Abstract
The chemokine receptor CXCR4 and the μ-opioid receptor (MOR) are G-protein–coupled receptors (GPCRs) essential to normal function of the nervous and immune systems. Several studies suggest that MOR is a key regulator of CXCR4 in the brain; however, the molecular basis of the opioid/chemokine interaction are not fully understood and may involve different mechanisms in neuronal and glial cells. Our previous studies demonstrated that MOR stimulation specifically up-regulates the protein ferritin heavy chain (FHC) - an inhibitor of CXCR4 - in neurons and suggested additional mechanisms could be operative in glial cells. In this study, we investigated CXCR4 function in brains and astroglia cultures deprived of MOR. Reduced coupling of CXCR4 to G proteins was found in brain slices and tissue homogenates of MOR−/− mice compared to wild type (WT) controls. CXCR4-induced signaling was also reduced in glial cultures from MOR−/− mice, as shown by analysis of CXCR4 downstream targets (Akt and ERK1/2). Pharmacological studies with δ-opioid receptor (DOR)-specific ligands suggested that DOR-CXCR4 interactions are implicated in the inhibition of CXCR4 in MOR-deficient cells both in vitro and in vivo. Moreover, increased CXCR4/DOR co-immunoprecipitation was found in brain tissue and cultured glia from MOR−/− mice. Importantly, CXCR4 function was restored by pretreatment with a DOR antagonist. Overall, these findings indicate that DOR play a crucial role in the regulation of CXCR4 in glia, likely via silent receptor heterodimers. The data also suggest that the opiate system interferes with normal CXCR4 function in different ways and depending on receptor subtypes.
Keywords: opioid, chemokine, G-protein coupled receptor, CXCR4, glia
INTRODUCTION
Chemokines play important roles in the central nervous system (CNS) under both healthy and pathological conditions. CXCL12 (a.k.a. SDF-1α) is one of the best-characterized chemokines in the CNS, involved in development and regulation of essential neuronal/glial functions, including neurotransmission, neuron-glia communication, and cell survival (Lazarini et al 2003; Li & Ransohoff 2008). The effects of CXCL12 in the brain are mainly mediated by its specific receptor, CXCR4, as demonstrated by studies using CXCR4 or CXCL12 knockout animals (Ma et al 1998; Zou et al 1998). The recently de-orphanized receptor CXCR7 can modulate CXCL12 function (Levoye et al 2009; Zabel et al 2009), but evidence about its signaling role in the CNS is scanty. CXCL12/CXCR4 signaling in neurons promotes their survival and protects them from excitotoxic stimuli, such as overactivation of glutamate receptors and exposure to HIV-1 proteins (Cook et al 2010; Meucci et al 1998; Nicolai et al 2010). However, neurotoxic actions of CXCR4 have also been reported and usually involve glia (Cali’ et al 2010). Thus, it is important to identify specific pathways that regulate CXCR4 in different CNS cells.
Opiates can alter the effects of chemokines in the brain via complex interactions that involve effects on all major cell types (i.e. neurons, astroglia, microglia) or cell-to-cell communication (Bell et al 2002; Donahoe & Vlahov 1998; Hauser et al 2005; Hauser et al 2006). Opioid and chemokine receptors are widely expressed in the CNS and immune system (Burbassi et al 2008; Hauser et al 2006; Sharp 2006). As other G-protein-coupled receptors (GPCRs), these receptors may form dimers, resulting in the modulation of their function (Devi 2001). For instance, μ-opioid receptors (MOR) form heterodimers with δ-opioid receptor (DOR), creating a functional unit distinct from MOR or DOR monomer/homodimer (Decaillot et al 2008; Rozenfeld & Devi 2007). Moreover, studies in immune cells suggest that CXCR4 can form silent heterodimers with DORs (Pello et al 2008). However, the clinical relevance of these interactions is still unclear due to paucity of studies investigating the behavior of endogenous receptors and to the intrinsic difficulty of addressing this issue in vivo.
We recently found that prolonged exposure of neurons to MOR agonists (i.e. morphine, endomorphin, or DAMGO) inhibits CXCR4 pro-survival signaling both in vitro and in vivo (Patel et al 2006; Sengupta et al 2009). This inhibition is due to specific increase in intracellular levels of the protein ferritin heavy chain (FHC) - a negative regulator of CXCR4 (Li et al 2006; Sengupta et al 2009). Interestingly, MOR stimulation did not alter FHC in glia (Sengupta et al 2009), suggesting that opiate-mediated regulation of CXCR4 may be cell-specific. To further characterize the interaction between endogenous CXCR4 and MOR, we used biochemical and pharmacological approaches to study CXCL12/CXCR4 signaling in brain and astroglia cultures from MOR-deficient mice. The results show that lack of MOR promotes a physical association of endogenous CXCR4 and DOR in glial cells, which ultimately results in reduced CXCL12 signaling. Thus, changes in MOR expression can alter the CXCL12/CXCR4 axis via multiple mechanisms.
MATERIALS AND METHODS
Animals
MOR−/− mice (C57BL/6 × 129/Ola, Harlan Laboratories, Frederick, MD, USA) and their wild-type (WT) littermates (The Jackson Laboratories, Sacramento, CA, USA) were used in this study (Loh et al 1998). All experiments were performed according to the guidelines for animal experimentation of the National Institutes of Health (United States). All procedures were approved by the Drexel University Animal Research Committee for handling experimental animals.
[35S]GTPγS incorporation in Brain Homogenates
The effect of opioid receptors or CXCR4 activation in cortex/hippocampus was determined using DAMGO (0.01–10 μM, Bachem Bioscience, King of Prussia, PA, USA), SNC80 (1–10 μM), naltrindole hydrochloride (1 μM, both from Tocris Bioscience, Ellisville, MO, USA), and CXCL12 (0.01–10 nM, PeproTech, Inc., Rocky Hill, NJ, USA). [35S]GTPγS autoradiography procedure was based on methods reported previously (Burbassi et al 2008). Briefly, animals were sacrificed and the brain removed; cortex and hippocampus were dissected and immediately frozen at −80°C. The tissue was homogenized to give a final concentration of 1 mg tissue/ml in 50 mM Tris–HCl buffer (pH 7.4) at room temperature. [35S]GTPγS “binding” (i.e. incorporation) was determined by incubating washed membranes for 45–60 min at 30°C in a total volume of 1 ml of 50 mM Tris buffer, with or without the indicated drugs. Nonspecific binding was defined by the addition of excess unlabeled GTPγS (10 μM, final concentration). Furthermore, 1 μM DPCPX (Tocris Bioscience) was added to each experimental group as reported previously (Burbassi et al 2008). The reaction was terminated by rapid filtration through Whatman GF/B filters, using a Brandel Cell Harvester (Brandel, Gaithersburg, MD, USA); the filters were washed (3 × 5 ml) with ice-cold 20 mM Tris–HCl buffer (pH 7.4); the radioactivity retained on the filters was counted by liquid scintillation spectrophotometry. Results were mean ± SEM of three to six independent experiments performed in duplicate.
