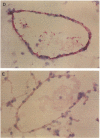
Abstract
Neutrophil emigration during an inflammatory response is mediated through interactions between adhesion molecules on endothelial cells and neutrophils. P-Selectin mediates rolling or slowing of neutrophils, while intercellular adhesion molecule-1 (ICAM-1) contributes to the firm adhesion and emigration of neutrophils. Removing the function of either molecule partially prevents neutrophil emigration. To analyze further the role of P-selectin and ICAM-1, we have generated a line of mice with mutations in both of these molecules. While mice with either mutation alone show a 60-70% reduction in acute neutrophil emigration into the peritoneum during Streptococcus pneumoniae-induced peritonitis, double mutant mice show a complete loss of neutrophil emigration. In contrast, neutrophil emigration into the alveolar spaces during acute S. pneumoniae-induced pneumonia is normal in double mutant mice. These data demonstrate organ-specific differences, since emigration into the peritoneum requires both adhesion molecules while emigration into the lung requires neither. In the peritoneum, P-selectin-independent and ICAM-1-independent adhesive mechanisms permit reduced emigration when one of these molecules is deficient, but P-selectin-independent mechanisms cannot lead to ICAM-1-independent firm adhesion and emigration.
Full text
PDF

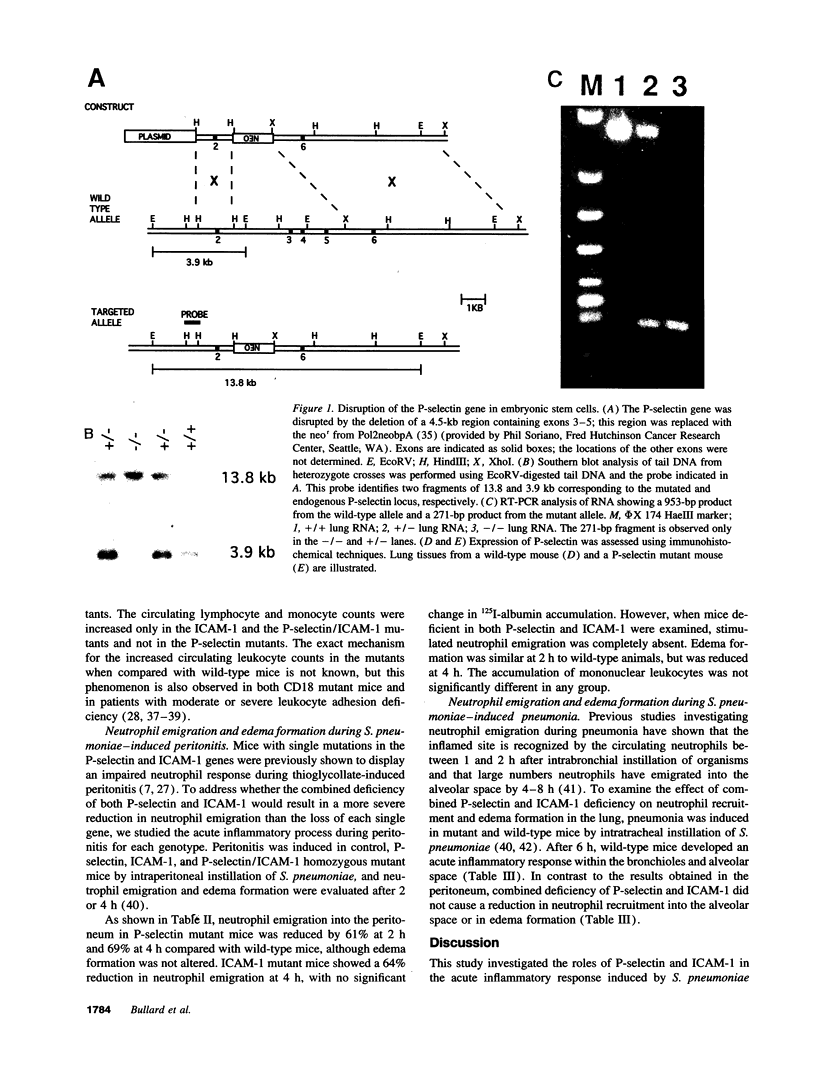




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Smith C. W., Ward P. A. Adhesion molecules and inflammatory injury. FASEB J. 1994 May;8(8):504–512. [PubMed] [Google Scholar]
- Anderson D. C., Springer T. A. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu Rev Med. 1987;38:175–194. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- Arbonés M. L., Ord D. C., Ley K., Ratech H., Maynard-Curry C., Otten G., Capon D. J., Tedder T. F. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994 Jul;1(4):247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Arfors K. E., Lundberg C., Lindbom L., Lundberg K., Beatty P. G., Harlan J. M. A monoclonal antibody to the membrane glycoprotein complex CD18 inhibits polymorphonuclear leukocyte accumulation and plasma leakage in vivo. Blood. 1987 Jan;69(1):338–340. [PubMed] [Google Scholar]
- Arfors K. E., Lundberg C., Lindbom L., Lundberg K., Beatty P. G., Harlan J. M. A monoclonal antibody to the membrane glycoprotein complex CD18 inhibits polymorphonuclear leukocyte accumulation and plasma leakage in vivo. Blood. 1987 Jan;69(1):338–340. [PubMed] [Google Scholar]
- Atherton A., Born G. V. Quantitative investigations of the adhesiveness of circulating polymorphonuclear leucocytes to blood vessel walls. J Physiol. 1972 Apr;222(2):447–474. doi: 10.1113/jphysiol.1972.sp009808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Nelson R. M. Selectins. J Clin Invest. 1993 Feb;91(2):379–387. doi: 10.1172/JCI116210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns A. R., Takei F., Doerschuk C. M. Quantitation of ICAM-1 expression in mouse lung during pneumonia. J Immunol. 1994 Oct 1;153(7):3189–3198. [PubMed] [Google Scholar]
