaterials for tissue regeneration. Biomaterials. 2007;28(31):4628–4634. doi: 10.1016/j.biomaterials.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 63.Waldmann H. Transplantation tolerance-where do we stand? Nat Med. 1999;5(11):1245–1248. doi: 10.1038/15197. [DOI] [PubMed] [Google Scholar]
- 64.Korsgren O, Nilsson B. Improving islet transplantation: a road map for a widespread application for the cure of persons with type I diabetes. Curr Opin Organ Transplant. 2009;14(6):683–687. doi: 10.1097/MOT.0b013e328332c44c. [DOI] [PubMed] [Google Scholar]
- 65.Reach G, Jaffrin MY. Kinetic modelling as a tool for the design of a vascular bioartificial pancreas: feedback between modelling and experimental validation. Comput Methods Programs Biomed. 1990;32(3–4):277–285. doi: 10.1016/0169-2607(90)90110-u. [DOI] [PubMed] [Google Scholar]
- 66.Silva AI, Mateus M. Development of a polysulfone hollow fiber vascular bio-artificial pancreas device for in vitro studies. J Biotechnol. 2009;139(3):236–249. doi: 10.1016/j.jbiotec.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 67.Maki T, Ubhi CS, Sanchez-Farpon H, Sullivan SJ, Borland K, Muller TE, Solomon BA, Chick WL, Monaco AP. Successful treatment of diabetes with the biohybrid artificial pancreas in dogs. Transplantation. 1991;51(1):43–51. doi: 10.1097/00007890-199101000-00006. [DOI] [PubMed] [Google Scholar]
- 68.Petruzzo P, Pibiri L, De Giudici MA, Basta G, Calafiore R, Falorni A, Brunetti P, Brotzu G. Xenotransplantation of microencapsulated pancreatic islets contained in a vascular prosthesis: preliminary results. Transpl Int. 1991;4(4):200–204. doi: 10.1007/BF00649103. [DOI] [PubMed] [Google Scholar]
- 69.Maki T, Ubhi CS, Sanchez-Farpon H, Sullivan SJ, Borland K, Muller TE, Solomon BA, Chick WL, Monaco AP. The biohybrid artificial pancreas for treatment of diabetes in totally pancreatectomized dogs. Transplant Proc. 1991;23(1 Pt 1):754–755. [PubMed] [Google Scholar]
- 70.Altman JJ, Penfornis A, Boillot J, Maletti M. Bioartificial pancreas in autoimmune nonobese diabetic mice. ASAIO Trans. 1988;34(3):247–249. [PubMed] [Google Scholar]
- 71.Delaunay C, Darquy S, Honiger J, Capron F, Rouault C, Reach G. Glucose-insulin kinetics of a bioartificial pancreas made of an AN69 hydrogel hollow fiber containing porcine islets and implanted in diabetic mice. Artif Organs. 1998;22(4):291–299. doi: 10.1046/j.1525-1594.1998.05087.x. [DOI] [PubMed] [Google Scholar]
- 72.Suzuki K, Bonner-Weir S, Trivedi N, Yoon KH, Hollister-Lock J, Colton CK, Weir GC. Function and survival of macroencapsulated syngeneic islets transplanted into streptozocin-diabetic mice. Transplantation. 1998;66(1):21–28. doi: 10.1097/00007890-199807150-00004. [DOI] [PubMed] [Google Scholar]
- 73.Desai TA, Chu WH, Rasi G, Sinibaldi-Vallebona P, Guarino E, Ferrari M. Microfabricated biocapsules provide short-term immunoisolation of insulinoma xenografts. Biomed Microdevices. 1999;1(2):131–138. doi: 10.1023/A:1009948524686. [DOI] [PubMed] [Google Scholar]
- 74.Olivares E, Piranda S, Malaisse WJ. Long-term correction of hyperglycaemia in streptozotocin-induced diabetic rats transplanted with islets placed in an implantation device. Horm Metab Res. 2001;33(11):687–688. doi: 10.1055/s-2001-18685. [DOI] [PubMed] [Google Scholar]
- 75.Young TH, Chuang WY, Hsieh MY, Chen LW, Hsu JP. Assessment and modeling of poly(vinyl alcohol) bioartificial pancreas in vivo. Biomaterials. 2002;23(16):3495–3501. doi: 10.1016/s0142-9612(02)00075-3. [DOI] [PubMed] [Google Scholar]
