Abstract
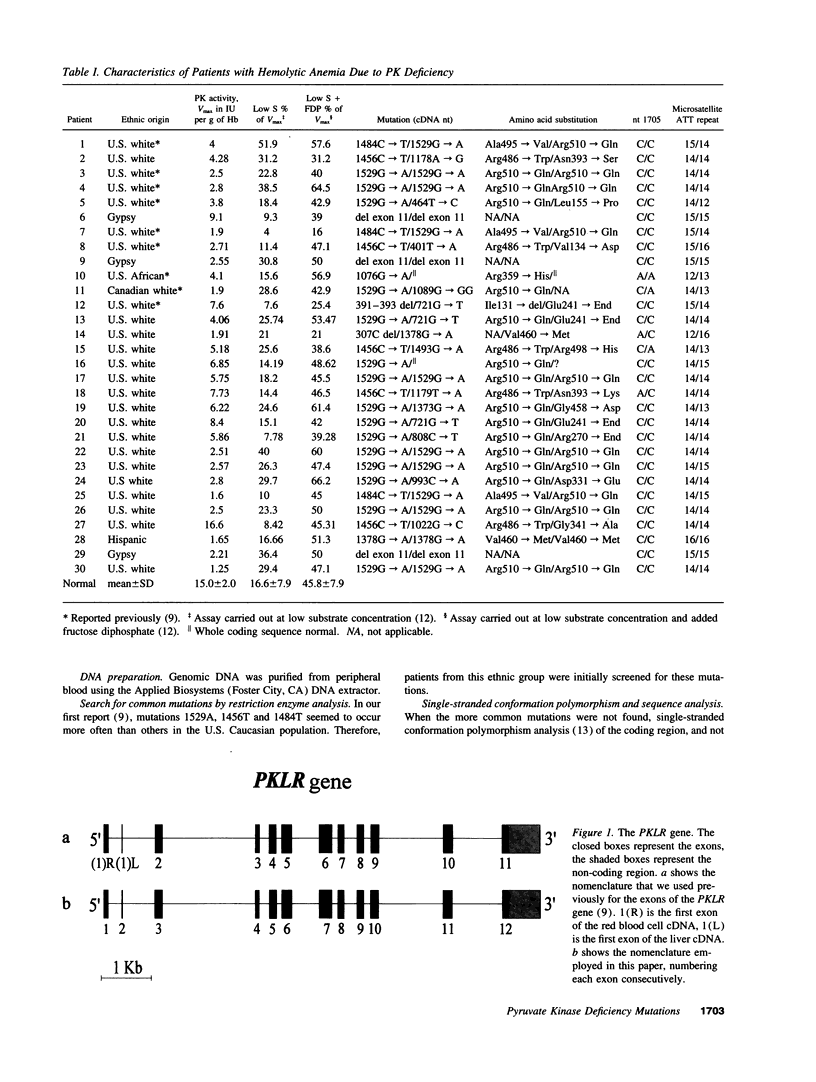
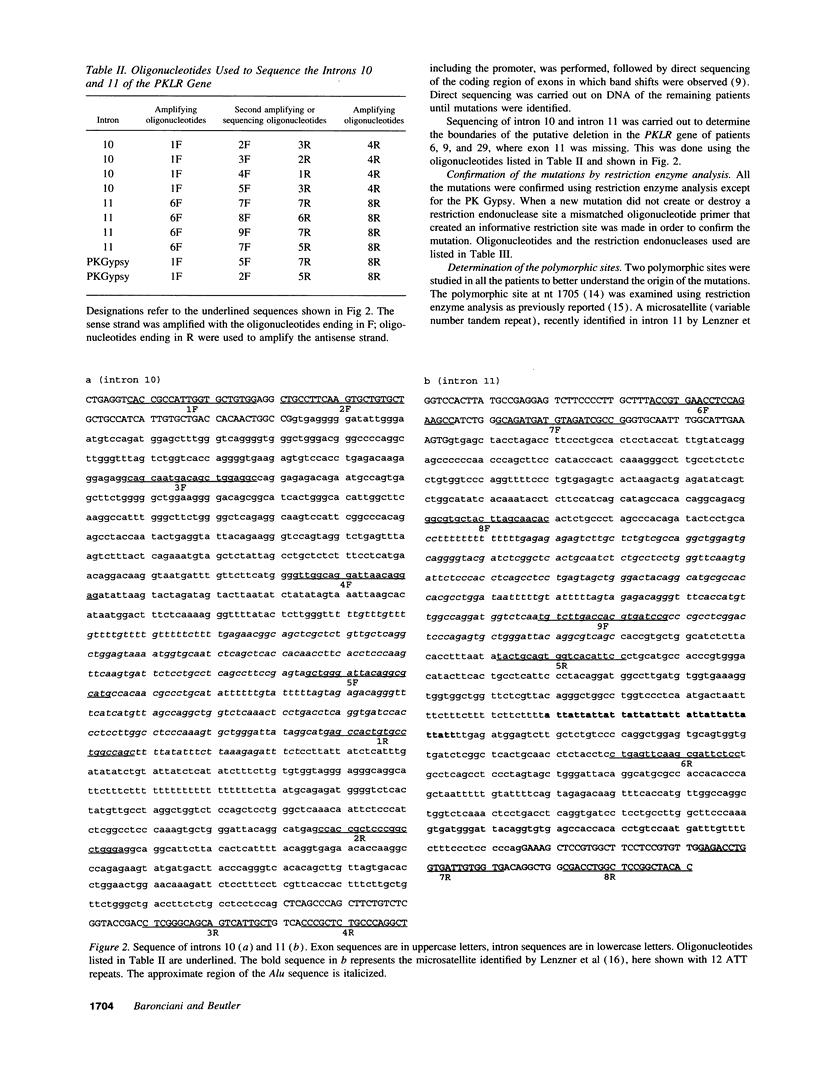
DNA analysis was performed on 30 unrelated patients with hereditary nonspherocytic hemolytic anemia (HNSHA) who had been found to be pyruvate kinase (PK) deficient by enzyme assay. 19 different mutations were identified among 58 of the 60 alleles at risk. 13 of these were missense mutations that caused single amino acid changes. Included were the following nucleotide substitutions: 401A, 464C, 993A, 1022C, 1076A, 1178G, 1179A, 1373A, 1378A, 1456T, 1484T, 1493A, 1529A. The remaining six mutations were as follows: two nonsense mutations, 721T and 808T; a nucleotide deletion, 307C; a nucleotide insertion, 1089GG; a three nucleotide in frame deletion, 391-392-393 and a deletion of 1149 bp from the PKLR gene that resulted in the loss of exon 11. All the patients were studied for two polymorphic sites, nucleotide (nt) 1705 A/C and a microsatellite in intron 11, to better understand the origin of the mutations. The 1529A mutation, which is the most common mutation in the European population, was found in 25 alleles. With a single exception this mutation was in linkage disequilibrium with both of the polymorphic markers, i.e., found with 1705C and 14 repeats in the microsatellite. This finding is consistent with a single origin of this common mutation. Other mutations occurring more than once were of much lower frequency than the 1529A mutation.
