Abstract
Sensitization to mechanical stimuli is important in most pain syndromes. We evaluated the populations of nociceptors mediating mechanical hyperalgesia and those mediating mu-opioid receptor (MOR) and delta-opioid receptor (DOR) agonist-induced inhibition of hyperalgesia, in the rat. We found that: 1) intradermal injection of both the endogenous ligand for the Ret receptor, GDNF, and the ligand for the TrkA receptor, NGF – which are present on distinct populations of nociceptors – both produce mechanical hyperalgesia; 2) DOR agonist SNC but not MOR agonist DAMGO inhibit GDNF-induced hyperalgesia; 3) both DAMGO and SNC inhibit NGF hyperalgesia, even in rats pretreated with IB4-saporin, a toxin that destroys IB4-binding neurons; 4) co-administration of low doses of DAMGO and SNC produce enhanced analgesia, and; 5) repeated administration of DAMGO produces cross-tolerance to the analgesic effect of SNC. These findings demonstrate that, most nociceptors have a role in mechanical hyperalgesia, only the DOR agonist inhibits GDNF hyperalgesia, and MOR and DOR are co-localized on a functionally important population of TrkA-positive nociceptors.
Keywords: Pain, Neuronal population, Mouse, Mechanical transduction, Analgesia, Tolerance
The primary afferent nociceptor, the first neuron in the pain pathway, transmits nocifensive information from injured tissue to the central nervous system or in the case of neuropathic pain generates the pain signal. While nociceptors are a heterogeneous group of sensory neurons, as demonstrated by a variety of anatomical markers and physiological properties (Basbaum and Woolf, 1999, Hucho and Levine, 2007, Scherrer et al., 2009, Wang et al., 2010), the importance of the subpopulations of nociceptors in pain syndromes remain poorly understood. In addition, given that opioid receptors are located on nociceptors (Aley and Levine, 1997c, Walwyn et al., 2007, Scherrer et al., 2009, Walwyn et al., 2009, Wang et al., 2010), the efficacy of these clinically important analgesics may also vary based on the population of nociceptors activated in a particular pain syndrome. This may help explain the marked variability in the different patients’ responses to this class of analgesics.
One of the major divisions of nociceptors is based on the spinal lamina in which they terminate. Thus, staining with isolectin B4 (IB4), a plant lectin, identifies a subset of nociceptors (IB4-positive, IB4(+)) that terminate in lamina IIi of the spinal dorsal horn (Kitchener et al., 1993, Molliver et al., 1995, Gerke and Plenderleith, 2004). In contrast, IB4(−) (TrkA/peptidergic) nociceptors terminate in lamina IIo and I (Molliver et al., 1995). Recently it has been suggested that this subdivision of nociceptors, into IB4(+) and IB4(−) (peptidergic), defines two clinically important features of pain, in the mouse, namely sensitivity to noxious mechanical versus thermal stimuli and response to mu- vs. delta-opioid analgesics (Scherrer et al., 2009). Scherrer and colleagues reported that mu-opioid (MOR) and delta-opioid (DOR) receptors are expressed on different subsets of nociceptors; MOR is located on IB4(−) (peptidergic) nociceptors while DOR occurs on IB4(+) (non peptidergic) nociceptors (Scherrer et al., 2009). And, intrathecal administration of DOR but not MOR agonists was able to produce analgesia for mechanical pain (Scherrer et al., 2009).
In the present experiments we analyzed the nociceptor population mediating mechanical hyperalgesia and the effects of MOR and DOR selective agonists on mechanical hyperalgesia, in a second species, the rat. To ensure that the effect of hyperalgesic agents and MOR and DOR agonists occurs by their action on the nociceptor, we administered these compounds into their peripheral receptive fields, rather than intrathecally, since spinal cord neurons also have opioid receptors (Zajac et al., 1989, Besse et al., 1990b, Gillberg and Askmark, 1991, Morinville et al., 2004, Kline and Wiley, 2008). Also, to establish that mu- and delta-opioid effects were on different populations of nociceptors, we used hyperalgesic ligands acting at receptors that occur on different populations of nociceptors (Bennett et al., 1998, Kashiba et al., 1998), namely nerve growth factor (NGF) and glia-derived growth factor (GDNF). These receptors are located on separate nociceptors (Malik-Hall et al., 2005, Bogen et al., 2008). Importantly, since peripherally administered opioids, in the absence of nociceptor sensitization, do not affect mechanical nociceptive threshold (Aley et al., 1995, Aley and Levine, 1997a, b, c) their effects are only to reverse NGF and/or GDNF hyperalgesia. Finally, we determined if MOR and DOR agonists might be able to act on the same nociceptor to produce inhibition of mechanical hyperalgesia by evaluating for enhanced analgesia after co-administration and cross-tolerance after sequential administration.
Experimental procedures
Animals
Experiments were performed on adult male Sprague–Dawley rats (200–250 g; Charles River, Hollister, CA). Animals were housed three per cage, under a 12-h light/dark cycle, in a temperature and humidity controlled environment. Food and water were available ad libitum. All nociceptive testing was done between 10:00 am and 4:00 pm. All experimental protocols were approved by the UCSF Committee on Animal Research and conformed to National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Nociceptive testing
The nociceptive flexion reflex was quantified with an Ugo Basile Analgesymeter® (Stoelting, Chicago, IL), which applies a linearly increasing mechanical force to the dorsum of the rat's hind paw. Nociceptive threshold, defined as the force in grams at which the rat withdrew its paw, was the mean of 3 readings taken at 5 min intervals. Rats are lightly restrained in cylindrical transparent acrylic restrainers that have triangular windows on the side, which allow extension of the hind leg from the restrainer for nociceptive threshold testing. All rats were acclimatized to the testing procedures to reduce variability in the paw-withdrawal threshold, which were determined before (baseline) and after administration of test agents. Each paw was treated as an independent measure and each experiment performed on separate groups of rats. The results are expressed as percentage change from baseline mechanical nociceptive threshold.
