Abstract
DNA is subjected to many endogenous and exogenous damages. All organisms have developed a complex network of DNA repair mechanisms. A variety of different DNA repair pathways have been reported: direct reversal, base excision repair, nucleotide excision repair, mismatch repair, and recombination repair pathways. Recent studies of the fundamental mechanisms for DNA repair processes have revealed a complexity beyond that initially expected, with inter- and intrapathway complementation as well as functional interactions between proteins involved in repair pathways. In this paper we give a broad overview of the whole DNA repair system and focus on the molecular basis of the repair machineries, particularly in Thermus thermophilus HB8.
1. Introduction
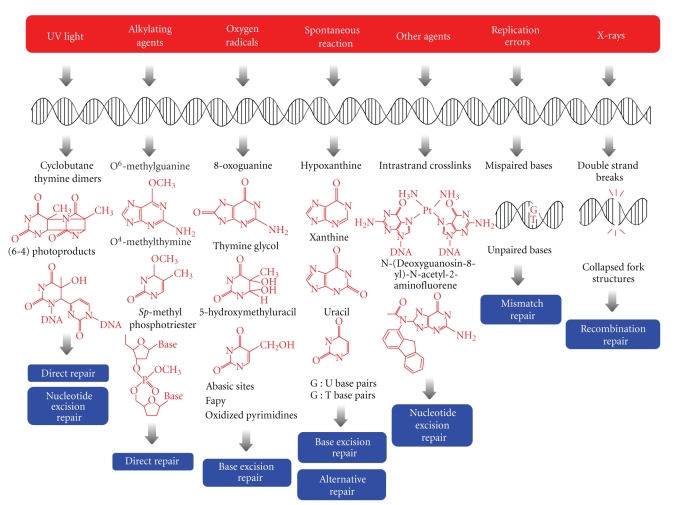
It is essential for all living organisms to warrant accurate functioning and propagation of their genetic information. However, the genome is constantly exposed to various environmental and endogenous agents, which produce a large variety of DNA lesions (Figure 1) [1, 2]. Environmental damage can be induced by several chemical reactive species and physical agents. Endogenous damages occur spontaneously and continuously even under normal physiologic conditions through intrinsic instability of chemical bonds in DNA structure. The biological consequences of these damages usually depend on the chemical nature of the lesion. Most of these lesions affect the fidelity of DNA replication, which leads to mutations. Some of human genetic diseases are associated to defects in DNA repair (Table 1).
Figure 1.
Different repair systems for the principal types of DNA lesion produced by a wide range of factors. UV-light induces cyclobutane pyrimidine dimers or (6-4) photoproducts that are repaired by nucleotide excision repair and direct reversal systems. Alkylating agents can modify all of the bases and the phosphates of the DNA, and some repair proteins remove these alkyl adducts in a direct manner. Oxygen radicals modify DNA, and the base excision repair system acts to reverse these changes. The main cause of spontaneous mutation is deamination, and base excision repair and alternative repair systems remove the lesions. Other bulky adducts or interstrand cross-links are repaired by the nucleotide excision repair system. The mismatch repair pathway repairs replication errors. Double-strand breaks and four-way junctions are induced by X-rays and are repaired by recombinational repair.
Table 1.
Distribution of DNA repair genes. ∗1Related human diseases are listed by referencing the following databases: KEGG disease (http://www.genome.jp/kegg/disease/), GeneCards (http://www.genecards.org/), and Online Mendelian Inheritance in Man (http://www.ncbi.nlm.nih.gov/omim). ∗2Descriptions in the parentheses indicate the subunit organizations of holoenzymes.