Full text
PDF


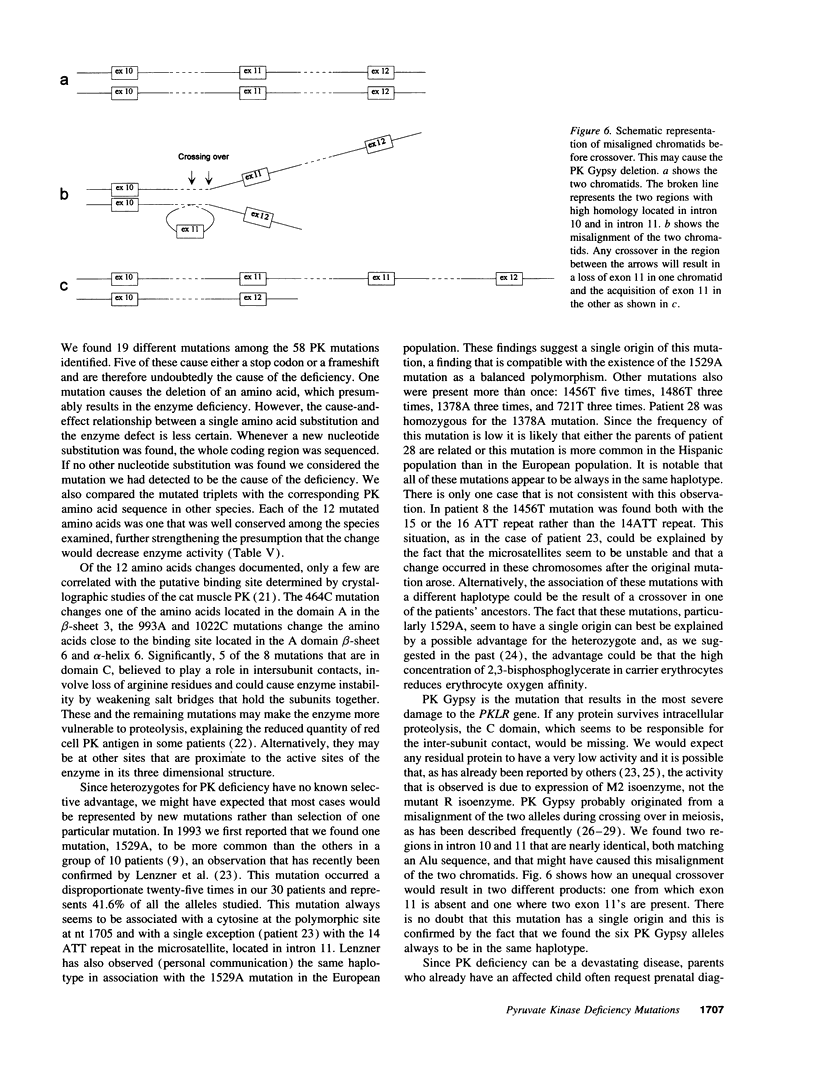
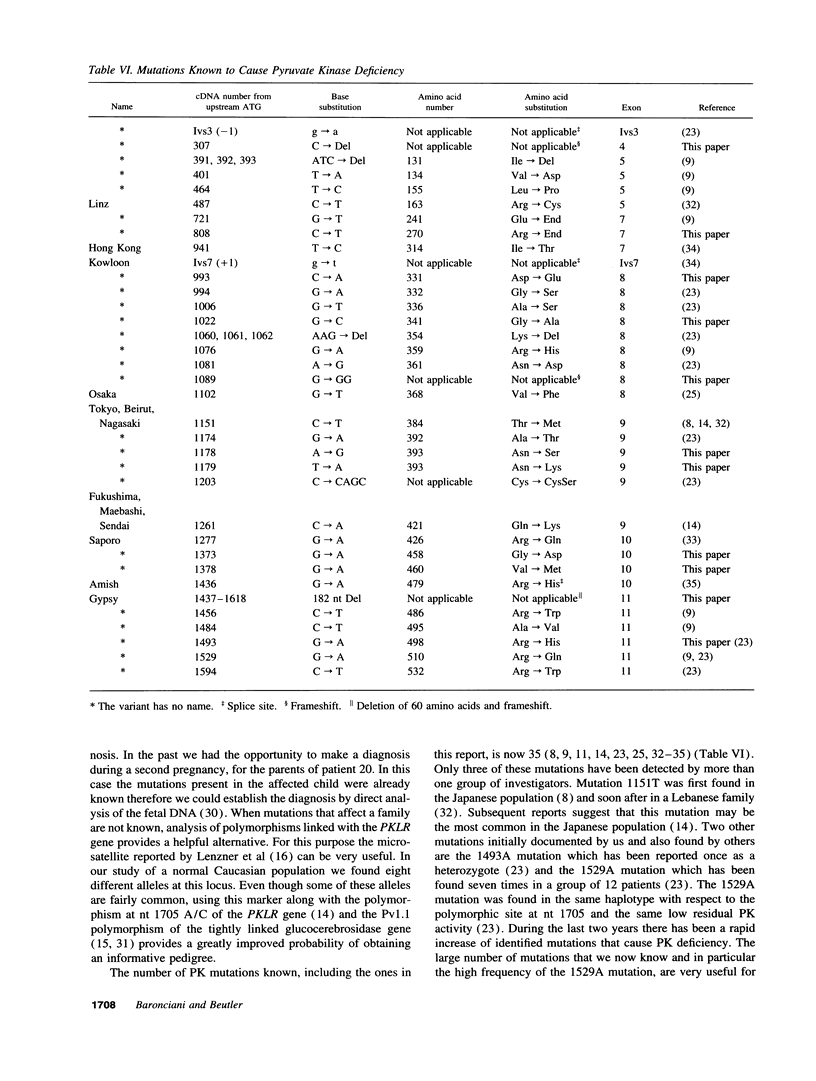

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baronciani L., Beutler E. Analysis of pyruvate kinase-deficiency mutations that produce nonspherocytic hemolytic anemia. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4324–4327. doi: 10.1073/pnas.90.9.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baronciani L., Beutler E. Prenatal diagnosis of pyruvate kinase deficiency. Blood. 1994 Oct 1;84(7):2354–2356. [PubMed] [Google Scholar]
- Beutler E., West C., Blume K. G. The removal of leukocytes and platelets from whole blood. J Lab Clin Med. 1976 Aug;88(2):328–333. [PubMed] [Google Scholar]
- Beutler E., West C., Gelbart T. Polymorphisms in the human glucocerebrosidase gene. Genomics. 1992 Apr;12(4):795–800. doi: 10.1016/0888-7543(92)90311-f. [DOI] [PubMed] [Google Scholar]
- Glenn D., Gelbart T., Beutler E. Tight linkage of pyruvate kinase (PKLR) and glucocerebrosidase (GBA) genes. Hum Genet. 1994 Jun;93(6):635–638. doi: 10.1007/BF00201562. [DOI] [PubMed] [Google Scholar]
- Hobbs H. H., Brown M. S., Goldstein J. L., Russell D. W. Deletion of exon encoding cysteine-rich repeat of low density lipoprotein receptor alters its binding specificity in a subject with familial hypercholesterolemia. J Biol Chem. 1986 Oct 5;261(28):13114–13120. [PubMed] [Google Scholar]
- Kahn A., Marie J., Galand C., Boivin P. Molecular mechanism of erythrocyte pyruvate kinase deficiency. Humangenetik. 1975 Oct 7;29(4):271–280. doi: 10.1007/BF00394188. [DOI] [PubMed] [Google Scholar]
- Kanno H., Ballas S. K., Miwa S., Fujii H., Bowman H. S. Molecular abnormality of erythrocyte pyruvate kinase deficiency in the Amish. Blood. 1994 Apr 15;83(8):2311–2316. [PubMed] [Google Scholar]
- Kanno H., Fujii H., Hirono A., Miwa S. cDNA cloning of human R-type pyruvate kinase and identification of a single amino acid substitution (Thr384----Met) affecting enzymatic stability in a pyruvate kinase variant (PK Tokyo) associated with hereditary hemolytic anemia. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8218–8221. doi: 10.1073/pnas.88.18.8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno H., Fujii H., Hirono A., Omine M., Miwa S. Identical point mutations of the R-type pyruvate kinase (PK) cDNA found in unrelated PK variants associated with hereditary hemolytic anemia. Blood. 1992 Mar 1;79(5):1347–1350. [PubMed] [Google Scholar]