In Vivo Treatments and in Situ [35S]GTPγS Autoradiography
MOR−/− mice and WT littermates in their second postnatal week (both genders) were used in this study. Morphine sulfate (20mg/kg, Sigma-Aldrich, St. Louis, MO, USA) and naltrindole hydrochloride (1mg/kg, Tocris Bioscience) were dissolved in physiological saline. All drugs were subcutaneously injected in a volume of 1 ml/kg. Mice were euthanized at indicated times after injection of the drugs. The brain was rapidly removed, frozen, and stored at −80°C. The procedure for [35S]GTPγS autoradiography was based on established methods (Burbassi et al 2008). Briefly, 20-μm frozen coronal sections were thaw-mounted onto slides subbed with chrome-aluminum. Slides were preincubated in mailers for 40 min at 25°C in assay buffer (Tris–HCl, 50 mM; MgCl2, 4 mM; EGTA, 0.3 mM; NaCl, 100 mM; pH 7.4) followed by 20 min incubation in 2 mM guanosine diphosphate (GDP) assay buffer. Sections were then incubated for 2 hr at 25°C in assay buffer containing both [35S]GTPγS (0.04 nM, 1250 Ci mmol) and 2 mM GDP. Each mailer contained vehicle (basal condition), 50 nM CXCL12 (Peprotech), 1 μM DAMGO (Bachem Bioscence), 1 μM SNC80 (Tocris Bioscience), or unlabeled GTPγS (10 μM, nonspecific binding). After incubation, slides were rinsed twice in cold Tris–HCl (50 mM, pH 7.4), then briefly in cold deionized water, dried immediately, and desiccated overnight. Slides were apposed to Kodak Biomax MS film (Eastman Kodak Company, Rochester, NY, USA) for 48 hr. Images from the developed films were scanned and quantified using Image Pro-Plus Version 4.5 software (MediaCybernetics, Bethesda, MD, USA).
Cell Cultures
Primary glial cells were harvested from brain cortex of 2–4 days old mice and cultured as previously described (Meucci & Miller 1996; Shimizu et al 2007) in 75-cm2 flasks in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal calf serum and 50 μg/ml gentamicin. On day 14 in vitro (DIV) cells were harvested, and secondary glia, mainly astroglia cells (>95%, as determined by GFAP staining) were plated on 60-mm culture dishes (0.5 × 106/per plate). Unless otherwise specified, cell culture products were purchased from Invitrogen (Carlsbad, CA, USA).
Western Blots Analysis
a) Whole cell lysates: Cell lysates were prepared following drug treatment as described previously (Sengupta et al 2009). Briefly, secondary glia was washed with ice-cold phosphate-buffered saline (PBS), collected in lysis buffer (150 mM NaCl; 50 mM Tris; 0.5% sodium deoxycholate; 0.1% SDS; 10 mM Na4P2O7; 5 mM EDTA; 1% Triton X; and 1% protease and phosphatase inhibitor cocktail), and then incubated on ice, for 30 min. Lysates were spun and protein concentration of the supernatants was determined using the bicinchoninic acid protein assay according to manufacturer’s instructions (Pierce, Thermo Scientific, Rockford, IL, USA). Equal amounts of protein (40 μg/lane) were loaded for SDS-PAGE followed by immunoblotting. b) Tissue Homogenates: Mouse brain hippocampi/cortices were washed once with ice-cold PBS and incubated in TNN buffer (50mM Tris/HCl pH 7.4′ 50mM Hepes; 150mM NaCl; 1%Triton; 1.5mM MgCl2; 1% protease and phosphatase inhibitor cocktail) on ice for 10 min followed by centrifugation. Protein concentration of the supernatant was measured by bicinchoninic acid. We used 40μg/lane of protein for SDS-PAGE followed by immunoblotting. c) Cell Surface Protein Isolation: The Cell Surface Protein Isolation Kit (Pierce, Thermo Scientific, USA) was used according to manufacturer’s protocol as we previously described (Sengupta et al 2009). A sample of the initial cell lysate was also retained for analysis of total proteins. Proteins were eluted from the column and loaded for SDS-PAGE, followed by immunoblotting.
The following antibodies were used: anti-CXCR4 [H-118 and G-19, raised against amino acids 176 to 293 and N-terminus, respectively (Abcam, Cambridge, MA, USA) (1:1000)] and anti FHC (Y-16) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) (1:500); anti-Akt, anti-phosphoAkt (Ser473), anti-ERK, and anti-phosphoERK (Cell Signaling, Boston, MA, USA) (1:2000); anti-β-actin (Sigma-Aldrich, 1:5000); anti-MOR (Santa Cruz Biotecnology) (1:1000); anti-DOR (residues L(3)VPSARAELQSSPLV(17), ABR Affinity BioReagents, Golden, CA, USA) (1:1000); and anti-KOR (residues S(262)REKDRNLRRITKL(275), ABR Affinity BioReagents) (1:1000). An image acquisition- (Alpha Innotech, San Leandro, CA, USA) and software-analysis system (UN-SCAN IT software, Silk Scientific, Orem, UT, USA) was used for detection of chemiluminescent bands and densitometric analysis. Values from actin, Akt, ERK, or total CXCR4 bands were used to verify equal protein loading and/or normalization as reported in the text. All experiments were repeated at least three times.
RNA Extraction and Reverse Transcription PCR (RT-PCR)
Total RNA was isolated from mice brain tissue (cortex/hippocampus) using TRIzol Reagent (Invitrogen) and purified using an RNeasy midi kit (Qiagen, Valencia, CA, USA) as reported previously (Sengupta et al 2009; Shimizu et al 2007). Total RNA was reverse transcribed to cDNA. cDNA was amplified by PCR for 35 cycles using specific primers for mouse CXCR4 (For: GGT CTG GAG ACT ATG ACT CC; Rev: CAC AGA TGT ACC TGT CAT CC), MOR (For: GTG TGT AGT GGG CCT CTT TGG; Rev: TCT ATG GAC CCC TGC CTG TATT), DOR (For: CCG GTA CAC CAA ATT GAA GACC; Rev: AAC TGG AGC ATG CAT ACC ACTG), KOR (For: TGT TTG TCA TCA TCC GAT ACA CG; Rev: AAA CTG CAA GGA GCA TTC AATG). Products were run on a 3% agarose gel and visualized under ultraviolet light using ethidium bromide. Samples in which no reverse transcriptase (no RT) was added were used as negative control. Values from the housekeeping gene β-actin (For: TGG AAT CCT GTG GCA TCC ATG AAAC; Rev: TAA AAC GCA GCT CAG TAA CAG TCCG) were used to verify equal mRNA loading.
Quantitative PCR (qPCR)
Total RNA was extracted from brains (cortex/hippocampus), and cDNA was synthesized as mentioned previously. TaqMan primer setsfor the amplification of MOR, DOR, KOR, and CXCR4 (Applied Biosystems, Foster City, CA, USA #Mm01188089_m1, Mm00443063_m1; Mm00440561_m1, Mm99999055_m1, respectively) and mouse β-actin binding protein (Applied Biosystems, Mm00607939_m1) cDNAs as internal standard were used. Amplification was carried out on an ABI Prism 7300 Real-Time PCR System (Applied Biosystems Inc.) using TaqMan Universal PCR Master Mix (Applied Biosystems Inc.) according to manufacturer’s instructions. Reactions were carried out in optical 96-well fast-reaction plates (Applied Biosystems Inc.) with 20μl reaction volume containing 50 ng of reverse-transcribed mRNA. A standard curve for each gene was generated to determine the linear range and amplification efficiency of each sample. The ΔΔCT method was used to analyze the data. Level of each gene was expressed relative to fold change after normalization to that of the housekeeping gene β-actin. Real-time PCR quantifications were performed in quadruplicate from six independent experiments.
Immunoprecipitation Assay
500 μg of total cell lysate or homogenized tissue was transferred to a 1.5-ml microfuge tube on ice and precleared using the rabbit ExactaCruz FLIP matrix (Santa Cruz Biotechnology) for 30 min at 4°C. An immunoprecipitation (IP) antibody–IP matrix complex was prepared according to manufacturer’s instructions using primary anti-CXCR4 or anti-DOR antibody as we previously reported (Sengupta et al 2009). Samples were boiled for 2–3 min and resolved on a 10% polyacrylamide gel.