| Repair pathways | Molecular functions | T. thermophilus | E. coli | S. cerevisiae | A. thaliana | M. musculus | H. sapiens | Related disease∗1 |
|---|---|---|---|---|---|---|---|---|
| Direct reversal | ||||||||
| Photoreactivation | Photoreactivation | Phr (TTHB102) | PhrA | PHR1 | PhrB | |||
|
| ||||||||
| Alkyltransfer | Alkyltransfer or recognition | ATL (TTHA1564) | Ada, AGT, ATL | MGT1 | MGMT | MGMT | ||
| alkyltransfer | AlkB | AlkBH1 | ALKBH2, ALKBH3 | |||||
|
| ||||||||
|
| ||||||||
| Base excision repair | ||||||||
| Base excision | Remove ring-saturated or fragmented pyrimidines | EndoIII (TTHA0112) | Nth (EndoIII) | Ntg1p, Ntg2p | AT1G05900 | Nthl1 | NTHL1 | |
| remove 3-meA, ethenoA, hypoxanthine | AlkA (TTHA0329) | AlkA, TagA | Mag1p | AT3G12040 (MAG) | Mpg | MPG, (MAG, AAG) | ||
| Remove U | UDGA (TTHA0718) | Ung | Ung1p | AT3G18630 uracil DNA glycosylase family protein | Ung | Ung | Hyper IgM syndromes, autosomal recessive type | |
| UDGB (TTHA1149) | ||||||||
| Remove U, hydroxymethyl U | Smug1 | SMUG1 | ||||||
| Remove U or T opposite G at CpG sequences | Mbd4 | MBD4 (MED1) | ||||||
| Remove U, T, or ethenoC opposite G | Mug | Tdg | TDG | |||||
| Remove 8-oxoG opposite C | MutM (TTHA1806) | Fpg (MutM) | Ogg1p | OGG1 | Ogg1 | OGG1 | Lung cancer | |
| Remove A opposite 8-oxoG | MutY (TTHA1898) | MutY | AT4G12740 (MYH-related) | MUTYH | MUTYH | |||
| Remove thymine glycol | Nei (EndoVIII) | Neil1 | NEIL1 | |||||
| Remove oxidative products of C, U | NEIL2 | |||||||
| Not known | NEIL3 | |||||||
| Alternative strand incision | Incision 3′ of hypoxanthine and uracil | EndoV (TTHA1347) | Nfi (EndoV) | EndoV | EndoV | EndoV | ||
|
| ||||||||
| AP site processing and resynthesis | AP endonuclease | EndoIV (Nfo) (TTHA0834) | Nfo (EndoIV) | Apn1p | ||||
| AP endonuclease | XthA (ExoIII) | Apn2p (Eyh1) | ARP | Apex1 | APEX1 (APE1, APEX, HAP1, REF1), | |||
| AP endonuclease | AT4G36050 | Apex2 | APEX2 | |||||
| gap-filling DNA polymerase | PolX (TTHA1150) | Polβ | Polβ | |||||
| DNA polymerase, 5′ flap endonuclease | PolI (TTHA1054) | PolI | ||||||
| replication and BER in mitochondrial DNA | Mip1p | Polγ | Polγ | |||||
| NAD-dependent DNA ligase | LigA (TTHA1097) | LigA | ||||||
| ATP-dependent DNA ligase | AT1G66730 (ATP-dependent) | Lig3 (ATP-dependent) | LIG3 (ATP-dependent) | |||||
| accessory factor for LIG3 and BER | AT1G80420 (putative XRCC1) | Xrcc1 | XRCC1 | |||||
| poly (ADP-ribose) polymerase | PARP2 | Parp1 | PARP1 (ADPRT) | |||||
| ADPRT-like enzyme | APP (Arabidopsis poly(ADP-ribose) polymerase) | Parp2 | PARP2 (ADPRTL2) | |||||
|
| ||||||||
|
| ||||||||
| Nucleotide excision repair | ||||||||
| DNA binding | Bind damaged DNA in complex with UvrB | UvrA (TTHA1440) | UvrA | |||||
| Catalyze unwinding in preincision complex | UvrB (TTHA1892) | UvrB | ||||||
| Bind disordered DNA as complex | RAD4 | RAD4 | Xpc | XPC | Xeroderma pigmentosum (XP) | |||