Drugs
Drugs employed in this study were NGF, D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO) and 4-[(R)-[(2S,5R)-4-allyl-2,5-dimethylpiperazin-1-yl](3-methoxyphenyl)methyl]-N,N-diethylbenzamide (SNC), from Sigma (St. Louis, MO), and GDNF from EMD Biosciences (La Jolla, CA). NGF and GDNF are direct-acting hyperalgesic agents (Malik-Hall et al., 2005, Bogen et al., 2008). Drugs were applied by intradermal injection on the dorsum of the hind paw. A stock solution of NGF (1 µg/µl in 0.9% NaCl containing 0.5% bovine serum albumin) was diluted in 0.9% NaCl just prior to injection (dose 1 µg) (Malik-Hall et al., 2005); GDNF was similarly prepared (Malik-Hall et al., 2005). DAMGO was dissolved in normal saline. SNC was dissolved in DMSO; the final concentration of DMSO was less than 5%. IB4-saporin, which consists of isolectin B4 coupled to saporin, a potent neurotoxin, was purchased from Advanced Targeting Systems (San Diego, CA). All drugs, except IB4-saporin, which was administered by the spinal intrathecal route, were administered intradermally in a volume of 5 µl using a 30-gauge hypodermic needle attached to a micro-syringe (Hamilton, Reno, NV). The selection of the drug doses used in this study was based on previous dose–response curves (Khasar et al., 1993, Malik-Hall et al., 2005, Joseph et al., 2007, Bogen et al., 2008).
Intrathecal administration of IB4-saporin
IB4-saporin was diluted with saline and a dose of 3.2 µg/20 µL administered intrathecally 10 days prior to an experiment (Bogen et al., 2008, Joseph et al., 2008, Bogen et al., 2009, Joseph and Levine, 2010). With the use of an insulin syringe, IB4-saporin was injected between the L4 and L5 vertebrae, into the subarachnoid space. For this procedure, rats were briefly anesthetized with 2.5% isoflurane/97.5% O2.
Statistical analysis
In all experiments, the dependent variable was change in paw withdrawal threshold, represented as percentage change from baseline paw withdrawal threshold. Group data are presented as mean±standard error of the mean (SEM). Statistical significance was determined by ANOVA followed by Tukey's post hoc test; p < 0.05 was considered statistically significant.
Results
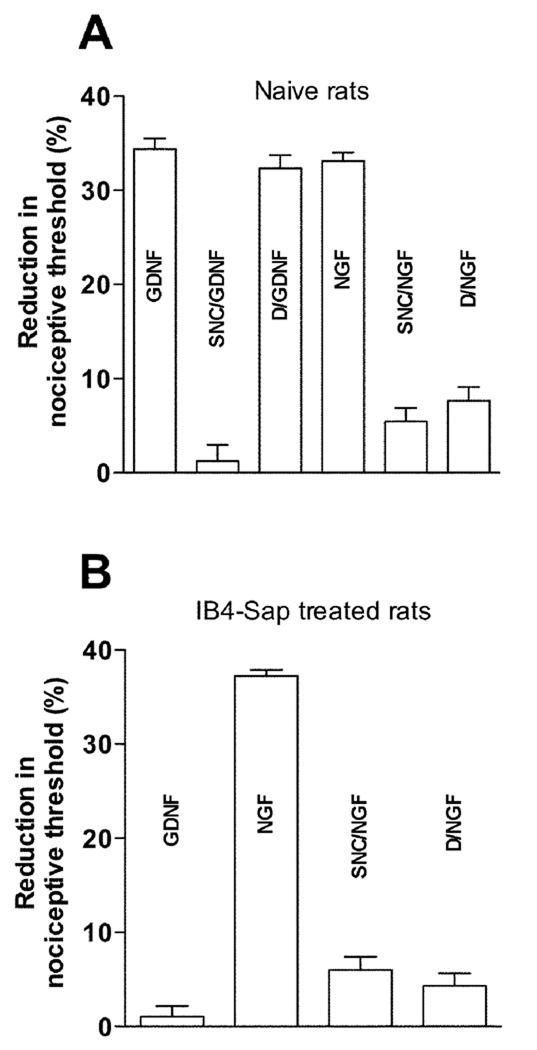
Intradermally injected GDNF acts at its receptor, Ret, on the peripheral terminal of the primary afferent nociceptor to produce mechanical hyperalgesia in the rat (Bogen et al., 2008). In this species Ret is restricted to IB4(+) neurons (Bennett et al., 1998). Therefore, we first compared the effects of intradermal administration of the MOR and DOR selective agonists, DAMGO and SNC, respectively, on the mechanical hyperalgesia induced by GDNF. While the DOR selective agonist, SNC (100 ng) markedly inhibited GDNF (10 ng; n=8)-induced mechanical hyperalgesia (Fig. 1A), the MOR selective agonist DAMGO (1 µg) at a dose that strongly inhibits NGF (1 µg, n=8) hyperalgesia (Fig. 1A) had no effect on similar magnitude GDNF-induced hyperalgesia (Fig. 1). Thus, for Ret(+) nociceptors in the rat, as recently demonstrated for the IB4(+) population of nociceptors in the mouse (Basbaum et al., 2009), DOR but not MOR agonists attenuate GDNF mechanical hyperalgesia. In contrast, for mechanical hyperalgesia induced by NGF, the endogenous ligand for the TrkA receptor (Malik-Hall et al., 2005), both MOR (DAMGO, 1 µg, n=8) and DOR (SNC, 100 ng, n=6) agonists attenuated NGF-induced mechanical hyperalgesia (Fig. 1A).
Figure 1.
A Effect of delta and mu-opioid receptor agonists on GDNF and NGF-induced mechanical hyperalgesia Intradermal injections of GDNF (10 ng, n = 8) and NGF (1 µg, n = 8) produced significant (p < 0.001) mechanical hyperalgesia (reduction in the paw withdrawal threshold, measured on the dorsal side of the hind paw of the rat. Administration of SNC (delta-opioid receptor agonist, 100ng, n = 8), prior (15') to GDNF significantly (p < 0.001) attenuated GDNF-induced mechanical hyperalgesia, while DAMGO (mu-opioid receptor agonist, 1 µg, n = 8) failed to attenuate GDNF-induced mechanical hyperalgesia. However, similar treatment with both SNC (100 ng, n =6), and DAMGO (1 µg, n = 8) prior (15’) to NGF resulted in significant attenuation of NGF-induced hyperalgesia.