Statistical Analysis
Data are reported as mean ± SEM and total number of experiments (performed at least three times). Statistical analysis was performed by one-way analysis of variance followed by Newman Keuls post-test or paired/unpaired Student t test. P and F values are reported in the figure legends.
RESULTS
CXCR4 Coupling To G Proteins Is Reduced in the Brain of MOR−/− Mice
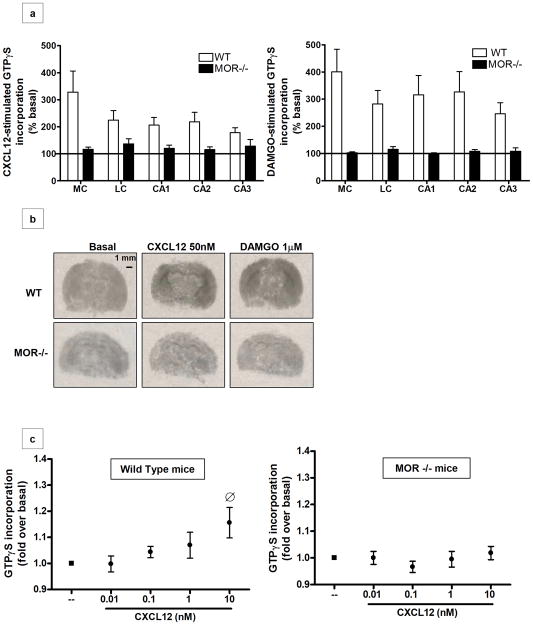
To establish whether the physical absence of MORs alters coupling of CXCR4 to G proteins, CXCL12-induced G-protein activation was measured using in situ GTPγS autoradiography as previously reported (Burbassi et al 2008). This assay assesses receptor activation by measuring guanine nucleotide exchange, an early event in GPCR-mediated signaling (Sim et al 1996). As reported in Figure 1a (left graph), CXCL12-induced GTPγS incorporation was abolished in MOR−/− brain slices; the CXCR4 impairment was observed in different brain areas, including the medial and lateral cortex and the hippocampus (CA1, CA2, and CA3). Furthermore, as expected, G-protein stimulation by the potent and selective MOR agonist DAMGO was also inhibited in these mice (Figure 1a, right graph). Representative images for CXCL12 and DAMGO stimulation in brain slices of WT and MOR−/− animals are presented in Figure 1b, indicating a loss in normal CXCR4 function associated with the lack of MOR in vivo. These data were confirmed by parallel studies in brain homogenates of MOR−/− and WT mice pups (P2-P11) testing various concentrations of CXCL12 (0.01–10nM) or DAMGO (0.01–10 μM). In line with the previous data, no GTPγS incorporation was induced by these ligands in cortical/hippocampal membranes derived from MOR deficient mice (Figure 1c, right panel); a response to CXCL12 was observed only in the samples from WT animals, reaching the maximum stimulation at 10 nM (Figure 1c, left panel). DAMGO increased GTPγS incorporation in WT-derived tissue but had no effect in the MOR−/− mice, confirming the absence of functional MOR in these animals (Supplementary Figure S1).
Figure 1. CXCR4 coupling to G proteins is reduced in the brains of MOR−/− animals.
(a) Coronal brain sections of wild type and MOR−/− mice were treated with CXCL12 (50nM) or DAMGO (1μM) and processed for GTPγS autoradiography. Analysis was performed in different areas of the brain: the medial cortex (MC), lateral cortex (LC), and hippocampus (fields CA1, CA2 and CA3). Data are expressed as mean ± SEM of n=3 animals per group. A statistically significant difference was observed in MOR−/− compared to WT brain sections in all of these areas (CXCL12-induced GTPγS incorporation: t8=4.159, P=0.0032; DAMGO-induced GTPγS incorporation: t8= 7.984, P < 0.0001). (b) Representative autoradiographs of GTPγS incorporation after vehicle-(i.e. basal), CXCL12- (center panels), and DAMGO- (right panels) stimulation of WT and MOR−/− pups are shown. Scale bar, 1mm. (c) Brain homogenates (cortices and hippocampi) of P2-P11 WT (left panel) and MOR−/− (right panel) pups were exposed to different doses of CXCL12, as indicated. Graphs represent the mean ratio of agonist-stimulated GTPγS binding over basal (n=5) ± SEM. [F4, 95= 2.876, O P > 0.05 versus basal].
Lack of MOR Impairs CXCL12/CXCR4 signaling without changing FHC levels
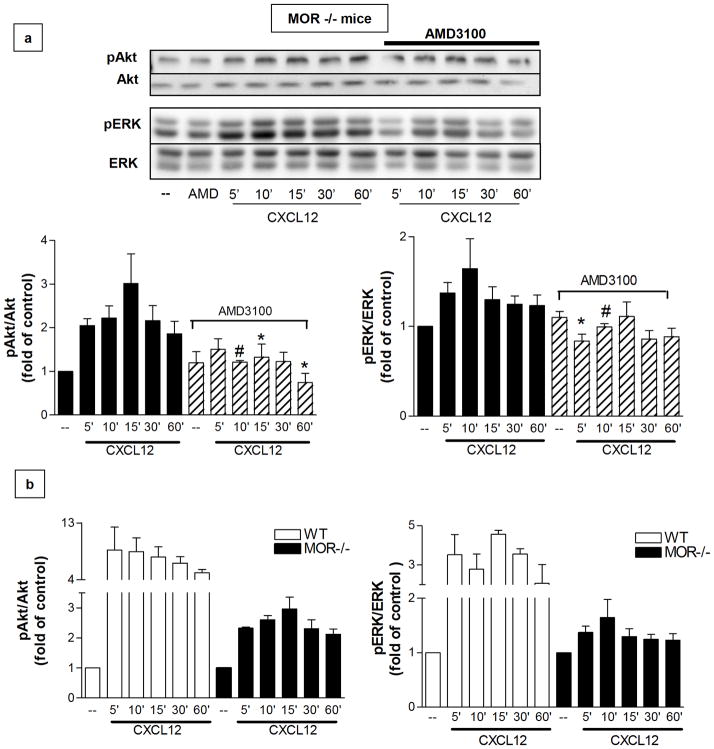
As previously mentioned, prolonged stimulation of MOR increases intracellular levels of the protein FHC in rat neurons, thus inhibiting CXCR4 signaling (Li et al 2006; Sengupta et al 2009). However, DAMGO treatment did not affect FHC protein levels in glial cells (Sengupta et al 2009), which also express functional MOR and CXCR4 and are a major target of opiates in the CNS (Hauser et al 2007). To investigate the mechanisms involved in opioid regulation of glial CXCR4, we compared CXCL12/CXCR4 signaling in glial cultures from MOR−/− and WT mice. CXCL12 treatment induced only a modest and delayed increase in Akt and ERK1/2 phosphorylation in cultures from MOR-deficient animals (Figure 2a). Compared to the level of Akt and ERK1/2 activation in WT cultures, CXCL12-induced phosphorylation was reduced by at least threefold in MOR−/− glia (Figure 2b). The CXCR4 antagonist AMD3100 blocked CXCL12 effects, confirming the role of CXCR4 in mediating CXCL12-induced responses in MOR-deficient cells (Figure 2a).
Figure 2. Lack of MOR affects the kinetics and magnitude of CXCR4-mediated ERK1/2 and Akt phosphorylation.