| Bind disordered DNA as complex | RAD23 | RAD23 | Rad23b (Hr23b) | RAD23B (HR23B) | ||||
| RAD23B paralog | Rad23a (Hr23a) | RAD23A (HHR23A) | ||||||
| Bind DNA and proteins in preincision complex | RAD14 | Xpa | XPA | XP, | ||||
|
| ||||||||
| TFIIH subunits | 3′-5′ DNA helicase TFIIH subunit | SSL2 (RAD25) | XPB2 | Xpb (Ercc3) | XPB (ERCC3) | XP, Cockayne syndrome (CS), Trichothiodystrophy (TTD) | ||
| 5′-3′ DNA helicase TFIIH subunit | RAD3 | UVH6 | Xpd (Ercc2) | ERCC2 | XP, CS, TTD | |||
| TFIIH subunit p62 | TFB1 | AT1G55750 | Gtf2h1 | GTF2H1 | ||||
| TFIIH subunit p44 | SSL1 | GTF2H2 | Gtf2h2 | GTF2H2 | ||||
| TFIIH subunit p34 | TFB4 | AT1G18340 | Gtf2h3 | GTF2H3 | ||||
| TFIIH subunit p52 | TFB2 | AT4G17020 | Gtf2h4 | GTF2H4 | ||||
| TFIIH subunit p8 | TFB5 | AT1G12400 | Gtf2h5 | GTF2H5 (TTDA) | TTD | |||
| Kinase subunits of TFIIH | KIN28 | CDKD1;3 | Cdk7 | CDK7 | ||||
| Kinase subunits of TFIIH | CCL1 | CYCH;1 | Ccnh | CCNH | ||||
| THIIH subunit | TFB3 | AT4G30820 | Mnat1 (Mat1) | MNAT1 (MAT1) | ||||
|
| ||||||||
| Strand incision and excision | 3′ and 5′ incision nuclease | UvrC (TTHA1548) | UvrC | |||||
| 3′ incision nuclease | Cho | |||||||
| 3′ incision nuclease | RAD2 | UVH3 | Xpg (Ercc5) | ERCC5 | XP, CS | |||
| 5′ incision nuclease subunits | RAD10 | ERCC1 | Ercc1 | ERCC1 | XP | |||
| 5′ incision nuclease subunits | RAD1 | UVH1 | Xpf (Ercc4) | ERCC4 | XP | |||
|
| ||||||||
| Separating two annealed strands | DNA helicase | UvrD (TTHA1427) | UvrD | |||||
| Other factors | Transcription- repair coupling factor | Mfd (TTHA0889) | Mfd | |||||
| Cockayne syndrome, needed for TC-NER | ERCC6 | Csb (Ercc6) | CSB (ERCC6) | CS, UV-sensitive syndrome (UVS) | ||||
| Cockayne syndrome, needed for TC-NER | AT1G19750 | Csa (Ckn1, Ercc8) | CSA (ERCC8) | CS | ||||
| P127 subunit of DDB | DDB1 | Ddb1 | DDB1 (XPE) | |||||
| P48 subunit of DDB, defective in XP-E | DDB2 | Ddb2 (Xpe) | DDB2 (XPE) | XP | ||||
| transcription and NER | AT5G48120 | Mms19 | MMS19 | |||||
|
| ||||||||
|
| ||||||||
| Mismatch repair | ||||||||
| Mismatch recognition | DNA-binding ATPase | MutS (TTHA1324) | MutS | MutSα
(MSH2/MSH6) MutSβ (MSH2/MSH3) |
MutSα
(MSH2/MSH6) MutSβ (MSH2/MSH3) |
MutSα
(MSH2/MSH6) MutSβ (MSH2/MSH3) |
MutSα
(MSH2/MSH6) MutSβ (MSH2/MSH3) |
Colorectal cancer, Ovarian cancer |
|
| ||||||||
| Strand incision | Activation of MutL homologue | DNA polymerase III, β subunit (TTHA0001) | — | PCNA | PCNA | PCNA | PCNA | |
| Activation of MutL homologue | DNA polymerase III, δ, δ′, γ, τ subunits (TTHA0788, 1860, 1952) | RFC (RFC1-5)∗2 | RFC (RFC1-5)∗2 | RFC (RFC1-5)∗2 | RFC (RFC1-5)∗2 | |||
| Endonuclease ATPase | MutL (TTHA1323) | — | MutLα (MLH1/PMS1) MutLβ (MLH1/MLH2) MutLγ (MLH1/MLH3) | MutLα (MLH1/PMS1) MutLγ (MLH1/MLH3) | MutLα (MLH1/PMS2) MutLβ (MLH1/PMS1) MutLγ (MLH1/MLH3) | MutLα (MLH1/PMS2) MutLβ (MLH1/PMS1) MutLγ (MLH1/MLH3) | Colorectal cancer, Endometrial cancer, Ovarian cancer | |
