Abstract
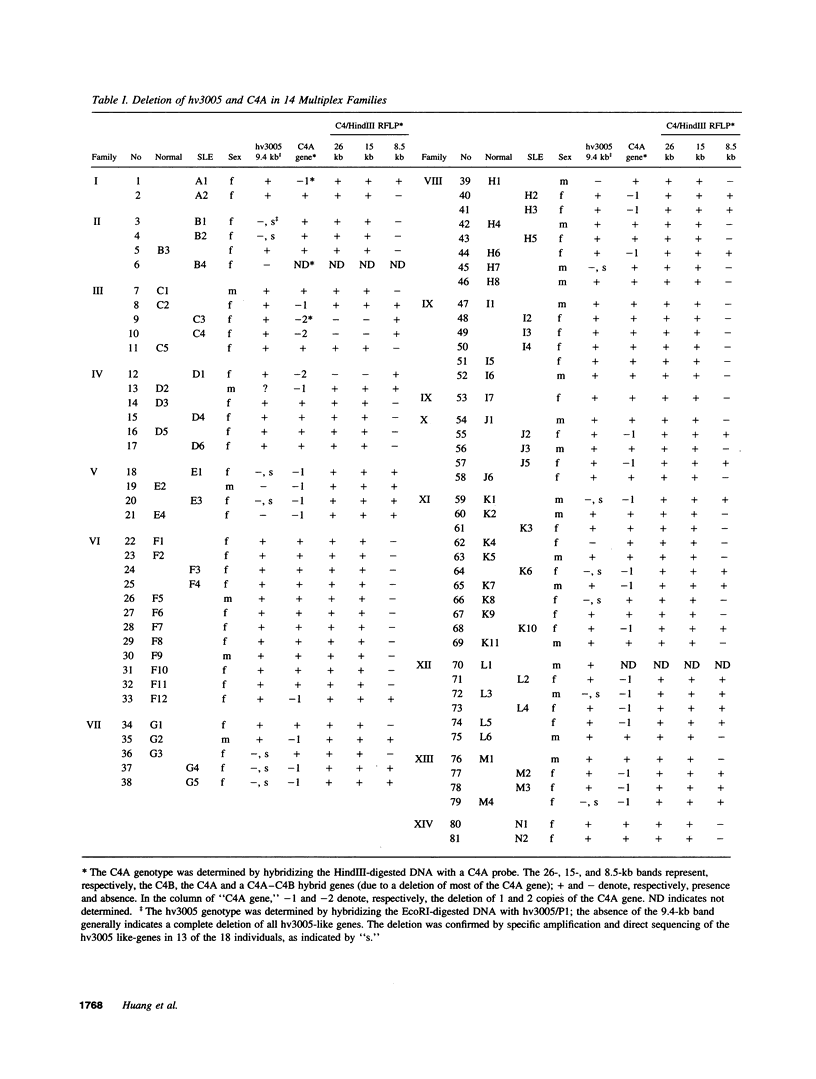
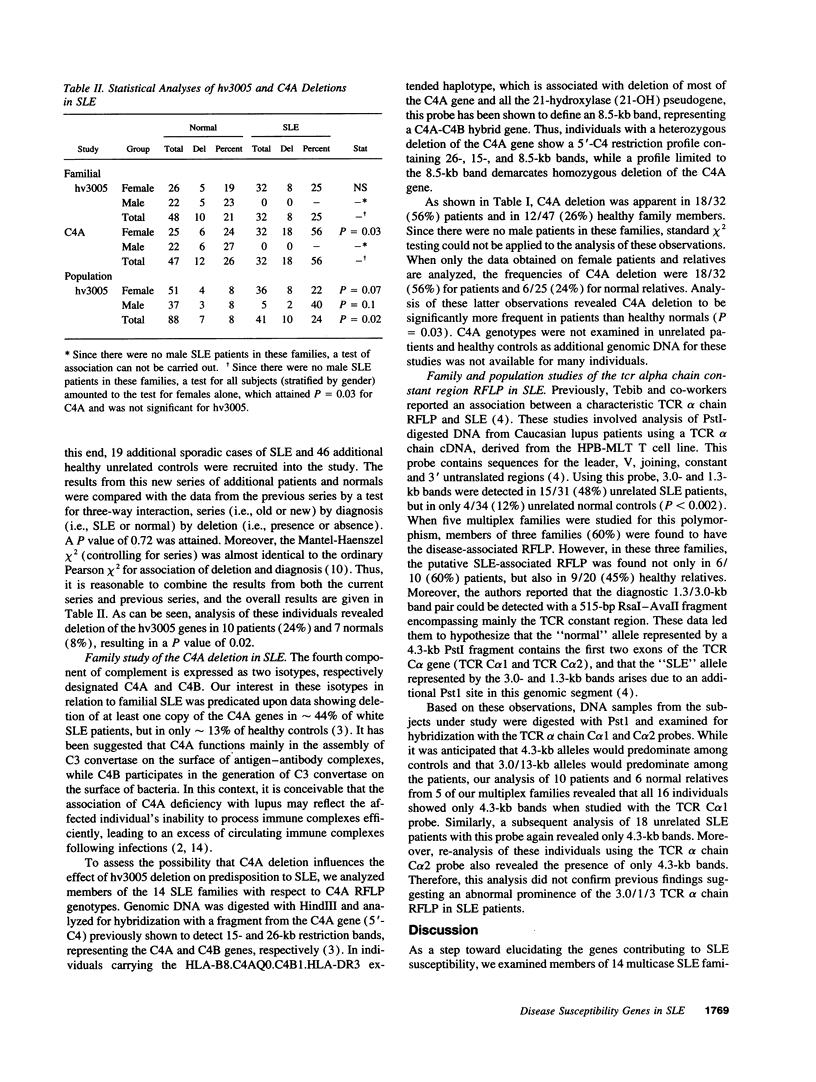
The contribution to systemic lupus erythematosus (SLE) of three lupus-associated polymorphisms (involving the C4A2 complement component, Humhv3005 and the T cell antigen receptor alpha chain gene) are investigated in 81 individuals from 14 multiplex SLE families, 41 unrelated lupus patients, and 88 unrelated healthy controls. The results show a strong association between C4A deletion and SLE in these families. While the current study confirms the previously reported association between hv3005 deletion and sporadic SLE, the study fails to support this association in familial SLE patients. Moreover, no correlation is detected between the occurrence of hv3005 deletion and C4A null alleles in lupus patients, suggesting that the effects of these genetic polymorphisms on predisposition to lupus are independent. The previously reported lupus-associated T cell receptor (TCR) alpha chain polymorphism is not detected in any of the individuals studied here. The combined data suggest that C4A null alleles predispose strongly to development of lupus, whereas the influence of hv3005 deletion is relatively weak. The results also suggest that contributions of weak susceptibility genes such as hv3005 to disease predisposition may be obscured by the effects of stronger genetic factors and thus need to be examined in patients lacking these factors.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman J. E., Mellis S. J., Pollock R., Smith C. L., Suh H., Heinke B., Kowal C., Surti U., Chess L., Cantor C. R. Content and organization of the human Ig VH locus: definition of three new VH families and linkage to the Ig CH locus. EMBO J. 1988 Mar;7(3):727–738. doi: 10.1002/j.1460-2075.1988.tb02869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson D. A., Chen P. P., Fox R. I., Kipps T. J., Jirik F., Goldfien R. D., Silverman G., Radoux V., Fong S. Rheumatoid factor and immune networks. Annu Rev Immunol. 1987;5:109–126. doi: 10.1146/annurev.iy.05.040187.000545. [DOI] [PubMed] [Google Scholar]
- Casali P., Notkins A. L. Probing the human B-cell repertoire with EBV: polyreactive antibodies and CD5+ B lymphocytes. Annu Rev Immunol. 1989;7:513–535. doi: 10.1146/annurev.iy.07.040189.002501. [DOI] [PubMed] [Google Scholar]
- Chen P. P., Fong S., Goni F., Silverman G. J., Fox R. I., Liu M. F., Frangione B., Carson D. A. Cross-reacting idiotypes on cryoprecipitating rheumatoid factor. Springer Semin Immunopathol. 1988;10(1):35–55. doi: 10.1007/BF02054022. [DOI] [PubMed] [Google Scholar]