(a) Effect of 20nM CXCL12 for indicated amounts of time on Akt and ERK phosphorylation in MOR−/− glial cultures as determined by immunoblotting for pAkt and pERK. Membranes were stripped and reprobed for Akt/ERK to confirm equal loading. Pretreatment with the CXCR4 antagonist AMD3100 (100nM, 15 min) abolishes CXCL12-induced phosphorylation. The bar graphs represent the mean ± SEM of pAkt or pERK densities normalized to total protein levels and represented with respect to control levels from four independent identical experiments. (pAkt/Akt: F11, 408= 4.333, n=4, *P < 0.05; #P < 0.01 vs respective CXCL12 alone; pERK/ERK: F11, 408= 3.113, n=4, *P < 0.05; #P < 0.01 vs respective CXCL12 alone). (b) WT and MOR−/− glial cultures were treated with CXCL12 (20 nM) for the indicated times and processed for pAkt/Akt (left) or pERK/ERK (right) immunoblotting as reported above. The bar graphs represent the mean ± SEM of pAkt or pERK densities normalized to total protein levels and represented with respect to control levels from three independent identical experiments. In both graphs and for every treatment, CXCL12-induced phosphorylation is significantly higher in WT cultures than in MOR−/− cultures (pAkt/Akt: F11,408= 6.008, p<0.0001; pERK/ERK: F11,408= 6.110, p<0.0001).
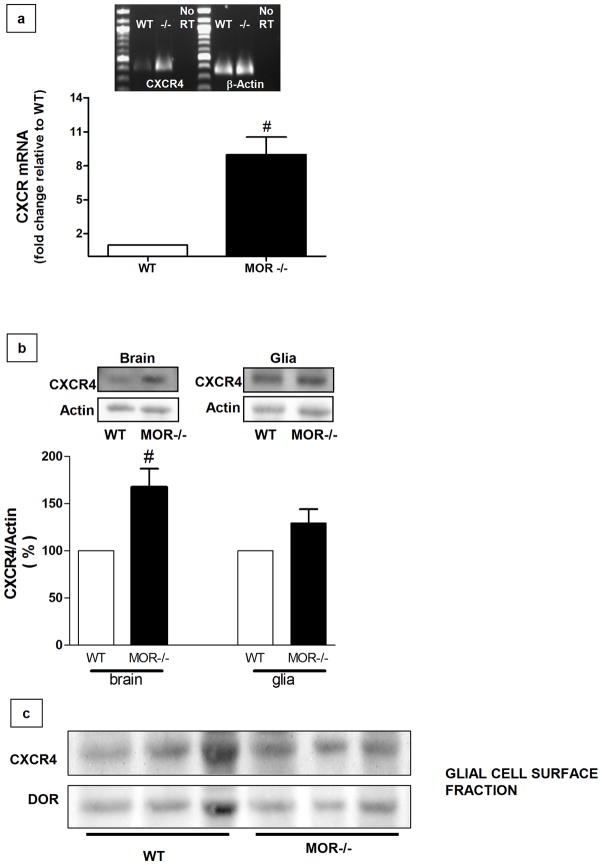
To evaluate the possibility that CXCR4 impairment in MOR-deficient cells was due to a decrease in receptor expression, we analyzed CXCR4 protein/mRNA levels in brain homogenates and glial cultures from WT and MOR−/− mice. Though an overall increase in CXCR4 mRNA (figure 3a) and protein (figure 3b) was found in brain homogenates from MOR−/− mice as compared to wild type, CXCR4 protein levels were not affected in MOR-deficient cortical glia (figure 3b). This suggests that presence of MOR may control expression of CXCR4 in specific brain cells population. Furthermore, surface expression of CXCR4 was comparable in wild type and MOR−/− glia cultures (figure 3c). Overall, these data show that CXCR4 impairment in MOR−/− tissue is not merely caused by diminished CXCR4 protein. Moreover, the different results from brain tissue and cell cultures suggest that regulation of CXCR4 by MOR is cell (and/or region) specific.
Figure 3. Impairment of CXCR4 activity is not due to a reduction in its expression in MOR−/− mice.
(a) Expression of the mRNA encoding for CXCR4 was examined via RT-PCR and quantitative PCR in brains (cortices + hippocampus) from WT and MOR−/− mice (n=6 animals per group). The graph shows relative changes in CXCR4 expression as assessed by qPCR (t10= 5.018, ≠ P =0.0003 versus WT, Student’s t-test). All values were normalized to the housekeeping gene β-actin. The image shows representative results from RT- PCR analysis (n=4). (b) CXCR4 protein levels in WT and MOR−/− brains (left) and glial cultures (right). Brain extracts and whole-cell extracts were run on 10% SDS-PAGE, followed by immunoblotting using a CXCR4-specific antibody. Membranes were stripped and reprobed for actin to confirm equal loading. The bar graphs represent CXCR4 band density (mean ± SEM), represented with respect to WT levels (t4= 3.443, ≠ P = 0.0131, Student’s t-test, n=3). (c) Immunoblots of cell-surface proteins showing no significant differences between the levels of CXCR4 and DOR in WT and MOR−/− glial cells collected from three separate experiments (each lane contains extracts from a different WT or MOR−/− cell culture).
Finally, to determine whether stimulation of MOR modulates levels of FHC in mice as we previously observed in rat (Sengupta et al 2009), MOR−/− and WT mice were treated with morphine (single injection, 20 mg/kg subcutaneously) and sacrificed after 30 min or 6hr. In agreement with our observations in rat brain, morphine treatment led to a significant increase in FHC levels in WT mice only in the 6hr group (Figure 4a/b) and it had no effect on the regulation of FHC in the MOR-deficient animals (Figure 4b). Furthermore, morphine inhibited CXCL12-induced GTPγS binding in the brain of WT mice (Supplementary Figure S2), replicating our results in rats (Sengupta et al 2009). Notably, levels of FHC in saline-treated MOR−/− and WT mice did not differ (Figure 4c). These findings show that the MOR-FHC pathway is functional in the mouse brain and suggest that an additional mechanism must be involved in the regulation of CXCR4 by opioids in glia cells.
Figure 4. Morphine does not increase the levels of FHC in MOR−/− mice.
(a, b) WT and MOR−/− mice were injected with saline (1 ml/kg) or morphine (20 mg/kg, 1 injection) and sacrificed after 30 min or 6 hr. Tissue homogenates (cortices and hippocampi) were run on a 10% SDS-PAGE followed by immunoblotting using an FHC-specific antibody. The bar graph shows FHC band density normalized to actin and represented with respect to the control (mean ± SEM from 3 separate immunoblots using a total of 3 animals/group); in WT animals FHC band density in the morphine-pretreated (6hr) mice is significantly higher than in the control (F2,15= 9.539, * P < 0.005). (c) Comparison between the basal levels of FHC in WT and MOR−/− mice: Cortical/hippocampal tissue homogenates prepared from MOR−/− mice showed no differences in FHC levels compared to WT (P > 0.05, n=6 animals/each group, i.e. WT and MOR−/−).
