Abstract
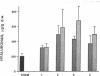
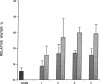
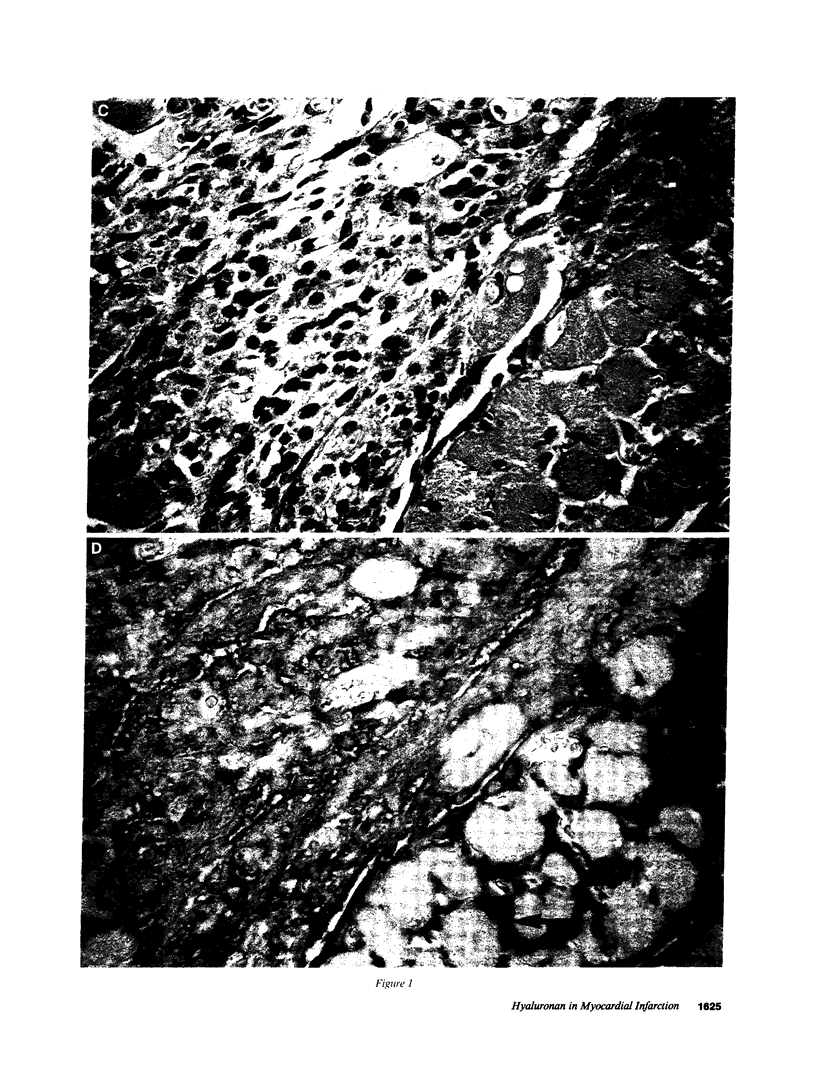
Experimental myocardial infarction was induced in rats. The myocardial accumulation of hyaluronan (HA) and water during the development of infarction was measured. The extractable HA content of the infarcted area increased progressively from day 1 and on day 3 reached a threefold increase compared with the HA amounts in myocardium of sham operated controls. The relative water content of infarcted areas also increased progressively reaching a maximum value by day 3 and was strongly correlated with the HA accumulation. Affinity histochemistry visualized a thin rim of HA in the endoperimysium in healthy myocardium. By day 2 an interstitial edema with inflammatory cells was apparent. The widened endoperimysium stained extensively for HA. By its water-binding ability, interstitial accumulation of HA will contribute to the interstitial edema in infarcted myocardial tissue. An interstitial edema is likely to influence the electromechanical characteristics of the myocardium and facilitate reentry phenomena due to a loss of contact between muscle cells. The edema also induces an increased extracellular pressure and an altered myocardial wall compliance that might impair myocardial microcirculation. The findings are relevant to an understanding of the beneficial effect of hyaluronidase treatment in limiting cellular damage during myocardial ischemia.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjermer L., Engström-Laurent A., Lundgren R., Rosenhall L., Hällgren R. Hyaluronate and type III procollagen peptide concentrations in bronchoalveolar lavage fluid as markers of disease activity in farmer's lung. Br Med J (Clin Res Ed) 1987 Oct 3;295(6602):803–806. doi: 10.1136/bmj.295.6602.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comper W. D., Laurent T. C. Physiological function of connective tissue polysaccharides. Physiol Rev. 1978 Jan;58(1):255–315. doi: 10.1152/physrev.1978.58.1.255. [DOI] [PubMed] [Google Scholar]
- Engström-Laurent A., Feltelius N., Hällgren R., Wasteson A. Raised serum hyaluronate levels in scleroderma: an effect of growth factor induced activation of connective tissue cells? Ann Rheum Dis. 1985 Sep;44(9):614–620. doi: 10.1136/ard.44.9.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haft J. I., Fani K. Stress and the induction of intravascular platelet aggregation in the heart. Circulation. 1973 Jul;48(1):164–169. doi: 10.1161/01.cir.48.1.164. [DOI] [PubMed] [Google Scholar]
- Hamerman D., Wood D. D. Interleukin 1 enhances synovial cell hyaluronate synthesis. Proc Soc Exp Biol Med. 1984 Oct;177(1):205–210. doi: 10.3181/00379727-177-1-rc1. [DOI] [PubMed] [Google Scholar]
- Hascall V. C. Interaction of cartilage proteoglycans with hyaluronic acid. J Supramol Struct. 1977;7(1):101–120. doi: 10.1002/jss.400070110. [DOI] [PubMed] [Google Scholar]
- Hällgren R., Gerdin B., Tengblad A., Tufveson G. Accumulation of hyaluronan (hyaluronic acid) in myocardial interstitial tissue parallels development of transplantation edema in heart allografts in rats. J Clin Invest. 1990 Mar;85(3):668–673. doi: 10.1172/JCI114490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. L., Ziff M. Lymphokine stimulation of collagen accumulation. J Clin Invest. 1976 Jul;58(1):240–252. doi: 10.1172/JCI108455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin N. M. Cell migrations in embryos. Cell. 1984 Sep;38(2):353–360. doi: 10.1016/0092-8674(84)90490-2. [DOI] [PubMed] [Google Scholar]