- 76.Qi M, Gu Y, Sakata N, Kim D, Shirouzu Y, Yamamoto C, Hiura A, Sumi S, Inoue K. PVA hydrogel sheet macroencapsulation for the bioartificial pancreas. Biomaterials. 2004;25(27):5885–5892. doi: 10.1016/j.biomaterials.2004.01.050. [DOI] [PubMed] [Google Scholar]
- 77.Yang KC, Wu CC, Sumi S, Tseng CL, Wu YH, Kuo TF, Lin FH. Calcium phosphate cement chamber as an immunoisolative device for bioartificial pancreas: in vitro and preliminary in vivo study. Pancreas. 2010;39(4):444–451. doi: 10.1097/MPA.0b013e3181be2f95. [DOI] [PubMed] [Google Scholar]
- 78.Storrs R, Dorian R, King SR, Lakey J, Rilo H. Preclinical development of the Islet Sheet. Ann N Y Acad Sci. 2001;944:252–266. doi: 10.1111/j.1749-6632.2001.tb03837.x. [DOI] [PubMed] [Google Scholar]
- 79.Edamura K, Itakura S, Nasu K, Iwami Y, Ogawa H, Sasaki N, Ohgawara H. Xenotransplantation of porcine pancreatic endocrine cells to total pancreatectomized dogs. J Vet Med Sci. 2003;65(5):549–556. doi: 10.1292/jvms.65.549. [DOI] [PubMed] [Google Scholar]
- 80.Grundfest-Broniatowski SF, Tellioglu G, Rosenthal KS, Kang J, Erdodi G, Yalcin B, Cakmak M, Drazba J, Bennett A, Lu L, Kennedy JP. A new bioartificial pancreas utilizing amphiphilic membranes for the immunoisolation of porcine islets: a pilot study in the canine. ASAIO J. 2009;55(4):400–405. doi: 10.1097/MAT.0b013e3181a8deba. [DOI] [PubMed] [Google Scholar]
- 81.Dulong JL, Legallais C. Contributions of a finite element model for the geometric optimization of an implantable bioartificial pancreas. Artif Organs. 2002;26(7):583–589. doi: 10.1046/j.1525-1594.2002.07080.x. [DOI] [PubMed] [Google Scholar]
- 82.Dulong JL, Legallais C, Darquy S, Reach G. A novel model of solute transport in a hollow-fiber bioartificial pancreas based on a finite element method. Biotechnol Bioeng. 2002;78(5):576–582. doi: 10.1002/bit.10230. [DOI] [PubMed] [Google Scholar]
- 83.Dulong JL, Legallais C. A theoretical study of oxygen transfer including cell necrosis for the design of a bioartificial pancreas. Biotechnol Bioeng. 2007;96(5):990–998. doi: 10.1002/bit.21140. [DOI] [PubMed] [Google Scholar]
- 84.Dulong JL, Legallais C. What are the relevant parameters for the geometrical optimization of an implantable bioartificial pancreas? J Biomech Eng. 2005;127(7):1054–1061. doi: 10.1115/1.2073407. [DOI] [PubMed] [Google Scholar]
- 85.Gimi B, Kwon J, Kuznetsov A, Vachha B, Magin RL, Philipson LH, Lee JB. A nanoporous, transparent microcontainer for encapsulated islet therapy. J Diabetes Sci Technol. 2009;3(2):297–303. doi: 10.1901/jaba.2009.3-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lim F, Moss RD. Microencapsulation of living cells and tissues. J Pharm Sci. 1981;70(4):351–354. doi: 10.1002/jps.2600700402. [DOI] [PubMed] [Google Scholar]
- 87.Van Schilfgaarde R, de Vos P. Factors influencing the properties and performance of microcapsules for immunoprotection of pancreatic islets. J Mol Med. 1999;77(1):199–205. doi: 10.1007/s001090050336. [DOI] [PubMed] [Google Scholar]
- 88.Safley SA, Cui H, Cauffiel S, Tucker-Burden C, Weber CJ. Biocompatibility and immune acceptance of adult porcine islets transplanted intraperitoneally in diabetic NOD mice in calcium alginate poly-L-lysine microcapsules versus barium alginate microcapsules without poly-L-lysine. J Diabetes Sci Technol. 2008;2(5):760–767. doi: 10.1177/193229680800200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iwata H, Takagi T, Amemiya H, Shimizu H, Yamashita K, Kobayashi K, Akutsu T. Agarose for a bioartificial pancreas. J Biomed Mater Res. 1992;26(7):967–977. doi: 10.1002/jbm.820260711. [DOI] [PubMed] [Google Scholar]