B Effect of intraspinal IB4-Saporin (neurotoxin) treatment Intradermal injection of GDNF (10 ng, n = 6) to rats, previously (10 days prior) treated with IB4-Saporin intraspinally failed to induce mechanical hyperalgesia, while in IB4-Sap treated rats NGF (1 µg, n = 6) still produced robust hyperalgesia, which was significantly (p < 0.001) attenuated by both SNC (100 ng, n = 8) and DAMGO (1 µg, n = 8).
Since approximately one third of TrkA(+) (peptidergic) nociceptors in the rat are IB4(+) (Kashiba et al., 2001, Fang et al., 2006) – unlike in the mouse where 90% TrkA(+) (peptidergic) nociceptors are IB4(−) (Price and Flores, 2007) – we next evaluated the ability of DAMGO and SNC to produce analgesia in the IB4(−) subpopulation of TrkA(+) nociceptors. Ten days after intrathecal administration of IB4-saporin, to destroy IB4(+) sensory neurons (Bogen et al., 2008, Joseph et al., 2008, Bogen et al., 2009, Joseph and Levine, 2010), NGF was injected intradermally. In these rats, in which IB4(+) nociceptors have been destroyed, NGF (1 µg, n=6), but not GDNF (10 ng), still induced robust hyperalgesia (Fig. 1B), and both the MOR and DOR agonists, DAMGO (1 µg, n=8) and SNC (100 ng, n=8), respectively, still inhibited NGF hyperalgesia (Fig. 1B). These experiments establish the ability of IB4(−) neurons to detect noxious mechanical stimuli and be sensitized, producing mechanical hyperalgesia, and the ability of a DOR selective agonist (SNC) as well as a MOR selective agonist (DAMGO) to inhibit NGF-induced mechanical hyperalgesia in rats that no longer have IB4(+) nociceptors. This contrasts with what has recently been reported in the mouse, where IB4(−) nociceptors do not mediate mechanical pain (Scherrer et al., 2009). Although MOR and DOR agonists each alone almost completely eliminate NGF hyperalgesia in IB4-saporin-treated rats, compatible with MOR and DOR being co-expressed on many of these nociceptors, these experiments do not specifically test the establish that MOR and DOR are co-localized on nociceptors.
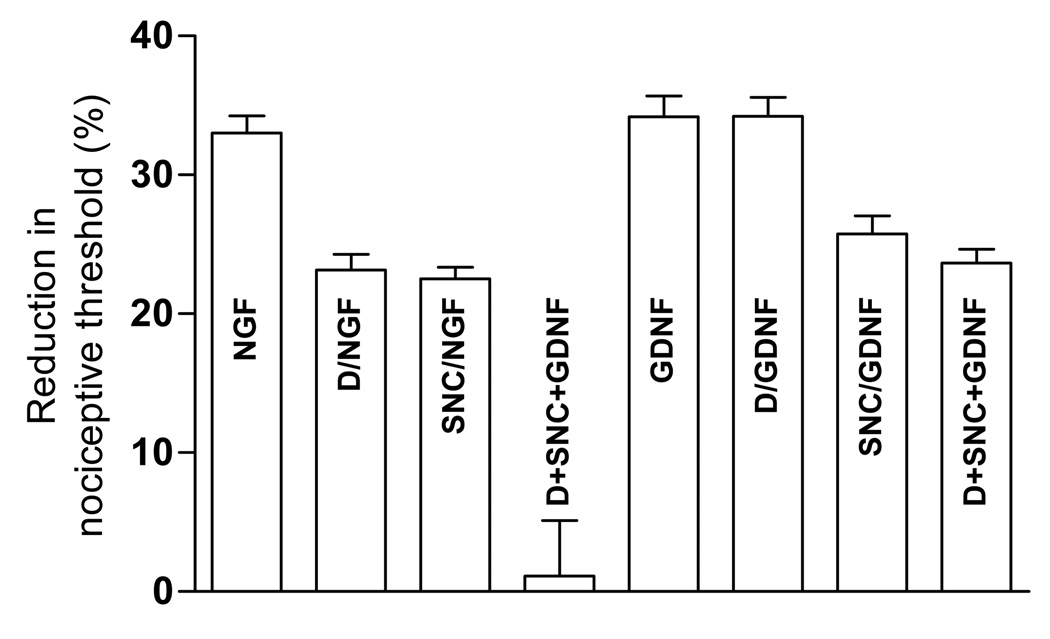
To test the hypothesis that MOR and DOR are co-expressed on nociceptor terminals, we evaluated for an interaction between intradermally injected MOR and DOR agonists to produce enhanced analgesia (Aley et al., 1995, Aley and Levine, 1997a, b, c). While a low dose of DAMGO (100 ng, n=6) or SNC (10 ng, n=6) each alone produced weak inhibition of NGF-induced mechanical hyperalgesia, the combination of the two produced almost complete inhibition of NGF (1 µg, n=6), but not GDNF (10 ng, n=6), hyperalgesia (Fig. 2). That DAMGO had no effect on GDNF hyperalgesia indicates its analgesia is DOR independent. While not proving that MOR and DOR are co-localized on nociceptors, these experiments are compatible with this interpretation.
Figure 2. Synergistic attenuating effect.
Sub-effective doses of SNC (10ng) and DAMGO (100ng), both administered separately but 15' prior to NGF (1 µg, n = 8) produced synergistic attenuation (p<0.001) of NGF induced hyperalgesia, while similar treatment prior to GDNF (10 ng, n =6) did not increase the attenuating effect more than that produced by SNC (10 ng) alone.