|
| ||||||||
| Match making | ATPase | — | MutL | — | — | — | — | |
| Strand excision | 5′-3′ exonuclease | RecJ (TTHA1167) | RecJ | |||||
| 3′-5′ exonuclease | ExoI (TTHB178) | ExoI | ||||||
| 5′-3′ exonuclease | ExoVII | |||||||
| 3′-5′ exonuclease | ExoX | |||||||
| 5′-3′ exonuclease | EXO1 | AT1G29630 | Exo1 | EXO1 | ||||
| Single-stranded DNA binding protein | SSB (TTHA0244) | SSB | ||||||
| Single-stranded DNA binding protein complex | RFA (RFA1-3)∗2 | RPA (RPA1-3)∗2 | Rpa (Rpa1-3)∗2 | RPA (RPA1-3)∗2 | ||||
| DNA helicase | UvrD (TTHA1427) | UvrD | ||||||
| Recombination repair | ||||||||
|
| ||||||||
| End resection and recombinase loading | 5′-3′ exonuclease | RecJ (TTHA1167) | RecJ | |||||
| 5′-3′ exonuclease | EXO1 | AT1G29630 | Exo1 | EXO1 | ||||
| 5′-flap endonuclease | DNA2 | AT1G08840 | Dna2 | DNA2 | ||||
| RECQ family DNA helicase | RecQ | SGS1 | AT1G10930 (RECQ4A) | Blm | BLM | Bloom syndrome | ||
| Endonuclease, interact with MRN complex | SAE2 | AT3G52115 (ATGR1) | CtIP (Rbbp8) | CTIP (RBBP8) | ||||
| SMC-like ATPase, complex with SbcD (Mre11) | SbcC (TTHA1288) | SbcC | RAD50 | AT2G31970 (RAD50) | Rad50 | RAD50 | Nijmegen breakage syndrome-like disorder | |
| 3′-5′ exonuclease, endonuclease, complex with SbcC (Rad50) | SbcD (TTHA1289) | SbcD | MRE11 | AT5G54260 (MRE11) | Mre11a | MRE11A | Ataxia telangiectasia -like disorder | |
| Accessory protein for MR complex | XRS2 | AT3G02680 (NBS1) | Nbn (Nbs1) | NBN (NBS1) | Nijmegen breakage syndrome | |||
| SMC-like ATPase | RecN (TTHA1525) | RecN | ||||||
| Helicase/nuclease complex | RecB | |||||||
| Helicase/nuclease complex | RecC | |||||||
| Helicase/nuclease complex | RecD | |||||||
| 5′-3′ exonuclease | RecE | |||||||
| ssDNA annealing | RecT | |||||||
| Single-stranded DNA binding protein | SSB (TTHA0244) | Ssb | ||||||
| Single-stranded DNA binding protein complex | RFA (RFA1-3) | RPA (RPA1-3) | Rpa (Rpa1-3) | RPA (RPA1-3) | ||||
| ATPase, complex with RecR | RecF (TTHA0264) | RecF | ||||||
| Recombinase mediator, ssDNA annealing | RecO (TTHA0623) | RecO | ||||||
| DNA binding, complex with RecF and RecO | RecR (TTHA1600) | RecR | ||||||
| Recombinase mediator, ssDNA annealing | Rad52-like (TTHA0081) | RAD52 | Rad52 | RAD52 | ||||
| Recombinase mediator | AT5G01630 (BRCA2B) | Brca2 | BRCA2 | Pancreatic cancer, Ovarian cancer, Breast cancer, Fanconi anemia | ||||
| RAD54 family DNA translocase, recombinase mediator | RAD54 | AT3G19210 (ATRad54) | Rad54l | RAD54L | Adenocarcinoma, Non-Hodgkin lymphoma | |||
| RAD54 family DNA translocase, recombinase mediator | RDH54 | Rad54b | RAD54B | Colon cancer, Non-Hodgkin lymphoma | ||||
| RAD51-like, recombinase mediator | AT5G64520 (XRCC2) | Xrcc2 | XRCC2 | Breast cancer | ||||