- 90.Gin H, Dupuy B, Baquey C, Ducassou D, Aubertin J. Agarose encapsulation of islets of Langerhans: reduced toxicity in vitro. J Microencapsul. 1987;4(3):239–242. doi: 10.3109/02652048709021817. [DOI] [PubMed] [Google Scholar]
- 91.De Vos P, De Haan B, Pater J, Van Schilfgaarde R. Association between capsule diameter, adequacy of encapsulation, and survival of microencapsulated rat islet allografts. Transplantation. 1996;62(7):893–899. doi: 10.1097/00007890-199610150-00004. [DOI] [PubMed] [Google Scholar]
- 92.Dionne KE, Colton CK, Yarmush ML. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes. 1993;42(1):12–21. doi: 10.2337/diab.42.1.12. [DOI] [PubMed] [Google Scholar]
- 93.Trivedi N, Keegan M, Steil GM, Hollister-Lock J, Hasenkamp WM, Colton CK, Bonner-Weir S, Weir GC. Islets in alginate macrobeads reverse diabetes despite minimal acute insulin secretory responses. Transplantation. 2001;71(2):203–211. doi: 10.1097/00007890-200101270-00006. [DOI] [PubMed] [Google Scholar]
- 94.De Haan BJ, Faas MM, de Vos P. Factors influencing insulin secretion from encapsulated islets. Cell Transplant. 2003;12(6):617–625. doi: 10.3727/000000003108747226. [DOI] [PubMed] [Google Scholar]
- 95.Strand BL, Ryan TL, In't Veld P, Kulseng B, Rokstad AM, Skjak-Brek G, Espevik T. Poly-L-Lysine induces fibrosis on alginate microcapsules via the induction of cytokines. Cell Transplant. 2001;10(3):263–275. [PubMed] [Google Scholar]
- 96.Koo SK, Kim SC, Wee YM, Kim YH, Jung EJ, Choi MY, Park YH, Park KT, Lim DG, Han DJ. Experimental microencapsulation of porcine and rat pancreatic islet cells with air-driven droplet generator and alginate. Transplant Proc. 2008;40(8):2578–2580. doi: 10.1016/j.transproceed.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 97.Wolters GH, Fritschy WM, Gerrits D, van Schilfgaarde R. A versatile alginate droplet generator applicable for microencapsulation of pancreatic islets. J Appl Biomater. 1991;3(4):281–286. doi: 10.1002/jab.770030407. [DOI] [PubMed] [Google Scholar]
- 98.Klokk TI, Melvik JE. Controlling the size of alginate gel beads by use of a high electrostatic potential. J Microencapsul. 2002;19(4):415–424. doi: 10.1080/02652040210144234. [DOI] [PubMed] [Google Scholar]
- 99.Lewińska D, Rosiński S, Weryński A. Influence of process conditions during impulsed electrostatic droplet formation on size distribution of hydrogel beads. Artif Cells Blood Substit Immobil Biotechnol. 2004;32(1):41–53. doi: 10.1081/bio-120028667. [DOI] [PubMed] [Google Scholar]
- 100.Hallé JP, Leblond FA, Pariseau JF, Jutras P, Brabant MJ, Lepage Y. Studies on small (<300 microns) microcapsules: II--Parameters governing the production of alginate beads by high voltage electrostatic pulses. Cell Transplant. 1994;3(5):365–372. doi: 10.1177/096368979400300503. [DOI] [PubMed] [Google Scholar]
- 101.Zekorn T, Siebers U, Horcher A, Schnettler R, Zimmermann U, Bretzel RG, Federlin K. Alginate coating of islets of Langerhans: in vitro studies on a new method for microencapsulation for immuno-isolated transplantation. Acta Diabetol. 1992;29(1):41–45. doi: 10.1007/BF00572829. [DOI] [PubMed] [Google Scholar]
- 102.May MH, Sefton MV. Conformal coating of small particles and cell aggregates at a liquid-liquid interface. Ann N Y Acad Sci. 1999;875:126–134. doi: 10.1111/j.1749-6632.1999.tb08498.x. [DOI] [PubMed] [Google Scholar]