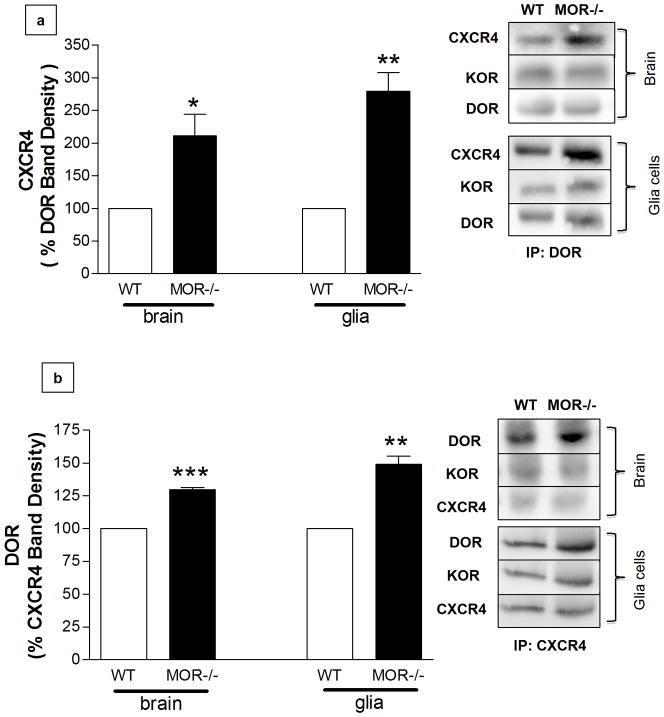
Increased Co-Immunoprecipitation of CXCR4 with DOR in MOR−/− cells
Previous studies provide biochemical, molecular, and pharmacological evidence about dimerization of MOR with DOR in various cell types (Cahill et al 2001; Decaillot et al 2008; Gomes et al 2000; Rozenfeld & Devi 2007; Traynor & Elliott 1993). Also, studies by Pello et al. (2008) suggest that DOR and CXCR4 can form “silent” heterodimers in immune cells. These authors proposed that simultaneous treatment with CXCR4 and DOR agonists (i.e. CXCL12 and DPDPE) further promotes the formation of CXCR4/DOR dimers, which are unable to signal (hence the term “silent”). Based on these findings, we hypothesized that endogenous CXCR4 could more easily interact with DOR in glia cells, as a consequence of MOR−/− absence. Immunoprecipitation experiments in brain tissue (Figure 5a/b, bars on the left) and glia cultures (Figure 5a/b, bars on the right) showed greater DOR-CXCR4 association in MOR−/− than in WT cells, whereas the amount of κ-opioid receptor (KOR) that immunoprecipitated with CXCR4 (or DOR) was similar in MOR−/− and WT samples. These data suggest that increased DOR-CXCR4 interaction may be responsible for the reduced CXCL12/CXCR4 signaling in MOR-deficient glia – a hypothesis we decided to test in vitro and in vivo using a pharmacological approach.
Figure 5. Increased co-immunoprecipitation (IP) of CXCR4 with DOR in brain tissue and glial cultures.
(a) After blotting for CXCR4 and KOR, membranes were stripped and reprobed for DOR to confirm equal loading (IP in brain homogenates: t6= 3.387, * P < 0.01, Student’s t-test, n=4 animals/group; IP in glia cultures: t6= 6.274, ** P < 0.005, Student’s t-test, n=4 glial cultures/group). (b) After blotting for DOR and KOR, membranes were stripped and reprobed for CXCR4 to confirm equal loading (IP in brain homogenates: t4= 19.35, ***P < 0.0001, Student’s t-test, n=3 animals/group; IP in glia cultures: t4= 7.771, *P < 0.005, Student’s t-test, n=3 glial cultures/group).
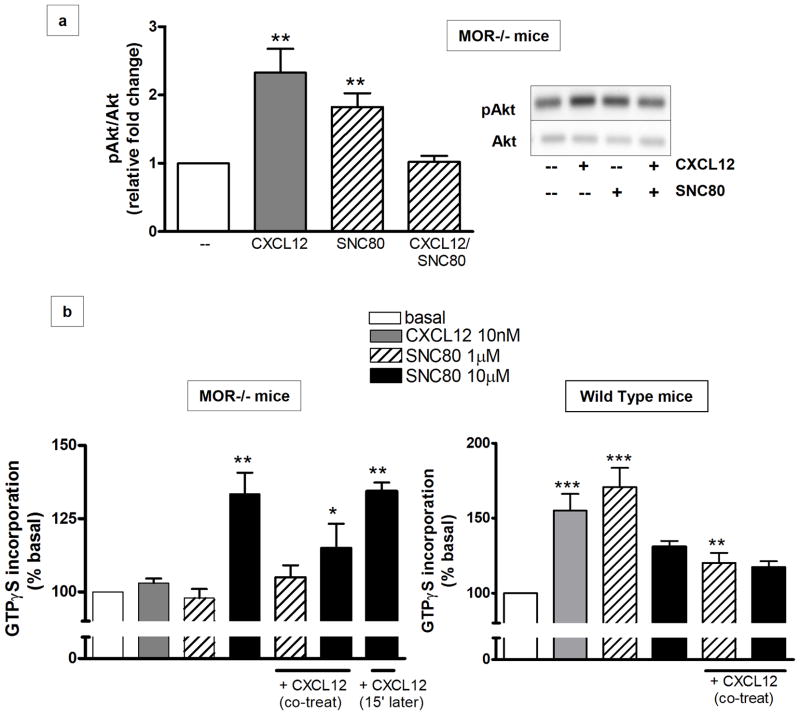
Pre-treatment with DOR Antagonist Restores CXCR4 Function in MOR−/− Mice
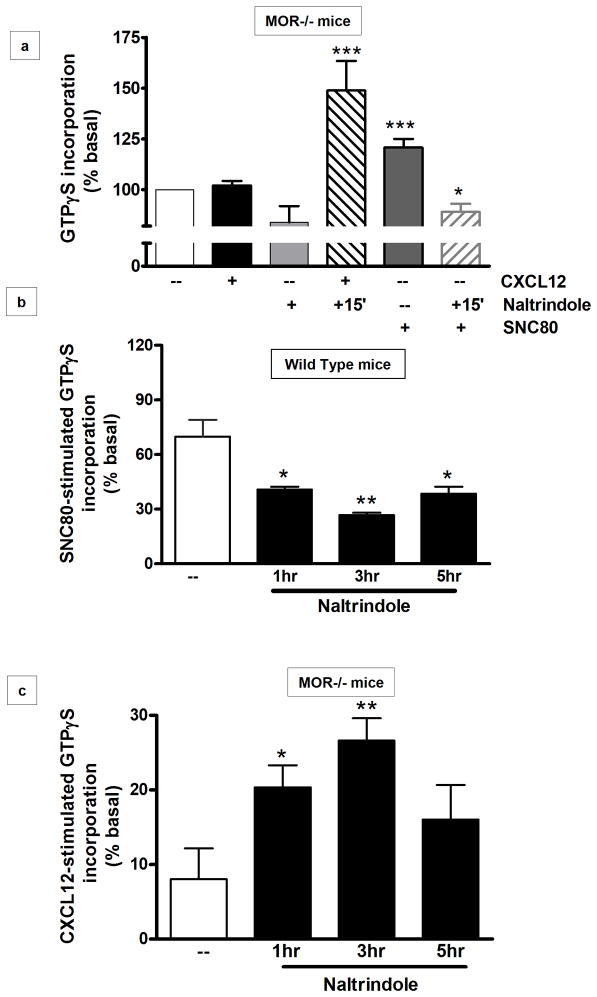
DOR agonists such as SNC80 (a selective, systemically active, nonpeptidic compound) induce Akt signaling in different cell types (Forster et al 2007; Heiss et al 2009), including glia (Fig 6a). Additionally, our data show that co-treatment of MOR−/− glia cultures with SNC80 and CXCL12 completely prevents Akt phosphorylation (Figure 6a), supporting the idea of silent heterodimers. Thus, we measured the levels of G-protein activation in homogenized brain tissues after co-treatment with the DOR- and CXCR4-specific ligands. MOR−/− homogenized brain tissues were treated with SNC80 (1–10μm) and/or CXCR4 (10nM) for 1hr. Two different concentrations of SNC80 were tested, as DOR activity may be partially reduced in mice lacking MOR (Kitchen et al 1997; Matthes et al 1998; Tien et al 2007). In the MOR−/− animals, the higher SNC80 concentration was necessary to induce GTPγS incorporation, which was still lower than the maximal response in WT mice (Figure 6b). Co-treatment with SNC80 and CXCL12 resulted in lower GTPγS “binding” as compared to SNC80 alone, in both WT and MOR deficient mice (Figure 6b). Also, pretreatment for 15 min with the DOR agonist followed by treatment with CXCL12 resulted in significant CXCL12-induced GTPγS incorporation. These results suggest that, due to the lack of MOR, DOR interacts more easily with CXCR4; also, previous engagement of DOR with its specific ligand may help restore CXCR4 function possibly by reducing the pool of DOR available to CXCR4. However, because both CXCR4 and DOR agonists normally induce coupling to G proteins, these studies could not determine which receptor was responsible for the restored GTPγS incorporation. To further investigate this issue, we used the highly specific DOR antagonist naltrindole. MOR−/− brain homogenate samples were treated with naltrindole (1μM 15 min) and then incubated with CXCL12 (10nM, 45 min). The data show that CXCL12-induced GTPγS incorporation was significantly higher in these samples (Figure 7a) and comparable to the WT levels. Block of DOR under these experimental conditions was confirmed by stimulating naltrindole-treated tissue with SNC80 (10μM, 45 min); as expected, GTPγS incorporation in the naltrindole+SNC80 group was lower than the one induced by SNC80 alone.