| RAD51-like, recombinase mediator | AT5G57450 (XRCC3) | Xrcc3 | XRCC3 | Breast cancer, Melanoma | ||||
| RAD51-like, recombinase mediator | RAD57 | AT2G28560 (RAD51B) | Rad51l1 | RAD51L1 | Uterine leiomyoma | |||
| RAD51-like, recombinase mediator | AT2G45280 (RAD51C) | Rad51c | RAD51C | Fanconi anemia-like disorder, Breast-Ovarian cancer | ||||
| RAD51-like, recombinase mediator | RAD55 | AT1G07745 (RAD51D) | Rad51l3 | RAD51L3 | ||||
|
| ||||||||
| Strand exchange | Recombinase | RecA (TTHA1818) | RecA | AT2G19490 (recA) | ||||
| Recombinase | RAD51 | AT5G20850 (ATRAD51) | Rad51 | RAD51 | ||||
|
| ||||||||
| Branch migration | Branch migration complex | RuvA (TTHA0291) | RuvA | |||||
| Branch migration complex | RuvB (TTHA0406) | RuvB | ||||||
| DNA helicase | RecG (TTHA1266) | RecG | AT2G01440 (RecG) | |||||
| RecA-like ATPase | RadA/Sms (TTHA0541) | RadA/Sms | AT5G50340 | |||||
| RAD54 family DNA translocase, recombinase mediator | RAD54 | AT3G19210 (ATRad54) | Rad54l | RAD54L | Adenocarcinoma, Non-Hodgkin lymphoma | |||
| RAD54 family DNA translocase, recombinase mediator | RDH54 | Rad54b | RAD54B | Colon cancer, Non-Hodgkin lymphoma | ||||
| RECQ family DNA helicase | RecQ | SGS1 | AT1G10930 (RECQ4A) | Blm | BLM | Bloom syndrome | ||
| RECQ family DNA helicase | Wrn | WRN | Werner syndrome | |||||
| RECQ family DNA helicase | AT1G31360 (RECQL2) | Recql | RECQL | |||||
| RECQ family DNA helicase | MPH1 | AT1G35530 | Fancm | FANCM | Fanconi anemia | |||
|
| ||||||||
| Holliday junction resolution | HJ resolvase | RuvC (TTHA1090) | RuvC | |||||
| HJ resolvase | RusA | |||||||
| HJ resolvase | YEN1 | AT1G01880 | Gen1 | GEN1 | ||||
| Structure-specific endonuclease | MUS81 | AT4G30870 (MUS81) | Mus81 | MUS81 | ||||
| complex with MUS81 | MMS4 | AT2G22140 (ATEME1B) | Eme1 | EME1 | ||||
| Structure-specific endonuclease | RAD1 | AT5G41150 (UVH1) | Ercc4 | ERCC4 | Xeroderma pigmentosum | |||
| complex with ERCC4 (RAD1) | RAD10 | AT3G05210 (ERCC1) | Ercc1 | ERCC1 | Cerebro-oculo-facio-skeletal syndrome | |||
| HJ resolvase | SLX1 | AT2G30350 | Slx1 (Giyd2) | SLX1 (GIYD2) | ||||
| Accessory protein for structure-specific nucleases | SLX4 | Slx4 (Btbd12) | SLX4 (BTBD12) | |||||
|
| ||||||||
| Anti-recombination | Recombinase inhibitor | RecX (TTHA0848) | RecX | AT3G13226 (RecX) | ||||
| DNA helicase | UvrD (TTHA1427) | UvrD | SRS2 | AT4G25120 | ||||
| Structure-specific endonuclease | MutS2 (TTHA1645) | AT1G65070 (MutS2) | ||||||
To cope with these DNA damages, all organisms have developed a complex network of DNA repair mechanisms [1, 3]. A variety of different DNA repair pathways have been reported: direct reversal, base excision repair, nucleotide excision repair, mismatch repair, and recombination repair pathways. Most of these pathways require functional interactions between multiple proteins. Furthermore, recent studies have revealed inter- and intra-pathway complementation.