- 103.Wyman JL, Kizilel S, Skarbek R, Zhao X, Connors M, Dillmore WS, Murphy WL, Mrksich M, Nagel SR, Garfinkel MR. Immunoisolating pancreatic islets by encapsulation with selective withdrawal. Small. 2007;3(4):683–690. doi: 10.1002/smll.200600231. [DOI] [PubMed] [Google Scholar]
- 104.Leung A, Ramaswamy Y, Munro P, Lawrie G, Nielsen L, Trau M. Emulsion strategies in the microencapsulation of cells: pathways to thin coherent membranes. Biotechnol Bioeng. 2005;92(1):45–53. doi: 10.1002/bit.20597. [DOI] [PubMed] [Google Scholar]
- 105.Cruise GM, Hegre OD, Scharp DS, Hubbell JA. A sensitivity study of the key parameters in the interfacial photopolymerization of poly(ethylene glycol) diacrylate upon porcine islets. Biotechnol Bioeng. 1998;57(6):655–665. doi: 10.1002/(sici)1097-0290(19980320)57:6<655::aid-bit3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 106.Sawhney AS, Pathak CP, Hubbell JA. Modification of islet of langerhans surfaces with immunoprotective poly(ethylene glycol) coatings via interfacial photopolymerization. Biotechnol Bioeng. 1994;44(3):383–386. doi: 10.1002/bit.260440317. [DOI] [PubMed] [Google Scholar]
- 107.Abuchowski A, van Es T, Palczuk NC, Davis FF. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J Biol Chem. 1977;252(11):3578–3581. [PubMed] [Google Scholar]
- 108.Abuchowski A, McCoy JR, Palczuk NC, van Es T, Davis FF. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem. 1977;252(11):3582–3586. [PubMed] [Google Scholar]
- 109.Scott MD, Murad KL, Koumpouras F, Talbot M, Eaton JW. Chemical camouflage of antigenic determinants: stealth erythrocytes. Proc Natl Acad Sci U S A. 1997;94(14):7566–7571. doi: 10.1073/pnas.94.14.7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Panza JL, Wagner WR, Rilo HL, Rao RH, Beckman EJ, Russell AJ. Treatment of rat pancreatic islets with reactive PEG. Biomaterials. 2000;21(11):1155–1164. doi: 10.1016/s0142-9612(99)00283-5. [DOI] [PubMed] [Google Scholar]
- 111.Lee DY, Yang K, Lee S, Chae SY, Kim KW, Lee MK, Han DJ, Byun Y. Optimization of monomethoxy-polyethylene glycol grafting on the pancreatic islet capsules. J Biomed Mater Res. 2002;62(3):372–377. doi: 10.1002/jbm.10246. [DOI] [PubMed] [Google Scholar]
- 112.Lee DY, Nam JH, Byun Y. Effect of polyethylene glycol grafted onto islet capsules on prevention of splenocyte and cytokine attacks. J Biomater Sci Polym Ed. 2004;15(6):753–766. doi: 10.1163/156856204774196144. [DOI] [PubMed] [Google Scholar]
- 113.Jang JY, Lee DY, Park SJ, Byun Y. Immune reactions of lymphocytes and macrophages against PEG-grafted pancreatic islets. Biomaterials. 2004;25(17):3663–3669. doi: 10.1016/j.biomaterials.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 114.Yun Lee D, Hee Nam J, Byun Y. Functional and histological evaluation of transplanted pancreatic islets immunoprotected by PEGylation and cyclosporine for 1 year. Biomaterials. 2007;28(11):1957–1966. doi: 10.1016/j.biomaterials.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 115.Teramura Y, Iwata H. Surface modification of islets with PEG-lipid for improvement of graft survival in intraportal transplantation. Transplantation. 2009;88(5):624–630. doi: 10.1097/TP.0b013e3181b230ac. [DOI] [PubMed] [Google Scholar]
- 116.Teramura Y, Iwata H. Islets surface modification prevents blood-mediated inflammatory responses. Bioconjug Chem. 2008;19(7):1389–1395. doi: 10.1021/bc800064t. [DOI] [PubMed] [Google Scholar]