Figure 6. Effect of the DOR agonist SNC80 on CXCL12-induced responses in brain cells.
(a) MOR−/− mice glial cells were treated with 20nM CXCL12 or 1μM SNC80 for 10 min, alone or in combination. Whole-cell extracts were prepared and run on a 10% SDS-PAGE and immunoblotted for pAkt. Membranes were stripped and re-probed for total proteins to confirm equal loading (F3, 42: 5.033, ** P < 0.05 vs control and CXCL12/SNC80; n=3). A representative immunoblot is shown on the right. (b) MOR−/− (left panel) or WT (right panel) brain homogenates were exposed to 10nM CXCL12 and/or 1–10μM SNC80 as indicated. G-protein stimulation was determined by GTPγS binding assays. Graphs represent the mean ratio ± SEM of agonist-stimulated GTP binding over basal (MOR−/−: n=3, F6, 91= 13.06, ** P < 0.005 vs basal; * P < 0.05 vs SNC80 (10μM) and vs basal; WT: n=5, F5, 126= 12.14, *** P < 0.001 vs control; ** P < 0.05 vs CXCL12 alone and vs SNC80 (1μM)).
Figure 7. Pretreatment with DOR antagonist restores CXCR4 function in MOR−/− mice.
(a) MOR−/− mouse brain homogenates were exposed to CXCL12 or DOR agonist (SNC80)/antagonist (naltrindole) as indicated. The graph represents the mean ratio, ± SEM, of agonist-stimulated GTPγS incorporation over basal (n=3; F5, 66= 9.2, *** P < 0.001; * P < 0.05 vs SNC80 alone); (b) WT mice (P9) were pretreated with saline or naltrindole acutely (acute: single injection, 1 mg/kg, subcutaneously) and killed after the indicated time. Brain slices were treated with SNC80 (1μM) and processed for [35S]GTPγS autoradiography. Analysis was performed in the cortex and hippocampus; overall results are reported in the graphs. Data are expressed as mean ± SEM of three animals for experimental group (F3, 60= 12.87, * P < 0.05 and ** P < 0.005 vs saline-treated WT mice); (c) MOR−/− mice (P9) were pretreated with saline or naltrindole acutely (acute: single injection, 1mg/kg, subcutaneously) and killed after the indicated time. Brain slices were treated with CXCL12 (50nM) and processed for [35S]GTPγS autoradiography. Analysis was performed in the cortex and hippocampus and reported in the graphs. Data are expressed as mean ± SEM of three animals for experimental group (F3, 60= 4.358, * P < 0.05 and ** P < 0.01 vs saline-treated).
Finally, we examined whether pretreatment with naltrindole in vivo also resulted in recovery of CXCL12 responses. Naltrindole is a potent but reversible DOR antagonist showing 124-fold selectivity for δ versus μ (Ananthan et al 1998). To maintain specificity, we used a relatively low concentration of naltrindole (1mg/kg) (Hutchinson et al 2000) and we confirmed the ability of this systemic treatment to block DOR by testing SNC80-induced responses in brains of naltrindole-treated animals ex vivo. WT and MOR−/− mice received a single injection of naltrindole or saline (1mg/kg, subcutaneously) and were killed 1, 3, or 5 hr after the injection. Brain slices from all groups of animals were treated with SNC80, CXCL12, or vehicle and processed for GTPγS autoradiography according to the procedure described previously. As shown in Figure 7b, pretreatment in vivo with naltrindole led to a substantial and long-lasting reduction in the SNC80–induced incorporation of [35S]GTPγS in the brains of WT mice. Due to the steps involved in the GTPγS procedure (e.g. the lag time between in vivo treatment and the ex vivo assay), we do not expect full inhibition of the SNC80–induced incorporation in these experiments. Nevertheless, naltrindole markedly increased CXCL12-induced incorporation in MOR−/− mice (Figure 7c). This increase was time-dependent and reached a maximum at 3hr after injection of the DOR antagonist in vivo. Overall, these findings indicate that DOR plays a major role in opioid-mediated inhibition of CXCR4 both in vivo and in vitro.
DISCUSSION
The interaction between the chemokine and opioid systems has received considerable attention in recent years as it can significantly affect their ability to coordinate essential functions of the immune and nervous systems (Devi 2001; Steele et al 2002). The reciprocal regulation of chemokines by opioids and viceversa may also contribute to various pathological processes, including HIV infection, neuroAIDS, and pain (Hauser et al 2006). Building on previous reports in both native and recombinant systems, we have been focusing on the opiates inhibition of the CXCL12/CXCR4 axis in the brain. Alteration of normal CXCR4 signaling can contribute to neuropathology in different ways, including by preventing the neuroprotective function of CXCL12 and/or inducing neurotoxic pathways (Bezzi et al 2001; Khan et al 2008; Meucci et al 1998; Vergote et al 2006). The present study provides both in vitro and in vivo evidence highlighting multiple (and possibly cell-specific) mechanisms responsible for regulation of endogenous CXCR4 by opioid receptors. Here we have characterized an FHC-unrelated regulation of CXCR4 in glia cells that involves interaction of CXCR4 with DOR. In line with previous observations in immune cells (Pello et al 2008), the results suggest that CXCR4 and DOR form silent dimers (or multimeric complexes) in brain glia. Our IP studies indicate that these CXCR4-DOR complexes are expressed under basal (i.e. unstimulated) conditions but can be regulated by DOR- and CXCR4-specific ligands. More importantly, the data suggest that the CXCR4/DOR interactions are favored in the absence of MOR. Therefore, therapeutic administration of specific combinations of receptor agonists/antagonists may be potentially used to elicit selected receptors responses in vivo. However, additional studies are required to determine whether the CXCR4/DOR interaction is a direct (i.e. immediate) effect of MOR depletion or a consequence of long-term compensatory changes due to lack of MOR. In both cases these studies unveil an important link between endogenous CXCR4 and DOR in glia.
Unlike the MOR, which is primarily expressed on cell surfaces, DOR is stored mainly in dense core vesicles (Hack et al 2005; Ma et al 2006). Its redistribution to the plasma membrane is promoted by several stimuli including inflammatory pain, stress (Commons 2003), and chronic morphine treatment (Cahill et al 2001). Therefore, it is possible that DOR-CXCR4 dimers may contribute to the inhibition of CXCR4 observed in morphine-treated neurons (Sengupta et al 2009); the relationship with FHC remains to be established.