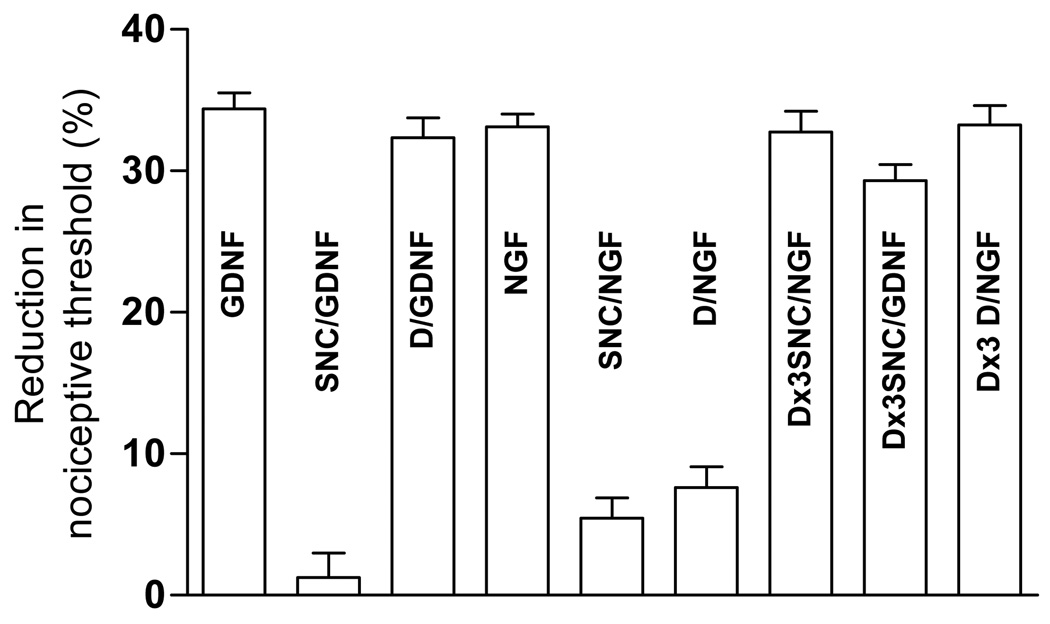
To provide more direct confirmation of an interaction between MOR and DOR in nociceptors, we determined if tolerance to the peripheral analgesic effect of DAMGO produced cross-tolerance to the analgesic effect of a subsequent injection of SNC. We have used this technique previously to demonstrate co-localization of MOR, A1-adenosine and α2-adrenergic receptors on nociceptors (Aley et al., 1995, Aley and Levine, 1997c). A protocol of repeated administration of DAMGO (1 µg), once per hour for 3 hours, produces tolerance at the MOR (Aley et al., 1995, Aley and Levine, 1997a, b, c). This protocol also produced cross-tolerance to the subsequent acute administration of SNC (100 ng) (Fig. 3). Since DAMGO does not act at DOR, the observation of cross-tolerance between MOR and DOR analgesia, in the periphery, can only be explained by the presence of MOR and DOR on the same nociceptor terminal.
Figure 3. Cross tolerance with repeated DAMGO treatment.
Both SNC (delta-opioid agonist), which significantly attenuated NGF and GDNF–induced hyperalgesia in naïve animals failed to attenuate NGF or GDNF-induced hyperalgesia in repeated DAMGO (1 µg, hourly, 3 times) treated rats (both n = 6). Similarly, DAMGO (mu-opioid agonist), which attenuated NGF-induced hyperalgesia in naïve animals, failed to attenuate NGF-induced hyperalgesia in repeated DAMGO (1 µg, hourly, 3 times) treated rats (n = 4).
Discussion
The first neuron in the pain pathway, the primary afferent nociceptor, is an extremely important target for the development of novel pain therapeutics since: 1) nociceptors contain functionally important molecules not found in other cells (e.g., voltage-gated sodium channel, NaV1.8 (Akopian et al., 1996, Sangameswaran et al., 1996, Renganathan et al., 2003)), 2) only a subpopulation of nociceptors may be involved in a given pain syndrome (Joseph et al., 2008, Joseph and Levine, 2010), which might allow for preservation of protective pain sensation, 3) analgesics working at this point in the pain pathway (i.e., in the primary afferent nociceptor) act before pain signals enter the central nervous system to diverge over multiple pathways (Braz et al., 2005, Bráz and Basbaum, 2009), 4) peripherally restricted analgesics avoid their many CNS-related side effects, and 5) blocking the pain signal in the periphery would prevent development of neuroplastic changes in the central nervous system (Dubner, 2004, Salter, 2005, Sharif Naeini et al., 2005, Eisenach, 2006, May, 2008, Descalzi et al., 2009, Latremoliere and Woolf, 2009, Seifert and Maihöfner, 2009, Toyoda et al., 2009, Asante et al., 2010).
The cardinal symptom in most pain syndromes is sensitivity to mechanical stimuli, pain made worse by movement or previous innocuous stimuli (e.g., mechanical hyperalgesia, mechanical allodynia, tenderness). Therefore, the present study focused on populations of nociceptors involved in mechanical hyperalgesia. We first determined which population(s) of nociceptors contributed to mechanical hyperalgesia. Our subdivision of nociceptors is based on the dorsal horn lamina in which they terminate. IB4(+) nociceptors terminate in lamina IIi (Kitchener et al., 1993, Molliver et al., 1995, Gerke and Plenderleith, 2004), while IB4(−) (peptidergic) nociceptors terminate in lamina IIo and I (Molliver et al., 1995). It has been suggested that these two populations of nociceptors contribute to different aspects of pain sensation (Braz et al., 2005). In this study we injected NGF and GDNF, ligands for the TrkA and Ret receptors, respectively, which produce hyperalgesia by direct action on nociceptor terminals (Malik-Hall et al., 2005, Bogen et al., 2008) and are found on different populations of nociceptors (Kashiba et al., 1998). In the rat Ret is only found on IB4(+) nociceptors while TrkA is on both IB4(+) and IB4(−) nociceptors (Kashiba et al., 2001). We observed that both GDNF and NGF produce mechanical hyperalgesia. However, in the rat approximately one-third of TrkA(+) nociceptors are IB4(+). Therefore, to confirm that IB4(−) nociceptors contribute to mechanical hyperalgesia, which has recently been brought into question, in the mouse (Basbaum et al., 2009), we demonstrated that a potent IB4(+) nociceptor toxin (Vulchanova et al., 2001, Tarpley et al., 2004) eliminated GDNF- but not NGF-induced mechanical hyperalgesia. Thus, in contrast to what has been suggested for the mouse (Basbaum et al., 2009), in the rat both the IB4(+) and IB4(−) nociceptors play a role in mechanical nociception and mechanical hyperalgesia. These observations may have clinical implications. For example, we have previously shown that IB4-saporin eliminates mechanical hyperalgesia in the early phase of the painful peripheral neuropathy produced by Oxaliplatin (Joseph et al., 2008), a clinically distinct phase of the painful peripheral neuropathy associated with this important cancer chemotherapeutic drug (Joseph et al., 2008). IB4-saporin also prevents development of hyperalgesic priming (Joseph and Levine, 2010) a neuroplastic change in nociceptors leading to enhanced and markedly prolonged inflammatory hyperalgesia. Unfortunately, few models of clinical pain syndromes have been studied in terms of the population(s) of nociceptors involved.