- MARTINS DE OLIVEIRA J., CARBALLO R., ZIMMERMAN H. A. Intravenous injection of hyaluronidase in acute myocardial infarction: preliminary report of clinical and experimental observations. Am Heart J. 1959 May;57(5):712–722. doi: 10.1016/0002-8703(59)90180-2. [DOI] [PubMed] [Google Scholar]
- Maclean D., Fishbein M. C., Maroko P. R., Braunwald E. Hyaluronidase-induced reductions in myocardial infarct size. Science. 1976 Oct 8;194(4261):199–200. doi: 10.1126/science.959848. [DOI] [PubMed] [Google Scholar]
- Maroko P. R., Libby P., Bloor C. M., Sobel B. E., Braunwald E. Reduction by hyaluronidase of myocardial necrosis following coronary artery occlusion. Circulation. 1972 Sep;46(3):430–437. doi: 10.1161/01.cir.46.3.430. [DOI] [PubMed] [Google Scholar]
- Mason R. M. Recent advances in the biochemistry of hyaluronic acid in cartilage. Prog Clin Biol Res. 1981;54:87–112. [PubMed] [Google Scholar]
- Nettelbladt O., Bergh J., Schenholm M., Tengblad A., Hällgren R. Accumulation of hyaluronic acid in the alveolar interstitial tissue in bleomycin-induced alveolitis. Am Rev Respir Dis. 1989 Mar;139(3):759–762. doi: 10.1164/ajrccm/139.3.759. [DOI] [PubMed] [Google Scholar]
- Ripellino J. A., Klinger M. M., Margolis R. U., Margolis R. K. The hyaluronic acid binding region as a specific probe for the localization of hyaluronic acid in tissue sections. Application to chick embryo and rat brain. J Histochem Cytochem. 1985 Oct;33(10):1060–1066. doi: 10.1177/33.10.4045184. [DOI] [PubMed] [Google Scholar]
- Roberts R., Braunwald E., Muller J. E., Croft C., Gold H. K., Hartwell T. D., Jaffe A. S., Mullin S. M., Parker C., Passamani E. R. Effect of hyaluronidase on mortality and morbidity in patients with early peaking of plasma creatine kinase MB and non-transmural ischaemia. Multicentre investigation for the limitation of infarct size (MILIS). Br Heart J. 1988 Oct;60(4):290–298. doi: 10.1136/hrt.60.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovetto M. J. Effect of hyaluronidase and methylprednisolone on myocardial function, glucose metabolism, and coronary flow in the isolated ischemic rat heart. Circ Res. 1977 Sep;41(3):373–379. doi: 10.1161/01.res.41.3.373. [DOI] [PubMed] [Google Scholar]
- Stern C. D. Mini-review: hyaluronidases in early embryonic development. Cell Biol Int Rep. 1984 Sep;8(9):703–717. doi: 10.1016/0309-1651(84)90108-5. [DOI] [PubMed] [Google Scholar]
- Sunnergren K. P., Rovetto M. J. The effects of hyaluronidase on interstitial hydration, plasma protein exclusion, and microvascular permeability in the isolated perfused rat heart. Microvasc Res. 1985 Nov;30(3):286–297. doi: 10.1016/0026-2862(85)90060-3. [DOI] [PubMed] [Google Scholar]
- Tengblad A. Affinity chromatography on immobilized hyaluronate and its application to the isolation of hyaluronate binding properties from cartilage. Biochim Biophys Acta. 1979 Jun 19;578(2):281–289. doi: 10.1016/0005-2795(79)90158-2. [DOI] [PubMed] [Google Scholar]
- Tengblad A. Quantitative analysis of hyaluronate in nanogram amounts. Biochem J. 1980 Jan 1;185(1):101–105. doi: 10.1042/bj1850101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker R. P., Erickson C. A. Morphology and behavior of quail neural crest cells in artificial three-dimensional extracellular matrices. Dev Biol. 1984 Aug;104(2):390–405. doi: 10.1016/0012-1606(84)90094-0. [DOI] [PubMed] [Google Scholar]
- Tucker V. L., Huxley V. H. O2 modulation of single-vessel hydraulic conductance. Am J Physiol. 1988 Feb;254(2 Pt 2):H317–H323. doi: 10.1152/ajpheart.1988.254.2.H317. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Wahl L. M., McCarthy J. B. Lymphocyte-mediated activation of fibroblast proliferation and collagen production. J Immunol. 1978 Sep;121(3):942–946. [PubMed] [Google Scholar]