Conversely, in line with previous studies (Morinville et al 2003), DOR expression on the surface of MOR−/− glia was similar to that of WT glia, suggesting that the presence of MOR is not required for expression of DOR under basal conditions. The finding of CXCR4/DOR complexes in both MOR−/− and WT cells also supports this conclusion. However, increased levels of CXCR4 mRNA and total protein (but not changes in surface CXCR4) were found in MOR−/− cells. This may be due to a feedback mechanism caused by reduced CXCR4 signaling and confirms the primary role of MOR in regulation of CXCR4. Altered intracellular trafficking of heterodimers can also account for the difference between total and surface CXCR4 protein as well as the reduced CXCR4 signaling, as GPCRs heterodimerization can change endocytic fate of individual receptors (Jordan et al 2001). It would be interesting to investigate whether chaperone proteins that contribute to cell surface expression of heterodimers - such as receptor transporting protein (RTP) and receptor expression enhancing protein (REEP) - are altered in MOR-deficient glia.
Overall, this study shows a dynamic interaction between DOR and CXCR4 in glia cells that differs from the MOR-FHC-CXCR4 pathway operative in neurons (Sengupta et al 2009). While our previous studies highlight a main role of FHC in the long-term cellular adaptations leading to inhibition of neuronal CXCR4 by MOR, CXCR4 regulation in glia cells heavily depends on DOR and does not seem to correlate with changes in FHC. On the other hand, the downregulation of CXCR4 by KOR agonists primarily involve heterologous desensitization mechanisms, as suggested by others (Rogers et al 2000) and by data not shown (Sengupta, Burbassi, Meucci et al, unpublished). Hence, though all opioid receptor subtypes can affect CXCR4 function in the brain, different mechanisms may predominate in glial and neuronal cells. However, the possibility that FHC may facilitate DOR-CXCR4 interactions in morphine-treated neurons cannot be excluded and it is currently being investigated.
To conclude, these findings contribute to our understanding of the behavior of endogenous chemokine and opioid receptors and the consequences of their interaction on the regulation of CXCR4 in vivo. Given the reported roles of CXCR4 in neuronal survival/differentiation as well as in neuronal-glial communication, alterations in CXCR4 may affect neuronal function and survival in different ways. Inhibition of the pro-survival signaling mediated by neuronal CXCR4 may “precipitate” neurotoxicity under those pathological conditions characterized by proteolityc cleavage of CXCL12 (which reduces its binding to CXCR4), such as HIV neuropathology (Jones et al 2007; Vergote et al 2006). This would be detrimental in certain patient populations that use or abuse opiates – i.e. patients treated with morphine for chronic pain or HIV-infected subjects using illicit drugs. However, the outcomes of CXCR4 regulation on glia may be quite different depending on their activation state. Therefore a clear understanding of the opioid-chemokine interaction in different CNS cell populations may have important therapeutic implications, as it would help identify the best course of action based on the relative contribution of neurons and glia to the underlying pathology. Additional investigations of native systems are necessary to possibly reach this goal.
Supplementary Material
Acknowledgments
This work was supported by NIH grants from NIDA (DA15014 and DA19808 to OM). The authors thank Dr. Kenny Simansky for sharing his scientific expertise and the laboratory instruments for the GTPγS binding assays.
Footnotes
Supplementary information is available at the European Journal of Neuroscience website.
References
- Ananthan S, Johnson CA, Carter RL, Clayton SD, Rice KC, et al. Synthesis, opioid receptor binding, and bioassay of naltrindole analogues substituted in the indolic benzene moiety. J Med Chem. 1998;41:2872–81. doi: 10.1021/jm980083i. [DOI] [PubMed] [Google Scholar]
- Bell JE, Arango JC, Robertson R, Brettle RP, Leen C, Simmonds P. HIV and drug misuse in the Edinburgh cohort. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S35–42. doi: 10.1097/00126334-200210012-00003. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, et al. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–10. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- Burbassi S, Aloyo VJ, Simansky KJ, Meucci O. GTPgammaS incorporation in the rat brain: a study on mu-opioid receptors and CXCR4. J Neuroimmune Pharmacol. 2008;3:26–34. doi: 10.1007/s11481-007-9083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Lee MC, Vincent JP, Collier B, Beaudet A. Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception. J Neurosci. 2001;21:7598–607. doi: 10.1523/JNEUROSCI.21-19-07598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cali’ C, Marchaland J, Mirante O, Bezzi P. Chemokines as neuromodulators: regulation of glutamatergic transmission by CXCR4-mediated glutamated release from astrocytes. In: Meucci O, editor. Chemokine receptors and neuroAIDS. Springer; 2010. pp. 271–300. [Google Scholar]
- Commons KG. Translocation of presynaptic delta opioid receptors in the ventrolateral periaqueductal gray after swim stress. J Comp Neurol. 2003;464:197–207. doi: 10.1002/cne.10788. [DOI] [PubMed] [Google Scholar]
- Cook A, Hippensteel R, Shimizu S, Nicolai J, Fatatis A, Meucci O. Interactions between chemokines: regulation of fractalkine/CX3CL1 homeostasis by SDF/CXCL12 in cortical neurons. J Biol Chem. 2010;285:10563–71. doi: 10.1074/jbc.M109.035477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaillot FM, Rozenfeld R, Gupta A, Devi LA. Cell surface targeting of mu-delta opioid receptor heterodimers by RTP4. Proc Natl Acad Sci U S A. 2008;105:16045–50. doi: 10.1073/pnas.0804106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi LA. Heterodimerization of G-protein-coupled receptors: pharmacology, signaling and trafficking. Trends Pharmacol Sci. 2001;22:532–7. doi: 10.1016/s0165-6147(00)01799-5. [DOI] [PubMed] [Google Scholar]
- Donahoe RM, Vlahov D. Opiates as potential cofactors in progression of HIV-1 infections to AIDS. J Neuroimmunol. 1998;83:77–87. doi: 10.1016/s0165-5728(97)00224-5. [DOI] [PubMed] [Google Scholar]
- Forster K, Kuno A, Solenkova N, Felix SB, Krieg T. The delta-opioid receptor agonist DADLE at reperfusion protects the heart through activation of pro-survival kinases via EGF receptor transactivation. Am J Physiol Heart Circ Physiol. 2007;293:H1604–8. doi: 10.1152/ajpheart.00418.2007. [DOI] [PubMed] [Google Scholar]
- Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of mu and delta opioid receptors: A role in opiate synergy. J Neurosci. 2000;20:RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack SP, Bagley EE, Chieng BC, Christie MJ. Induction of delta-opioid receptor function in the midbrain after chronic morphine treatment. J Neurosci. 2005;25:3192–8. doi: 10.1523/JNEUROSCI.4585-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, El-Hage N, Buch S, Berger JR, Tyor WR, et al. Molecular targets of opiate drug abuse in neuroAIDS. Neurotox Res. 2005;8:63–80. doi: 10.1007/BF03033820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, El-Hage N, Buch S, Nath A, Tyor WR, et al. Impact of opiate-HIV-1 interactions on neurotoxic signaling. J Neuroimmune Pharmacol. 2006;1:98–105. doi: 10.1007/s11481-005-9000-4. [DOI] [PubMed] [Google Scholar]