Although there are a number of model organisms representing different kingdoms, such as Escherichia coli, Saccharomyces cerevisiae, Arabidopsis thaliana, and Mus musculus (Table 1), we selected the bacterial species Thermus thermophilus HB8 for use in our studies of basic and essential biological processes. T. thermophilus is a Gram-negative eubacterium that can grow at temperatures over 75°C [4]. T. thermophilus HB8 was chosen for several reasons: (i) it has a smaller genome size than other model organisms; (ii) proteins from T. thermophilus HB8 are very stable suitable for in vitro analyses of molecular function; and (iii) the crystallization efficiency of the proteins is higher than for those of other organisms [5]. Moreover, since each biological system in T. thermophilus is only constituted of fundamentally necessary enzymes, in vitro reconstitution of a particular system should be easier and more understandable.
Our group has constructed overexpression plasmids for most T. thermophilus HB8 ORFs [6], and those plasmids are available from The DNA Bank, RIKEN Bioresource Center (Tsukuba, Japan) (http://www.brc.riken.jp/lab/dna/en/thermus_en.html). Approximately 80% of the ORFs have been completely cloned into the overexpression vectors pET-11a, pET-11b, pET-3a, and/or pET-HisTEV. Furthermore, plasmids for gene disruption are also available from the Structural-Biological Whole Cell Project (http://www.thermus.org/). Protein purification profiles and gene disruption methods can be downloaded from the RIKEN Bioresource Center. Therefore, it is a relatively simple matter to initiate an analysis of proteins of interest in this species.
T. thermophilus HB8 has all of the fundamental enzymes known to be essential for DNA repair, and most of these show homology to human enzymes. Biological and structural analyses of DNA repair in T. thermophilus will therefore provide a better understanding of DNA repair pathways in general. Moreover, these analyses are aided by the high efficiency of protein crystallization and stability of purified proteins in this species. In this paper we give a broad overview of the whole DNA repair system and focus on the molecular basis of the repair machineries, especially in T. thermophilus HB8.
2. Direct Reversal of DNA Damage
UV-induced pyrimidine dimers and alkylation adducts can be directly repaired by DNA photolyases and alkyl transferases, respectively. These repair systems are not followed by incision or resynthesis of DNA.
2.1. Photolyases
UV-induced pyrimidine dimers, such as cyclobutane pyrimidine dimers (CPDs) and (6-4) photoproducts, disturb DNA replication and transcription. Some species make use of DNA photolyases to repair these lesions (Figure 2(a)). The FADH− in the photolyase donates an electron to the CPD, which induces the breakage of the cyclobutane bond [7].