- Chen P. P., Liu M. F., Sinha S., Carson D. A. A 16/6 idiotype-positive anti-DNA antibody is encoded by a conserved VH gene with no somatic mutation. Arthritis Rheum. 1988 Nov;31(11):1429–1431. doi: 10.1002/art.1780311113. [DOI] [PubMed] [Google Scholar]
- Chen P. P., Olsen N. J., Yang P. M., Soto-Gil R. W., Olee T., Siminovitch K. A., Carson D. A. From human autoantibodies to the fetal antibody repertoire to B cell malignancy: it's a small world after all. Int Rev Immunol. 1990;5(3-4):239–251. doi: 10.3109/08830189009056732. [DOI] [PubMed] [Google Scholar]
- Chen P. P. Structural analyses of human developmentally regulated Vh3 genes. Scand J Immunol. 1990 Mar;31(3):257–267. doi: 10.1111/j.1365-3083.1990.tb02767.x. [DOI] [PubMed] [Google Scholar]
- Chen P. P., Yang P. M. A segment of human Vh gene locus is duplicated. Scand J Immunol. 1990 May;31(5):593–599. doi: 10.1111/j.1365-3083.1990.tb02810.x. [DOI] [PubMed] [Google Scholar]
- Coutinho A. Beyond clonal selection and network. Immunol Rev. 1989 Aug;110:63–87. doi: 10.1111/j.1600-065x.1989.tb00027.x. [DOI] [PubMed] [Google Scholar]
- Gatenby P. A. The role of complement in the aetiopathogenesis of systemic lupus erythematosus. Autoimmunity. 1991;11(1):61–66. doi: 10.3109/08916939108994709. [DOI] [PubMed] [Google Scholar]
- Hillson J. L., Oppliger I. R., Sasso E. H., Milner E. C., Wener M. H. Emerging human B cell repertoire. Influence of developmental stage and interindividual variation. J Immunol. 1992 Dec 1;149(11):3741–3752. [PubMed] [Google Scholar]
- Hochberg M. C. Systemic lupus erythematosus. Rheum Dis Clin North Am. 1990 Aug;16(3):617–639. [PubMed] [Google Scholar]
- Huang D. F., Olee T., Masuho Y., Matsumoto Y., Carson D. A., Chen P. P. Sequence analyses of three immunoglobulin G anti-virus antibodies reveal their utilization of autoantibody-related immunoglobulin Vh genes, but not V lambda genes. J Clin Invest. 1992 Dec;90(6):2197–2208. doi: 10.1172/JCI116105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp M. E., Atkinson J. P., Skanes V. M., Levine R. P., Chaplin D. D. Deletion of C4A genes in patients with systemic lupus erythematosus. Arthritis Rheum. 1987 Sep;30(9):1015–1022. doi: 10.1002/art.1780300908. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bookstein R., Hong F., Young L. J., Shew J. Y., Lee E. Y. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987 Mar 13;235(4794):1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- Matsuda F., Shin E. K., Nagaoka H., Matsumura R., Haino M., Fukita Y., Taka-ishi S., Imai T., Riley J. H., Anand R. Structure and physical map of 64 variable segments in the 3'0.8-megabase region of the human immunoglobulin heavy-chain locus. Nat Genet. 1993 Jan;3(1):88–94. doi: 10.1038/ng0193-88. [DOI] [PubMed] [Google Scholar]