A second important nociceptor mechanism, with well-established clinical significance, is as a site of action of opioid analgesics (Aley and Levine, 1997c, Walwyn et al., 2007, Scherrer et al., 2009, Stein and Zöllner, 2009, Walwyn et al., 2009). While the major analgesic effect of opioids is likely mediated by its action at the central terminal, we have used the peripheral terminal to distinguish the effect of DOR and MOR agonists on mechanical hyperalgesia in populations of nociceptors. This approach also allowed us to exclude action on opioid receptors in neurons intrinsic to the spinal dorsal horn (Zajac et al., 1989, Besse et al., 1990a, b, Gillberg and Askmark, 1991, Morinville et al., 2004, Kline and Wiley, 2008). Since centrally but not peripherally administered opioids elevate nociceptive threshold in the absence of mechanical hyperalgesia, it also allowed us to exclude actions other than reversal of NGF- or GDNF-induced hyperalgesia. We found that a DOR but not a MOR agonist produced analgesia by action on IB4(+)/Ret(+) nociceptors (i.e., for GDNF hyperalgesia), similar to what has recently been reported in the mouse (Scherrer et al., 2009). In contrast, both DAMGO and SNC inhibited mechanical hyperalgesia induced by NGF in IB4(−)/TrkA(+) nociceptors (i.e., in IB4-saporin treated rats).
To test the hypothesis that mu and delta opioid receptors are co-expressed on nociceptors, we performed two experiments. First, we tested the hypothesis that the combination of low doses of mu and delta opioid agonists would interact to produce enhanced analgesia. In these experiments we found that the combination of low doses of DAMGO and SNC produce markedly greater analgesia for NGF, but not GDNF, hyperalgesia. These findings suggest that the action of DAMGO is restricted to MOR, with no action at DOR. Thus, the interaction between DAMGO and SNC, to produce enhanced analgesia, is likely due to an interaction between MOR and DOR signaling. To more directly test the hypothesis that MOR and DOR signaling occur in the same neuron, we show that for NGF hyperalgesia, there is cross-tolerance between DAMGO- and SNC-induced analgesia. That is, repeated administration of DAMGO produced cross-tolerance to SNC. Thus, we conclude that MORs and DORs are co-expressed in a functionally important population of TrkA(+) nociceptors. These findings are compatible with previous studies providing evidence that, mu and delta opioid receptors dimerize (Lee et al., 1980, Schiller et al., 1999, Jordan et al., 2001, Gomes et al., 2004, Daniels et al., 2005), DOR modulates MOR analgesia (Standifer et al., 1994, Schiller et al., 1999, Zhu et al., 1999, Nitsche et al., 2002, Gomes et al., 2004, Fan et al., 2005, Gallantine and Meert, 2005, Chefer and Shippenberg, 2009, Xie et al., 2009), and MOR and DOR are co-expressed in DRG neurons (Ji et al., 1995, Rau et al., 2005, Wang et al., 2010). Using IB4-saporin to destroy IB4(+) nociceptors, we were able to show that this effect included action on IB4(−)/TrkA(+) nociceptors. These findings differ from those of Scherrer and colleagues (Scherrer et al., 2009), who found in the mouse that IB4(+)/nonpeptidergic but not IB4(−)/peptidergic neurons mediate mechanical pain, and DOR (SNC) but not MOR (DAMGO) agonists, administered intrathecally produce analgesia against mechanical pain (Scherrer et al., 2009).
Differences between the results in the present study and that of Scherrer and colleagues might be due, in part, to use of different species. Functional differences between the central and peripheral terminals of the nociceptor might also contribute, as Scherrer and colleagues used spinal and we intradermal administration of opioid agonists, since dorsal horn neurons also contain opioid receptors (Zajac et al., 1989, Besse et al., 1990a, b, Gillberg and Askmark, 1991, Morinville et al., 2004, Kline and Wiley, 2008). Also, differences in the pain models used, such as the use of the sensitized nociceptor in all the present experiments, may be important as CNS opioids have effects on nociceptive threshold in the absence of a sensitization state. Finally, use of VR1 as a selective marker for thermal nociceptors may also impact interpretation since VR1 may also function in mechanical transduction (Gevaert et al., 2007, Liedtke, 2007b, Liedtke, 2007a, Pedersen and Nilius, 2007, Bielefeldt and Davis, 2008, Yin and Kuebler, 2010). Of note, our findings in the rat are in agreement with a recent study in the mouse that reported coexpression of MOR and DOR on small-diameter dorsal root ganglion neurons, predominantly in IB4(+) population (Ji et al., 1995, Rau et al., 2005, Wang et al., 2010). Differences between rats and mice, with respect to which nociceptors mediate mechanotransduction, and MOR and DOR agonist-induced analgesia, will require considerable additional studies in both species.
In conclusion, in this study we have found that intradermal injection of both endogenous Ret ligand GDNF, and TrkA ligand NGF, present on distinct populations of nociceptors, both produce mechanical hyperalgesia. DOR agonist SNC but not MOR agonist DAMGO inhibits GDNF-induced hyperalgesia while both DAMGO and SNC both inhibit NGF hyperalgesia, even in rats pretreated with IB4-saporin. Co-administration of low doses of DAMGO and SNC produce marked analgesia, and repeatedly administered DAMGO produced cross-tolerance to the acute peripheral analgesic effect of SNC. These findings demonstrate that most nociceptors have a role in mechanical hyperalgesia, only the DOR agonist inhibits GDNF hyperalgesia mediated by IB4(+)/Ret(+) nociceptors, and MOR and DOR are co-expressed on a functionally important population of OB4(−)/TrkA(+) nociceptors. Since human DRG neurons do not bind IB4, the current use of NGF and GDNF to distinguish between physiologically important subpopulations of nociceptors, which have similar distribution to that in the rat (Wetmore and Olson, 1995, Josephson et al., 2001), may also allow more effective comparison of pain in preclinical models to pain syndromes in patients.