- Doerschuk C. M., Beyers N., Coxson H. O., Wiggs B., Hogg J. C. Comparison of neutrophil and capillary diameters and their relation to neutrophil sequestration in the lung. J Appl Physiol (1985) 1993 Jun;74(6):3040–3045. doi: 10.1152/jappl.1993.74.6.3040. [DOI] [PubMed] [Google Scholar]
- Doerschuk C. M., Markos J., Coxson H. O., English D., Hogg J. C. Quantitation of neutrophil migration in acute bacterial pneumonia in rabbits. J Appl Physiol (1985) 1994 Dec;77(6):2593–2599. doi: 10.1152/jappl.1994.77.6.2593. [DOI] [PubMed] [Google Scholar]
- Doerschuk C. M., Winn R. K., Coxson H. O., Harlan J. M. CD18-dependent and -independent mechanisms of neutrophil emigration in the pulmonary and systemic microcirculation of rabbits. J Immunol. 1990 Mar 15;144(6):2327–2333. [PubMed] [Google Scholar]
- Downey G. P., Worthen G. S., Henson P. M., Hyde D. M. Neutrophil sequestration and migration in localized pulmonary inflammation. Capillary localization and migration across the interalveolar septum. Am Rev Respir Dis. 1993 Jan;147(1):168–176. doi: 10.1164/ajrccm/147.1.168. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Etzioni A., Frydman M., Pollack S., Avidor I., Phillips M. L., Paulson J. C., Gershoni-Baruch R. Brief report: recurrent severe infections caused by a novel leukocyte adhesion deficiency. N Engl J Med. 1992 Dec 17;327(25):1789–1792. doi: 10.1056/NEJM199212173272505. [DOI] [PubMed] [Google Scholar]
- Frydman M., Etzioni A., Eidlitz-Markus T., Avidor I., Varsano I., Shechter Y., Orlin J. B., Gershoni-Baruch R. Rambam-Hasharon syndrome of psychomotor retardation, short stature, defective neutrophil motility, and Bombay phenotype. Am J Med Genet. 1992 Oct 1;44(3):297–302. doi: 10.1002/ajmg.1320440307. [DOI] [PubMed] [Google Scholar]
- Gallatin W. M., Weissman I. L., Butcher E. C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983 Jul 7;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Geng J. G., Bevilacqua M. P., Moore K. L., McIntyre T. M., Prescott S. M., Kim J. M., Bliss G. A., Zimmerman G. A., McEver R. P. Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature. 1990 Feb 22;343(6260):757–760. doi: 10.1038/343757a0. [DOI] [PubMed] [Google Scholar]
- Gie R. P., Doerschuk C. M., English D., Coxson H. O., Hogg J. C. Neutrophil-associated lung injury after the infusion of activated plasma. J Appl Physiol (1985) 1991 Jun;70(6):2471–2478. doi: 10.1152/jappl.1991.70.6.2471. [DOI] [PubMed] [Google Scholar]
- Hattori R., Hamilton K. K., Fugate R. D., McEver R. P., Sims P. J. Stimulated secretion of endothelial von Willebrand factor is accompanied by rapid redistribution to the cell surface of the intracellular granule membrane protein GMP-140. J Biol Chem. 1989 May 15;264(14):7768–7771. [PubMed] [Google Scholar]
- Hellewell P. G., Young S. K., Henson P. M., Worthen G. S. Disparate role of the beta 2-integrin CD18 in the local accumulation of neutrophils in pulmonary and cutaneous inflammation in the rabbit. Am J Respir Cell Mol Biol. 1994 Apr;10(4):391–398. doi: 10.1165/ajrcmb.10.4.7510985. [DOI] [PubMed] [Google Scholar]