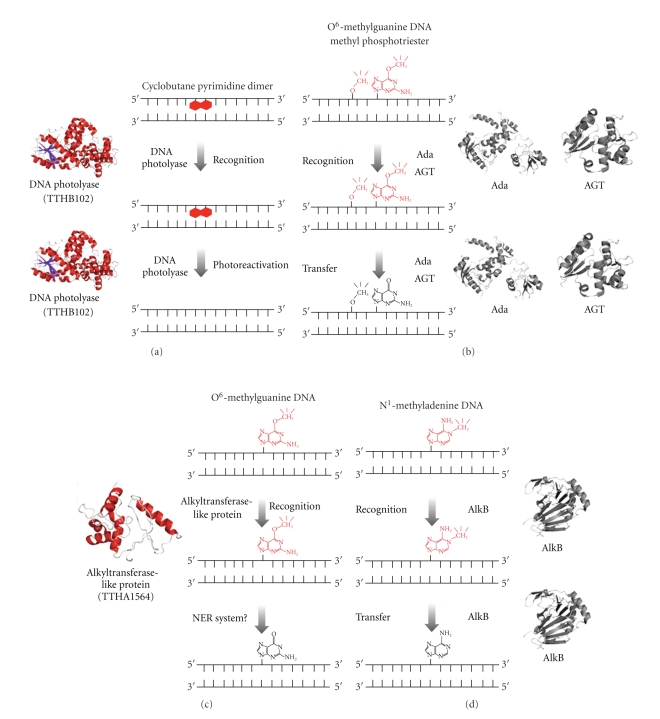
Figure 2.
A schematic representation of models for direct reversal of DNA damage. The structure of the ATL proteins was modeled by SWISS-MODEL (the template structure is Sulfolobus tokodaii Ogt) [19, 20]. AGT, Ada, and AlkB are not conserved in T. thermophilus. (a) Cyclobutane pyrimidine dimers are recognized by photolyase (TTHB102; PDB ID: 1IQR) and repaired by photolyase. (b) O 6-methylguanines are recognized by AGT (PDB ID: 1EH6) in most species and by the C-terminal domain of Ada (PDB ID: 1SFE) in E. coli. Methyl phosphotriesters are recognized by the N-terminal domain of Ada (PDB ID: 1WPK) in E. coli. These enzymes directly accept a methyl group, and the alkyl adducts are removed from the DNA. (c) O 6-alkyl adducts including O 6-methylguanines are recognized by ATL proteins (TTHA1564; predicted model) in several species. It is predicted that NER proteins are involved in this pathway after recognition of the adducts by ATL proteins. (d) N 1-methyladenines and N 3-methylcytosines are recognized by AlkB (PDB ID: 2IUW). Methyl group transfer by AlkB depends on α-ketoglutarate and Fe(II).
CPD photolyases repair UV-induced CPDs utilizing photon energy from blue or near-UV light [8]. To absorb light, CPD photolyases have two different chromophoric cofactors. One of these, FAD, acts as the photochemical reaction center in the repair process. An electron is transferred from an exogenous photoreductor to FAD, which is changed to the fully reduced, active form FADH− [9]. Although only this chromophore is necessary for the reaction, photolyases have a second chromophore as an auxiliary antenna to harvest light energy, which is transferred to the reaction center. The identity of the second chromophore differs among species. To date, reduced folate (5,10-methenyl-tetrahydrofolate, MTHF), 8-hydroxy-5-deazaflavin (8-HDF), FMN, and riboflavin have been identified as secondary chromophores.
A CPD photolyase (ORF ID, TTB102) of T. thermophilus (ttPhr) was identified as the first thermostable photolyase in 1997 [10]. The crystal structures of photolyases from E. coli and Aspergillus nidulans were reported in 1995 and 1997, respectively [11, 12]. Those of ttPhr and the complex it forms with thymine, a part of its substrate, were reported in 2001 [13]. NMR analysis showed that the CPD is flipped out from the double-stranded DNA (dsDNA) into a cavity in ttPhr [14]. Likewise, the thymine dimer interacts with the active site in the crystal structure of A. nidulans photolyase complexed with substrate dsDNA [15]. NMR analysis also showed the distance between FAD and CPD, which is important for understanding the CPD repair reaction by ttPhr [16]. In 2005, an overexpression analysis using E. coli identified the second chromophore of ttPhr as FMN [17]. Photolyases usually have a specific binding site for cofactors, but the second chromophore, FMN, of ttPhr shows promiscuous binding with riboflavin or 8-HDF [18].