- Kanno H., Fujii H., Miwa S. Low substrate affinity of pyruvate kinase variant (PK Sapporo) caused by a single amino acid substitution (426 Arg-->Gln) associated with hereditary hemolytic anemia. Blood. 1993 May 1;81(9):2439–2441. [PubMed] [Google Scholar]
- Kanno H., Fujii H., Tsujino G., Miwa S. Molecular basis of impaired pyruvate kinase isozyme conversion in erythroid cells: a single amino acid substitution near the active site and decreased mRNA content of the R-type PK. Biochem Biophys Res Commun. 1993 Apr 15;192(1):46–52. doi: 10.1006/bbrc.1993.1379. [DOI] [PubMed] [Google Scholar]
- Kogan S. C., Doherty M., Gitschier J. An improved method for prenatal diagnosis of genetic diseases by analysis of amplified DNA sequences. Application to hemophilia A. N Engl J Med. 1987 Oct 15;317(16):985–990. doi: 10.1056/NEJM198710153171603. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Goldstein J. L., Russell D. W., Brown M. S. Duplication of seven exons in LDL receptor gene caused by Alu-Alu recombination in a subject with familial hypercholesterolemia. Cell. 1987 Mar 13;48(5):827–835. doi: 10.1016/0092-8674(87)90079-1. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Russell D. W., Goldstein J. L., Brown M. S. Alu-Alu recombination deletes splice acceptor sites and produces secreted low density lipoprotein receptor in a subject with familial hypercholesterolemia. J Biol Chem. 1987 Mar 5;262(7):3354–3361. [PubMed] [Google Scholar]
- Lenzner C., Jacobasch G., Reis A., Thiele B., Nürnberg P. Trinucleotide repeat polymorphism at the PKLR locus. Hum Mol Genet. 1994 Mar;3(3):523–523. doi: 10.1093/hmg/3.3.523-a. [DOI] [PubMed] [Google Scholar]
- Lenzner C., Nürnberg P., Thiele B. J., Reis A., Brabec V., Sakalova A., Jacobasch G. Mutations in the pyruvate kinase L gene in patients with hereditary hemolytic anemia. Blood. 1994 May 15;83(10):2817–2822. [PubMed] [Google Scholar]
- Miwa S., Fujii H. Pyruvate kinase deficiency. Clin Biochem. 1990 Apr;23(2):155–157. doi: 10.1016/0009-9120(90)80029-i. [DOI] [PubMed] [Google Scholar]
- Miwa S., Kanno H., Fujii H. Concise review: pyruvate kinase deficiency: historical perspective and recent progress of molecular genetics. Am J Hematol. 1993 Jan;42(1):31–35. doi: 10.1002/ajh.2830420108. [DOI] [PubMed] [Google Scholar]