- Hsu-Lin S., Berman C. L., Furie B. C., August D., Furie B. A platelet membrane protein expressed during platelet activation and secretion. Studies using a monoclonal antibody specific for thrombin-activated platelets. J Biol Chem. 1984 Jul 25;259(14):9121–9126. [PubMed] [Google Scholar]
- Johnston G. I., Bliss G. A., Newman P. J., McEver R. P. Structure of the human gene encoding granule membrane protein-140, a member of the selectin family of adhesion receptors for leukocytes. J Biol Chem. 1990 Dec 5;265(34):21381–21385. [PubMed] [Google Scholar]
- Kishimoto T. K., Jutila M. A., Berg E. L., Butcher E. C. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989 Sep 15;245(4923):1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- Kuehn M. R., Bradley A., Robertson E. J., Evans M. J. A potential animal model for Lesch-Nyhan syndrome through introduction of HPRT mutations into mice. Nature. 1987 Mar 19;326(6110):295–298. doi: 10.1038/326295a0. [DOI] [PubMed] [Google Scholar]
- LEAKE E. S., GONZALEZ-OJEDA D., MYRVIK Q. N. ENZYMATIC DIFFERENCES BETWEEN NORMAL ALVEOLAR MACROPHAGES AND OIL-INDUCED PERITONEAL MACROPHAGES OBTAINED FROM RABBITS. Exp Cell Res. 1964 Feb;33:553–561. doi: 10.1016/0014-4827(64)90020-5. [DOI] [PubMed] [Google Scholar]
- Lawrence M. B., Springer T. A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991 May 31;65(5):859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Lawrence M. B., Springer T. A. Neutrophils roll on E-selectin. J Immunol. 1993 Dec 1;151(11):6338–6346. [PubMed] [Google Scholar]
- Lewinsohn D. M., Bargatze R. F., Butcher E. C. Leukocyte-endothelial cell recognition: evidence of a common molecular mechanism shared by neutrophils, lymphocytes, and other leukocytes. J Immunol. 1987 Jun 15;138(12):4313–4321. [PubMed] [Google Scholar]
- Ley K., Gaehtgens P., Fennie C., Singer M. S., Lasky L. A., Rosen S. D. Lectin-like cell adhesion molecule 1 mediates leukocyte rolling in mesenteric venules in vivo. Blood. 1991 Jun 15;77(12):2553–2555. [PubMed] [Google Scholar]
- Ley K., Tedder T. F., Kansas G. S. L-selectin can mediate leukocyte rolling in untreated mesenteric venules in vivo independent of E- or P-selectin. Blood. 1993 Sep 1;82(5):1632–1638. [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol. 1961 Dec;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayadas T. N., Johnson R. C., Rayburn H., Hynes R. O., Wagner D. D. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993 Aug 13;74(3):541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- McMahon A. P., Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990 Sep 21;62(6):1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- Mileski W., Harlan J., Rice C., Winn R. Streptococcus pneumoniae-stimulated macrophages induce neutrophils to emigrate by a CD18-independent mechanism of adherence. Circ Shock. 1990 Jul;31(3):259–267. [PubMed] [Google Scholar]