Acknowledgments
This research was funded by a grant from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- Aley KO, Green PG, Levine JD. Opioid and adenosine peripheral antinociception are subject to tolerance and withdrawal. J Neurosci. 1995;15:8031–8038. doi: 10.1523/JNEUROSCI.15-12-08031.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley KO, Levine JD. Different mechanisms mediate development and expression of tolerance and dependence for peripheral mu-opioid antinociception in rat. J Neurosci. 1997a;17:8018–8023. doi: 10.1523/JNEUROSCI.17-20-08018.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley KO, Levine JD. Dissociation of tolerance and dependence for opioid peripheral antinociception in rats. J Neurosci. 1997b;17:3907–3912. doi: 10.1523/JNEUROSCI.17-10-03907.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley KO, Levine JD. Multiple receptors involved in peripheral alpha 2, mu, and A1 antinociception, tolerance, and withdrawal. J Neurosci. 1997c;17:735–744. doi: 10.1523/JNEUROSCI.17-02-00735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asante CO, Wallace VC, Dickenson AH. Mammalian Target of Rapamycin Signaling in the Spinal Cord Is Required for Neuronal Plasticity and Behavioral Hypersensitivity Associated With Neuropathy in the Rat. J Pain. 2010 doi: 10.1016/j.jpain.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Woolf CJ. Pain. Curr Biol. 1999;9:R429–R431. doi: 10.1016/s0960-9822(99)80273-5. [DOI] [PubMed] [Google Scholar]
- Bennett DL, Michael GJ, Ramachandran N, Munson JB, Averill S, Yan Q, McMahon SB, Priestley JV. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besse D, Lombard MC, Zajac JM, Roques BP, Besson JM. Pre- and postsynaptic distribution of mu, delta and kappa opioid receptors in the superficial layers of the cervical dorsal horn of the rat spinal cord. Brain Res. 1990a;521:15–22. doi: 10.1016/0006-8993(90)91519-m. [DOI] [PubMed] [Google Scholar]
- Besse D, Lombard MC, Zajac JM, Roques BP, Besson JM. Pre- and postsynaptic location of mu, delta and kappa opioid receptors in the superficial layers of the dorsal horn of the rat spinal cord. Prog Clin Biol Res. 1990b;328:183–186. [PubMed] [Google Scholar]
- Bielefeldt K, Davis BM. Differential effects of ASIC3 and TRPV1 deletion on gastroesophageal sensation in mice. Am J Physiol Gastrointest Liver Physiol. 2008;294:G130–G138. doi: 10.1152/ajpgi.00388.2007. [DOI] [PubMed] [Google Scholar]
- Bogen O, Dina OA, Gear RW, Levine JD. Dependence of monocyte chemoattractant protein 1 induced hyperalgesia on the isolectin B4-binding protein versican. Neuroscience. 2009;159:780–786. doi: 10.1016/j.neuroscience.2008.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogen O, Joseph EK, Chen X, Levine JD. GDNF hyperalgesia is mediated by PLCgamma, MAPK/ERK, PI3K, CDK5 and Src family kinase signaling and dependent on the IB4-binding protein versican. Eur J Neurosci. 2008;28:12–19. doi: 10.1111/j.1460-9568.2008.06308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bráz JM, Basbaum AI. Triggering genetically-expressed transneuronal tracers by peripheral axotomy reveals convergent and segregated sensory neuron-spinal cord connectivity. Neuroscience. 2009;163:1220–1232. doi: 10.1016/j.neuroscience.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel "pain" pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47:787–793. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Shippenberg TS. Augmentation of morphine-induced sensitization but reduction in morphine tolerance and reward in delta-opioid receptor knockout mice. Neuropsychopharmacology. 2009;34:887–898. doi: 10.1038/npp.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels DJ, Lenard NR, Etienne CL, Law P-Y, Roerig SC, Portoghese PS. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc Natl Acad Sci U S A. 2005;102:19208–19213. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descalzi G, Kim S, Zhuo M. Presynaptic and postsynaptic cortical mechanisms of chronic pain. Mol Neurobiol. 2009;40:253–259. doi: 10.1007/s12035-009-8085-9. [DOI] [PubMed] [Google Scholar]
- Dubner R. The neurobiology of persistent pain and its clinical implications. Suppl Clin Neurophysiol. 2004;57:3–7. doi: 10.1016/s1567-424x(09)70337-x. [DOI] [PubMed] [Google Scholar]
- Eisenach JC. Treating and preventing chronic pain: a view from the spinal cord--Bonica Lecture, ASRA Annual Meeting, 2005. Reg Anesth Pain Med. 2006;31:146–151. doi: 10.1016/j.rapm.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Fan T, Varghese G, Nguyen T, Tse R, O'Dowd BF, George SR. A role for the distal carboxyl tails in generating the novel pharmacology and G protein activation profile of mu and delta opioid receptor hetero-oligomers. J Biol Chem. 2005;280:38478–38488. doi: 10.1074/jbc.M505644200. [DOI] [PubMed] [Google Scholar]
- Fang X, Djouhri L, McMullan S, Berry C, Waxman SG, Okuse K, Lawson SN. Intense isolectin-B4 binding in rat dorsal root ganglion neurons distinguishes C-fiber nociceptors with broad action potentials and high Nav1.9 expression. J Neurosci. 2006;26:7281–7292. doi: 10.1523/JNEUROSCI.1072-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallantine EL, Meert TF. A comparison of the antinociceptive and adverse effects of the mu-opioid agonist morphine and the delta-opioid agonist SNC80. Basic Clin Pharmacol Toxicol. 2005;97:39–51. doi: 10.1111/j.1742-7843.2005.pto_97107.x. [DOI] [PubMed] [Google Scholar]
- Gerke MB, Plenderleith MB. Ultrastructural analysis of the central terminals of primary sensory neurones labelled by transganglionic transport of bandeiraea simplicifolia I-isolectin B4. Neuroscience. 2004;127:165–175. doi: 10.1016/j.neuroscience.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Gevaert T, Vandepitte J, Hutchings G, Vriens J, Nilius B, De Ridder D. TRPV1 is involved in stretch-evoked contractile changes in the rat autonomous bladder model: a study with piperine, a new TRPV1 agonist. Neurourol Urodyn. 2007;26:440–450. doi: 10.1002/nau.20343. discussion 451-443. [DOI] [PubMed] [Google Scholar]