- Muirhead H., Clayden D. A., Barford D., Lorimer C. G., Fothergill-Gilmore L. A., Schiltz E., Schmitt W. The structure of cat muscle pyruvate kinase. EMBO J. 1986 Mar;5(3):475–481. doi: 10.1002/j.1460-2075.1986.tb04236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerowitz R., Hogikyan N. D. A deletion involving Alu sequences in the beta-hexosaminidase alpha-chain gene of French Canadians with Tay-Sachs disease. J Biol Chem. 1987 Nov 15;262(32):15396–15399. [PubMed] [Google Scholar]
- Neubauer B., Lakomek M., Winkler H., Parke M., Hofferbert S., Schröter W. Point mutations in the L-type pyruvate kinase gene of two children with hemolytic anemia caused by pyruvate kinase deficiency. Blood. 1991 May 1;77(9):1871–1875. [PubMed] [Google Scholar]
- Noguchi T., Yamada K., Inoue H., Matsuda T., Tanaka T. The L- and R-type isozymes of rat pyruvate kinase are produced from a single gene by use of different promoters. J Biol Chem. 1987 Oct 15;262(29):14366–14371. [PubMed] [Google Scholar]
- Orita M., Iwahana H., Kanazawa H., Hayashi K., Sekiya T. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2766–2770. doi: 10.1073/pnas.86.8.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recommended methods for the characterization of red cell pyruvate kinase variants. International Committee for Standardization in Haematology. Br J Haematol. 1979 Oct;43(2):275–286. doi: 10.1111/j.1365-2141.1979.tb03751.x. [DOI] [PubMed] [Google Scholar]
- Sorge J., Gross E., West C., Beutler E. High level transcription of the glucocerebrosidase pseudogene in normal subjects and patients with Gaucher disease. J Clin Invest. 1990 Oct;86(4):1137–1141. doi: 10.1172/JCI114818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani K., Fujii H., Nagata S., Miwa S. Human liver type pyruvate kinase: complete amino acid sequence and the expression in mammalian cells. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1792–1795. doi: 10.1073/pnas.85.6.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALENTINE W. N., TANAKA K. R., MIWA S. A specific erythrocyte glycolytic enzyme defect (pyruvate kinase) in three subjects with congenital non-spherocytic hemolytic anemia. Trans Assoc Am Physicians. 1961;74:100–110. [PubMed] [Google Scholar]
- Vettore L., De Matteis M. C., Zampini P. A new density gradient system for the separation of human red blood cells. Am J Hematol. 1980;8(3):291–297. doi: 10.1002/ajh.2830080307. [DOI] [PubMed] [Google Scholar]