- Muller W. A., Weigl S. A., Deng X., Phillips D. M. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993 Aug 1;178(2):449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. S., Polley M. J., Bayer R. J., Nunn M. F., Paulson J. C., Ward P. A. Neutrophil-dependent acute lung injury. Requirement for P-selectin (GMP-140). J Clin Invest. 1992 Oct;90(4):1600–1607. doi: 10.1172/JCI116029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Lapierre L. A., Mendrick D. L., Fiers W., Rothlein R., Springer T. A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986 Sep 15;137(6):1893–1896. [PubMed] [Google Scholar]
- Rothlein R., Dustin M. L., Marlin S. D., Springer T. A. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986 Aug 15;137(4):1270–1274. [PubMed] [Google Scholar]
- Schulman E. S. The role of mast cells in inflammatory responses in the lung. Crit Rev Immunol. 1993;13(1):35–70. [PubMed] [Google Scholar]
- Sligh J. E., Jr, Ballantyne C. M., Rich S. S., Hawkins H. K., Smith C. W., Bradley A., Beaudet A. L. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8529–8533. doi: 10.1073/pnas.90.18.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991 Feb 22;64(4):693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Takei F. Inhibition of mixed lymphocyte response by a rat monoclonal antibody to a novel murine lymphocyte activation antigen (MALA-2). J Immunol. 1985 Mar;134(3):1403–1407. [PubMed] [Google Scholar]
- VanOtteren G. M., Standiford T. J., Kunkel S. L., Danforth J. M., Burdick M. D., Abruzzo L. V., Strieter R. M. Expression and regulation of macrophage inflammatory protein-1 alpha by murine alveolar and peritoneal macrophages. Am J Respir Cell Mol Biol. 1994 Jan;10(1):8–15. doi: 10.1165/ajrcmb.10.1.8292385. [DOI] [PubMed] [Google Scholar]
- Vaporciyan A. A., DeLisser H. M., Yan H. C., Mendiguren I. I., Thom S. R., Jones M. L., Ward P. A., Albelda S. M. Involvement of platelet-endothelial cell adhesion molecule-1 in neutrophil recruitment in vivo. Science. 1993 Dec 3;262(5139):1580–1582. doi: 10.1126/science.8248808. [DOI] [PubMed] [Google Scholar]
- Watson S. R., Fennie C., Lasky L. A. Neutrophil influx into an inflammatory site inhibited by a soluble homing receptor-IgG chimaera. Nature. 1991 Jan 10;349(6305):164–167. doi: 10.1038/349164a0. [DOI] [PubMed] [Google Scholar]
- Weller A., Isenmann S., Vestweber D. Cloning of the mouse endothelial selectins. Expression of both E- and P-selectin is inducible by tumor necrosis factor alpha. J Biol Chem. 1992 Jul 25;267(21):15176–15183. [PubMed] [Google Scholar]
- Wilson R. W., Ballantyne C. M., Smith C. W., Montgomery C., Bradley A., O'Brien W. E., Beaudet A. L. Gene targeting yields a CD18-mutant mouse for study of inflammation. J Immunol. 1993 Aug 1;151(3):1571–1578. [PubMed] [Google Scholar]
- Winn R. K., Harlan J. M. CD18-independent neutrophil and mononuclear leukocyte emigration into the peritoneum of rabbits. J Clin Invest. 1993 Sep;92(3):1168–1173. doi: 10.1172/JCI116686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Andrian U. H., Chambers J. D., McEvoy L. M., Bargatze R. F., Arfors K. E., Butcher E. C. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]