- Naparstek Y., André-Schwartz J., Manser T., Wysocki L. J., Breitman L., Stollar B. D., Gefter M., Schwartz R. S. A single germline VH gene segment of normal A/J mice encodes autoantibodies characteristic of systemic lupus erythematosus. J Exp Med. 1986 Aug 1;164(2):614–626. doi: 10.1084/jem.164.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson K. G., Berman J., Glickman E., Chess L., Alt F. W. Early human IgH gene assembly in Epstein-Barr virus-transformed fetal B cell lines. Preferential utilization of the most JH-proximal D segment (DQ52) and two unusual VH-related rearrangements. J Exp Med. 1989 Apr 1;169(4):1391–1403. doi: 10.1084/jem.169.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olee T., Yang P. M., Siminovitch K. A., Olsen N. J., Hillson J., Wu J., Kozin F., Carson D. A., Chen P. P. Molecular basis of an autoantibody-associated restriction fragment length polymorphism that confers susceptibility to autoimmune diseases. J Clin Invest. 1991 Jul;88(1):193–203. doi: 10.1172/JCI115277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual V., Randen I., Thompson K., Sioud M., Forre O., Natvig J., Capra J. D. The complete nucleotide sequences of the heavy chain variable regions of six monospecific rheumatoid factors derived from Epstein-Barr virus-transformed B cells isolated from the synovial tissue of patients with rheumatoid arthritis. Further evidence that some autoantibodies are unmutated copies of germ line genes. J Clin Invest. 1990 Oct;86(4):1320–1328. doi: 10.1172/JCI114841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reveille J. D. The molecular genetics of systemic lupus erythematosus and Sjögren's syndrome. Curr Opin Rheumatol. 1991 Oct;3(5):722–730. doi: 10.1097/00002281-199110000-00002. [DOI] [PubMed] [Google Scholar]
- Sasso E. H., Willems van Dijk K., Bull A., van der Maarel S. M., Milner E. C. VH genes in tandem array comprise a repeated germline motif. J Immunol. 1992 Aug 15;149(4):1230–1236. [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Wang J. Y. Preferential utilization of conserved immunoglobulin heavy chain variable gene segments during human fetal life. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6146–6150. doi: 10.1073/pnas.87.16.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. S., Stollar B. D. Heavy-chain directed B-cell maturation: continuous clonal selection beginning at the pre-B cell stage. Immunol Today. 