- 117.Lee DY, Park SJ, Lee S, Nam JH, Byun Y. Highly poly(ethylene) glycolylated islets improve long-term islet allograft survival without immunosuppressive medication. Tissue Eng. 2007;13(8):2133–2141. doi: 10.1089/ten.2006.0009. [DOI] [PubMed] [Google Scholar]
- 118.Wilson JT, Krishnamurthy VR, Cui W, Qu Z, Chaikof EL. Noncovalent cell surface engineering with cationic graft copolymers. J Am Chem Soc. 2009;131(51):18228–18229. doi: 10.1021/ja908887v. [DOI] [PubMed] [Google Scholar]
- 119.Wilson JT, Cui W, Chaikof EL. Layer-by-layer assembly of a conformal nanothin PEG coating for intraportal islet transplantation. Nano Lett. 2008;8(7):1940–1948. doi: 10.1021/nl080694q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wilson JT, Haller CA, Qu Z, Cui W, Urlam MK, Chaikof EL. Biomolecular surface engineering of pancreatic islets with thrombomodulin. Acta Biomater. 2010;6(6):1895–1903. doi: 10.1016/j.actbio.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stabler CL, Sun XL, Cui W, Wilson JT, Haller CA, Chaikof EL. Surface re-engineering of pancreatic islets with recombinant azido-thrombomodulin. Bioconjug Chem. 2007;18(6):1713–1715. doi: 10.1021/bc7002814. [DOI] [PubMed] [Google Scholar]
- 122.Miura S, Teramura Y, Iwata H. Encapsulation of islets with ultra-thin polyion complex membrane through poly(ethylene glycol)-phospholipids anchored to cell membrane. Biomaterials. 2006;27(34):5828–5835. doi: 10.1016/j.biomaterials.2006.07.039. [DOI] [PubMed] [Google Scholar]
- 123.Wang RN, Rosenberg L. Maintenance of beta-cell function and survival following islet isolation requires re-establishment of the islet-matrix relationship. J Endocrinol. 1999;163(2):181–190. doi: 10.1677/joe.0.1630181. [DOI] [PubMed] [Google Scholar]
- 124.Weber LM, Cheung CY, Anseth KS. Multifunctional pancreatic islet encapsulation barriers achieved via multilayer PEG hydrogels. Cell Transplant. 2008;16(10):1049–1057. [PubMed] [Google Scholar]
- 125.Lin CC, Anseth KS. Glucagon-like peptide-1 functionalized PEG hydrogels promote survival and function of encapsulated pancreatic beta-cells. Biomacromolecules. 2009;10(9):2460–2467. doi: 10.1021/bm900420f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cabric S, Sanchez J, Lundgren T, Foss A, Felldin M, Källen R, Salmela K, Tibell A, Tufveson G, Larsson R, Korsgren O, Nilsson B. Islet surface heparinization prevents the instant blood-mediated inflammatory reaction in islet transplantation. Diabetes. 2007;56(8):2008–2015. doi: 10.2337/db07-0358. [DOI] [PubMed] [Google Scholar]
- 127.Cabric S, Sanchez J, Johansson U, Larsson R, Nilsson B, Korsgren O, Magnusson PU. Anchoring of vascular endothelial growth factor to surface-immobilized heparin on pancreatic islets: implications for stimulating islet angiogenesis. Tissue Eng Part A. 2010;16(3):961–970. doi: 10.1089/ten.tea.2009.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Su J, Hu BH, Lowe WL, Jr, Kaufman DB, Messersmith PB. Antiinflammatory peptide-functionalized hydrogels for insulin-secreting cell encapsulation. Biomaterials. 2010;31(2):308–314. doi: 10.1016/j.biomaterials.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lin CW, Chen LJ, Lee PL, Lee CI, Lin JC, Chiu JJ. The inhibition of TNF-alpha-induced E-selectin expression in endothelial cells via the JNK/NF-kappaB pathways by highly N-acetylated chitooligosaccharides. Biomaterials. 2007;28(7):1355–1366. doi: 10.1016/j.biomaterials.2006.11.006. [DOI] [PubMed] [Google Scholar]