- Gillberg PG, Askmark H. Changes in cholinergic and opioid receptors in the rat spinal cord, dorsal root and sciatic nerve after ventral and dorsal root lesion. J Neural Transm Gen Sect. 1991;85:31–39. doi: 10.1007/BF01244655. [DOI] [PubMed] [Google Scholar]
- Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci U S A. 2004;101:5135–5139. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55:365–376. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Ji RR, Zhang Q, Law PY, Low HH, Elde R, Hökfelt T. Expression of mu-, delta-, and kappa-opioid receptor-like immunoreactivities in rat dorsal root ganglia after carrageenan-induced inflammation. J Neurosci. 1995;15:8156–8166. doi: 10.1523/JNEUROSCI.15-12-08156.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan BA, Trapaidze N, Gomes I, Nivarthi R, Devi LA. Oligomerization of opioid receptors with beta 2-adrenergic receptors: a role in trafficking and mitogen-activated protein kinase activation. Proc Natl Acad Sci U S A. 2001;98:343–348. doi: 10.1073/pnas.011384898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Bogen O, Alessandri-Haber N, Levine JD. PLC-beta 3 signals upstream of PKC epsilon in acute and chronic inflammatory hyperalgesia. Pain. 2007;132:67–73. doi: 10.1016/j.pain.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Chen X, Bogen O, Levine JD. Oxaliplatin acts on IB4-positive nociceptors to induce an oxidative stress-dependent acute painful peripheral neuropathy. J Pain. 2008;9:463–472. doi: 10.1016/j.jpain.2008.01.335. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Hyperalgesic priming is restricted to isolectin B4-positive nociceptors. Neuroscience. 2010;169:431–435. doi: 10.1016/j.neuroscience.2010.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson A, Widenfalk J, Trifunovski A, Widmer HR, Olson L, Spenger C. GDNF and NGF family members and receptors in human fetal and adult spinal cord and dorsal root ganglia. J Comp Neurol. 2001;440:204–217. doi: 10.1002/cne.1380. [DOI] [PubMed] [Google Scholar]
- Kashiba H, Hyon B, Senba E. Glial cell line-derived neurotrophic factor and nerve growth factor receptor mRNAs are expressed in distinct subgroups of dorsal root ganglion neurons and are differentially regulated by peripheral axotomy in the rat. Neurosci Lett. 1998;252:107–110. doi: 10.1016/s0304-3940(98)00558-8. [DOI] [PubMed] [Google Scholar]
- Kashiba H, Uchida Y, Senba E. Difference in binding by isolectin B4 to trkA and c-ret mRNA-expressing neurons in rat sensory ganglia. Brain Res Mol Brain Res. 2001;95:18–26. doi: 10.1016/s0169-328x(01)00224-8. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Green PG, Levine JD. Comparison of intradermal and subcutaneous hyperalgesic effects of inflammatory mediators in the rat. Neurosci Lett. 1993;153:215–218. doi: 10.1016/0304-3940(93)90325-f. [DOI] [PubMed] [Google Scholar]
- Kitchener PD, Wilson P, Snow PJ. Selective labelling of primary sensory afferent terminals in lamina II of the dorsal horn by injection of Bandeiraea simplicifolia isolectin B4 into peripheral nerves. Neuroscience. 1993;54:545–551. doi: 10.1016/0306-4522(93)90274-j. [DOI] [PubMed] [Google Scholar]
- Kline RH, 4th, Wiley RG. Spinal mu-opioid receptor-expressing dorsal horn neurons: role in nociception and morphine antinociception. J Neurosci. 2008;28:904–913. doi: 10.1523/JNEUROSCI.4452-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NM, Leybin L, Chang JK, Loh HH. Opiate and peptide interaction: effect of enkephalins on morphine analgesia. Eur J Pharmacol. 1980;68:181–185. doi: 10.1016/0014-2999(80)90319-2. [DOI] [PubMed] [Google Scholar]
- Liedtke W. Role of TRPV ion channels in sensory transduction of osmotic stimuli in mammals. Exp Physiol. 2007a;92:507–512. doi: 10.1113/expphysiol.2006.035642. [DOI] [PubMed] [Google Scholar]
- Liedtke W. TRPV channels' role in osmotransduction and mechanotransduction. Handb Exp Pharmacol. 2007b:473–487. doi: 10.1007/978-3-540-34891-7_28. [DOI] [PubMed] [Google Scholar]
- Malik-Hall M, Dina OA, Levine JD. Primary afferent nociceptor mechanisms mediating NGF-induced mechanical hyperalgesia. Eur J Neurosci. 2005;21:3387–3394. doi: 10.1111/j.1460-9568.2005.04173.x. [DOI] [PubMed] [Google Scholar]
- May A. Chronic pain may change the structure of the brain. Pain. 2008;137:7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Radeke MJ, Feinstein SC, Snider WD. Presence or absence of TrkA protein distinguishes subsets of small sensory neurons with unique cytochemical characteristics and dorsal horn projections. J Comp Neurol. 1995;361:404–416. doi: 10.1002/cne.903610305. [DOI] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Aibak H, Rymar VV, Pradhan A, Hoffert C, Mennicken F, Stroh T, Sadikot AF, O'Donnell D, Clarke PBS, Collier B, Henry JL, Vincent J-P, Beaudet A. Morphine-induced changes in delta opioid receptor trafficking are linked to somatosensory processing in the rat spinal cord. J Neurosci. 2004;24:5549–5559. doi: 10.1523/JNEUROSCI.2719-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche JF, Schuller AGP, King MA, Zengh M, Pasternak GW, Pintar JE. Genetic dissociation of opiate tolerance and physical dependence in delta-opioid receptor-1 and preproenkephalin knock-out mice. J Neurosci. 2002;22:10906–10913. doi: 10.1523/JNEUROSCI.22-24-10906.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SF, Nilius B. Transient receptor potential channels in mechanosensing and cell volume regulation. Methods Enzymol. 2007;428:183–207. doi: 10.1016/S0076-6879(07)28010-3. [DOI] [PubMed] [Google Scholar]