1994 Jan;15(1):27–32. doi: 10.1016/0167-5699(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Siminovitch K. A., Chen P. P. The biologic significance of human natural autoimmune responses: relationship to the germline, early immune and malignant B cell variable gene repertoire. Int Rev Immunol. 1990;5(3-4):265–277. doi: 10.3109/08830189009056734. [DOI] [PubMed] [Google Scholar]
- Siminovitch K. A., Misener V., Kwong P. C., Song Q. L., Chen P. P. A natural autoantibody is encoded by germline heavy and lambda light chain variable region genes without somatic mutation. J Clin Invest. 1989 Nov;84(5):1675–1678. doi: 10.1172/JCI114347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Tebib J. G., Alcocer-Varela J., Alarcon-Segovia D., Schur P. H. Association between a T cell receptor restriction fragment length polymorphism and systemic lupus erythematosus. J Clin Invest. 1990 Dec;86(6):1961–1967. doi: 10.1172/JCI114930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Tomlinson I. M., Cook G. P., Winter G., Rabbitts T. H., Dear P. H. HAPPY mapping of a YAC reveals alternative haplotypes in the human immunoglobulin VH locus. Nucleic Acids Res. 1993 Sep 25;21(19):4524–4529. doi: 10.1093/nar/21.19.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weill J. C., Reynaud C. A. Early B-cell development in chickens, sheep and rabbits. Curr Opin Immunol. 1992 Apr;4(2):177–180. doi: 10.1016/0952-7915(92)90009-4. [DOI] [PubMed] [Google Scholar]
- Willems van Dijk K., Milner L. A., Sasso E. H., Milner E. C. Chromosomal organization of the heavy chain variable region gene segments comprising the human fetal antibody repertoire. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10430–10434. doi: 10.1073/pnas.89.21.10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P. M., Olsen N. J., Siminovitch K. A., Olee T., Kozin F., Carson D. A., Chen P. P. Possible deletion of a developmentally regulated heavy-chain variable region gene in autoimmune diseases. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7907–7911. doi: 10.1073/pnas.87.20.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikai Y., Clark S. P., Taylor S., Sohn U., Wilson B. I., Minden M. D., Mak T. W. Organization and sequences of the variable, joining and constant region genes of the human T-cell receptor alpha-chain. 1985 Aug 29-Sep 4Nature. 316(6031):837–840. doi: 10.1038/316837a0. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. Positive selection of lymphocytes. Cell. 1994 Jan 28;76(2):219–228. doi: 10.1016/0092-8674(94)90330-1. [DOI] [PubMed] [Google Scholar]