- Hauser KF, El-Hage N, Stiene-Martin A, Maragos WF, Nath A, et al. HIV-1 neuropathogenesis: glial mechanisms revealed through substance abuse. J Neurochem. 2007;100:567–86. doi: 10.1111/j.1471-4159.2006.04227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss A, Ammer H, Eisinger DA. delta-Opioid receptor-stimulated Akt signaling in neuroblastoma × glioma (NG108-15) hybrid cells involves receptor tyrosine kinase-mediated PI3K activation. Exp Cell Res. 2009;315:2115–25. doi: 10.1016/j.yexcr.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Hutchinson AC, Simpson GR, Randall JF, Zhang X, Calderon SN, et al. Assessment of SNC 80 and naltrindole within a conditioned taste aversion design. Pharmacol Biochem Behav. 2000;66:779–87. doi: 10.1016/s0091-3057(00)00278-1. [DOI] [PubMed] [Google Scholar]
- Jones GJ, Barsby NL, Cohen EA, Holden J, Harris K, et al. HIV-1 Vpr causes neuronal apoptosis and in vivo neurodegeneration. J Neurosci. 2007;27:3703–11. doi: 10.1523/JNEUROSCI.5522-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan BA, Trapaidze N, Gomes I, Nivarthi R, Devi LA. Oligomerization of opioid receptors with beta 2-adrenergic receptors: a role in trafficking and mitogen-activated protein kinase activation. Proc Natl Acad Sci U S A. 2001;98:343–8. doi: 10.1073/pnas.011384898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MZ, Brandimarti R, Shimizu S, Nicolai J, Crowe E, Meucci O. The chemokine CXCL12 promotes survival of postmitotic neurons by regulating Rb protein. Cell Death Differ. 2008;15:1663–72. doi: 10.1038/cdd.2008.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchen I, Slowe SJ, Matthes HW, Kieffer B. Quantitative autoradiographic mapping of mu-, delta- and kappa-opioid receptors in knockout mice lacking the mu-opioid receptor gene. Brain Res. 1997;778:73–88. doi: 10.1016/s0006-8993(97)00988-8. [DOI] [PubMed] [Google Scholar]
- Lazarini F, Tham TN, Casanova P, Arenzana-Seisdedos F, Dubois-Dalcq M. Role of the alpha-chemokine stromal cell-derived factor (SDF-1) in the developing and mature central nervous system. Glia. 2003;42:139–48. doi: 10.1002/glia.10139. [DOI] [PubMed] [Google Scholar]
- Levoye A, Balabanian K, Baleux F, Bachelerie F, Lagane B. CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood. 2009;113:6085–93. doi: 10.1182/blood-2008-12-196618. [DOI] [PubMed] [Google Scholar]
- Li M, Ransohoff RM. Multiple roles of chemokine CXCL12 in the central nervous system: a migration from immunology to neurobiology. Prog Neurobiol. 2008;84:116–31. doi: 10.1016/j.pneurobio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Luo C, Mines M, Zhang J, Fan GH. Chemokine CXCL12 induces binding of ferritin heavy chain to the chemokine receptor CXCR4, alters CXCR4 signaling, and induces phosphorylation and nuclear translocation of ferritin heavy chain. J Biol Chem. 2006;281:37616–27. doi: 10.1074/jbc.M607266200. [DOI] [PubMed] [Google Scholar]
- Loh HH, Liu HC, Cavalli A, Yang W, Chen YF, Wei LN. mu Opioid receptor knockout in mice: effects on ligand-induced analgesia and morphine lethality. Brain Res Mol Brain Res. 1998;54:321–6. doi: 10.1016/s0169-328x(97)00353-7. [DOI] [PubMed] [Google Scholar]
- Ma J, Zhang Y, Kalyuzhny AE, Pan ZZ. Emergence of functional delta-opioid receptors induced by long-term treatment with morphine. Mol Pharmacol. 2006;69:1137–45. doi: 10.1124/mol.105.019109. [DOI] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95:9448–53. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes HW, Smadja C, Valverde O, Vonesch JL, Foutz AS, et al. Activity of the delta-opioid receptor is partially reduced, whereas activity of the kappa-receptor is maintained in mice lacking the mu-receptor. J Neurosci. 1998;18:7285–95. doi: 10.1523/JNEUROSCI.18-18-07285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–5. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O, Miller RJ. gp120-induced neurotoxicity in hippocampal pyramidal neuron cultures: protective action of TGF-beta1. J Neurosci. 1996;16:4080–8. doi: 10.1523/JNEUROSCI.16-13-04080.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Esdaile MJ, Aibak H, Collier B, et al. Regulation of delta-opioid receptor trafficking via mu-opioid receptor stimulation: evidence from mu-opioid receptor knock-out mice. J Neurosci. 2003;23:4888–98. doi: 10.1523/JNEUROSCI.23-12-04888.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolai J, Burbassi S, Rubin J, Meucci O. CXCL12 inhibits expression of the NMDA receptor’s NR2B subunit through a Histone deacetylase-dependent pathway contributing to neuronal survival. Cell Death and Disease. 2010;1:e33. doi: 10.1038/cddis.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JP, Sengupta R, Bardi G, Khan MZ, Mullen-Przeworski A, Meucci O. Modulation of neuronal CXCR4 by the micro-opioid agonist DAMGO. J Neurovirol. 2006;12:492–500. doi: 10.1080/13550280601064798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pello OM, Martinez-Munoz L, Parrillas V, Serrano A, Rodriguez-Frade JM, et al. Ligand stabilization of CXCR4/delta-opioid receptor heterodimers reveals a mechanism for immune response regulation. Eur J Immunol. 2008;38:537–49. doi: 10.1002/eji.200737630. [DOI] [PubMed] [Google Scholar]
- Rogers TJ, Steele AD, Howard OM, Oppenheim JJ. Bidirectional heterologous desensitization of opioid and chemokine receptors. Ann N Y Acad Sci. 2000;917:19–28. doi: 10.1111/j.1749-6632.2000.tb05369.x. [DOI] [PubMed] [Google Scholar]
- Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: beta-arrestin2-mediated ERK activation by mu-delta opioid receptor heterodimers. Faseb J. 2007;21:2455–65. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta R, Burbassi S, Shimizu S, Cappello S, Vallee RB, et al. Morphine increases brain levels of ferritin heavy chain leading to inhibition of CXCR4-mediated survival signaling in neurons. J Neurosci. 2009;29:2534–44. doi: 10.1523/JNEUROSCI.5865-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp BM. Multiple opioid receptors on immune cells modulate intracellular signaling. Brain Behav Immun. 2006;20:9–14. doi: 10.1016/j.bbi.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Khan MZ, Hippensteel RL, Parkar A, Raghupathi R, Meucci O. Role of the transcription factor E2F1 in CXCR4-mediated neurotoxicity and HIV neuropathology. Neurobiol Dis. 2007;25:17–26. doi: 10.1016/j.nbd.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Dworkin SI, Childers SR. Effects of chronic morphine administration on mu opioid receptor-stimulated [35S]GTPgammaS autoradiography in rat brain. J Neurosci. 1996;16:2684–92. doi: 10.1523/JNEUROSCI.16-08-02684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele AD, Szabo I, Bednar F, Rogers TJ. Interactions between opioid and chemokine receptors: heterologous desensitization. Cytokine Growth Factor Rev. 2002;13:209–22. doi: 10.1016/s1359-6101(02)00007-2. [DOI] [PubMed] [Google Scholar]
- Tien LT, Ho IK, Loh HH, Ma T. Role of mu-opioid receptor in modulation of preproenkephalin mRNA expression and opioid and dopamine receptor binding in methamphetamine-sensitized mice. J Neurosci Res. 2007;85:673–80. doi: 10.1002/jnr.21145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor JR, Elliott J. delta-Opioid receptor subtypes and cross-talk with mu-receptors. Trends Pharmacol Sci. 1993;14:84–6. doi: 10.1016/0165-6147(93)90068-u. [DOI] [PubMed] [Google Scholar]
- Vergote D, Butler GS, Ooms M, Cox JH, Silva C, et al. Proteolytic processing of SDF-1alpha reveals a change in receptor specificity mediating HIV-associated neurodegeneration. Proc Natl Acad Sci U S A. 2006;103:19182–7. doi: 10.1073/pnas.0604678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel BA, Wang Y, Lewen S, Berahovich RD, Penfold ME, et al. Elucidation of CXCR7-mediated signaling events and inhibition of CXCR4-mediated tumor cell transendothelial migration by CXCR7 ligands. J Immunol. 2009;183:3204–11. doi: 10.4049/jimmunol.0900269. [DOI] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–9. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.