- Price TJ, Flores CM. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J Pain. 2007;8:263–272. doi: 10.1016/j.jpain.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau KK, Caudle RM, Cooper BY, Johnson RD. Diverse immunocytochemical expression of opioid receptors in electrophysiologically defined cells of rat dorsal root ganglia. J Chem Neuroanat. 2005;29:255–264. doi: 10.1016/j.jchemneu.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Renganathan M, Gelderblom M, Black JA, Waxman SG. Expression of Nav1.8 sodium channels perturbs the firing patterns of cerebellar Purkinje cells. Brain Res. 2003;959:235–242. doi: 10.1016/s0006-8993(02)03750-2. [DOI] [PubMed] [Google Scholar]
- Salter MW. Cellular signalling pathways of spinal pain neuroplasticity as targets for analgesic development. Curr Top Med Chem. 2005;5:557–567. doi: 10.2174/1568026054367638. [DOI] [PubMed] [Google Scholar]
- Sangameswaran L, Delgado SG, Fish LM, Koch BD, Jakeman LB, Stewart GR, Sze P, Hunter JC, Eglen RM, Herman RC. Structure and function of a novel voltage-gated, tetrodotoxin-resistant sodium channel specific to sensory neurons. J Biol Chem. 1996;271:5953–5956. doi: 10.1074/jbc.271.11.5953. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao Y-Q, Contet C, Mennicken F, O'Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PW, Fundytus ME, Merovitz L, Weltrowska G, Nguyen TM, Lemieux C, Chung NN, Coderre TJ. The opioid mu agonist/delta antagonist DIPP-NH(2)[Psi] produces a potent analgesic effect, no physical dependence, and less tolerance than morphine in rats. J Med Chem. 1999;42:3520–3526. doi: 10.1021/jm980724+. [DOI] [PubMed] [Google Scholar]
- Seifert F, Maihöfner C. Central mechanisms of experimental and chronic neuropathic pain: findings from functional imaging studies. Cell Mol Life Sci. 2009;66:375–390. doi: 10.1007/s00018-008-8428-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif Naeini R, Cahill CM, Ribeiro-da-Silva A, Ménard HA, Henry JL. Remodelling of spinal nociceptive mechanisms in an animal model of monoarthritis. Eur J Neurosci. 2005;22:2005–2015. doi: 10.1111/j.1460-9568.2005.04382.x. [DOI] [PubMed] [Google Scholar]
- Standifer KM, Chien CC, Wahlestedt C, Brown GP, Pasternak GW. Selective loss of delta opioid analgesia and binding by antisense oligodeoxynucleotides to a delta opioid receptor. Neuron. 1994;12:805–810. doi: 10.1016/0896-6273(94)90333-6. [DOI] [PubMed] [Google Scholar]
- Stein C, Zöllner C. Opioids and sensory nerves. Handb Exp Pharmacol. 2009:495–518. doi: 10.1007/978-3-540-79090-7_14. [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Goetzl EJ, Levine JD. Hyperalgesia onset latency suggests a hierarchy of action. Brain Res. 1987;423:333–337. doi: 10.1016/0006-8993(87)90858-4. [DOI] [PubMed] [Google Scholar]
- Tarpley JW, Kohler MG, Martin WJ. The behavioral and neuroanatomical effects of IB4-saporin treatment in rat models of nociceptive and neuropathic pain. Brain Res. 2004;1029:65–76. doi: 10.1016/j.brainres.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Toyoda H, Zhao M-G, Ulzhöfer B, Wu L-J, Xu H, Seeburg PH, Sprengel R, Kuner R, Zhuo M. Roles of the AMPA receptor subunit GluA1 but not GluA2 in synaptic potentiation and activation of ERK in the anterior cingulate cortex. Mol Pain. 2009;5:46. doi: 10.1186/1744-8069-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulchanova L, Olson TH, Stone LS, Riedl MS, Elde R, Honda CN. Cytotoxic targeting of isolectin IB4-binding sensory neurons. Neuroscience. 2001;108:143–155. doi: 10.1016/s0306-4522(01)00377-3. [DOI] [PubMed] [Google Scholar]
- Walwyn W, Evans CJ, Hales TG. Beta-arrestin2 and c-Src regulate the constitutive activity and recycling of mu opioid receptors in dorsal root ganglion neurons. J Neurosci. 2007;27:5092–5104. doi: 10.1523/JNEUROSCI.1157-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walwyn W, John S, Maga M, Evans CJ, Hales TG. Delta receptors are required for full inhibitory coupling of mu-receptors to voltage-dependent Ca(2+) channels in dorsal root ganglion neurons. Mol Pharmacol. 2009;76:134–143. doi: 10.1124/mol.109.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H-B, Zhao B, Zhong Y-Q, Li K-C, Li Z-Y, Wang Q, Lu Y-J, Zhang Z-N, He S-Q, Zheng H-C, Wu S-X, Hökfelt TGM, Bao L, Zhang X. Coexpression of {delta}-and {micro}-opioid receptors in nociceptive sensory neurons. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1008382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmore C, Olson L. Neuronal and nonneuronal expression of neurotrophins and their receptors in sensory and sympathetic ganglia suggest new intercellular trophic interactions. J Comp Neurol. 1995;353:143–159. doi: 10.1002/cne.903530113. [DOI] [PubMed] [Google Scholar]
- Xie W-Y, He Y, Yang Y-R, Li Y-F, Kang K, Xing B-M, Wang Y. Disruption of Cdk5-associated phosphorylation of residue threonine-161 of the delta-opioid receptor: impaired receptor function and attenuated morphine antinociceptive tolerance. J Neurosci. 2009;29:3551–3564. doi: 10.1523/JNEUROSCI.0415-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Kuebler WM. Mechanotransduction by TRP channels: general concepts and specific role in the vasculature. Cell Biochem Biophys. 2010;56:1–18. doi: 10.1007/s12013-009-9067-2. [DOI] [PubMed] [Google Scholar]
- Zajac JM, Lombard MC, Peschanski M, Besson JM, Roques BP. Autoradiographic study of mu and delta opioid binding sites and neutral endopeptidase-24.11 in rat after dorsal root rhizotomy. Brain Res. 1989;477:400–403. doi: 10.1016/0006-8993(89)91436-4. [DOI] [PubMed] [Google Scholar]
- Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Elde RP, Unterwald E, Pasternak GW, Pintar JE. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]