Placental mammals lack photoreactivation activity, but they do have nucleotide excision repair (NER) systems for repairing CPDs [21]. NER has two sub-pathways: global genomic repair (GGR) and transcription-coupled repair (TCR) [3]. These sub-pathways are versatile repair systems and are highly conserved across species. Thus, the absence of photoreactivation activity would not have a significant effect on DNA repair efficiency in placental mammals. The mechanisms of NER are detailed in the later section. It should be noted that mammals, birds, and plants have photolyase-like proteins, the so-called cryptochromes, which have no ability to repair damaged DNA but function as blue-light photoreceptors [22].
2.2. Reversal of O 6-Alkylguanine-DNA
O 6-alkylguanine is one of the most harmful alkylation adducts and can induce mutation and apoptosis [23–25]. Almost all species possess mechanisms to repair this adduct (Figures 2(b) and 2(c)). O 6-alkylguanine-DNA alkyltransferase (AGT) accepts an alkyl group on a cysteine residue at its active site (PCHR) in a stoichiometric fashion, and this alkylated AGT is inactive (Figure 2(b)) [26–28]. AGT acts as a monomer and transfers the alkyl group from DNA without a cofactor [29–31]. The structure of human AGT, MGMT, indicates that a helix-turn-helix motif mediates binding to the minor groove of DNA and that O 6-methylguanine (O 6-meG) is flipped out from the base stack into this active site [32, 33]. Tyrosine and arginine residues in the active site of the enzyme mediate nucleotide flipping.
The cysteine residue in the active site (PCHR) of AGT is necessary for the methyltransferase activity. Some AGT-like proteins lack cysteine residues in their active sites (PXHR) [34–40]. Alkyltransferase-like (ATL) proteins are a type of AGT homologue and are present in all three domains of life. ATL proteins from E. coli, Schizosaccharomyces pombe, and T. thermophilus can bind to DNA and show preferential binding to O 6-meG-containing DNA, but they are unable to transfer a methyl group from the modified DNA [37–39]. This binding activity inhibits AGT activity in a competitive manner [38]. E. coli has three AGT homologues, AGT, Ada, and the ATL protein, but S. pombe and T. thermophilus have only the ATL protein. Therefore, S. pombe or T. thermophilus are particularly suitable for studies of ATL proteins.
The tyrosine and arginine residues involved in base flipping are also conserved in ATL proteins. A fluorescence assay of the T. thermophilus ATL protein (TTHA1564) suggested that it can also recognize O 6-meG and flips out the target residue into its active site (Figure 2(c)) [37]. The crystal and NMR structures of ATL proteins indicate that the O 6-meG residue is flipped out from the base stacks into the active site [34, 40]. Mutational analysis demonstrated that the tyrosine and arginine residues of ATL proteins are also involved in base flipping [34].
A comparison of their 3D structures showed that the lesion-binding pocket of ATL proteins is approximately three times larger than that of AGTs [34, 40]. The S. pombe ATL protein (Atl1) can bind to the bulky O 6-adduct, O 6-4-(3-pyridyl)-4-oxobutylguanine (O 6-pobG), with higher affinity than to O 6-meG [34]. Additionally, AGT repairs O 6-pobG with lower efficiency than O 6-meG. In species that have both AGT and ATL protein, for example, E. coli, it is possible that AGT repairs O 6-meG while the ATL protein is involved in the repair of bulky O 6-adducts such as O 6-pobG.
It is known that the action of ATL proteins is linked with the NER pathway (Figure 2(c)) [34, 36, 37, 40]. The ATL protein of T. thermophilus, TTHA1564, can interact with UvrA, while that of E. coli can interact with UvrA and UvrC [36, 37, 40]. MNNG caused an increased mutation frequency in the ttha1564-deficient mutant compared with the wild type (unpublished data). Genetic analysis of S. pombe Atl1 showed that atl1 is epistatic to rad13 (the fission yeast orthologue of human ERCC5) and swi10 (the ERCC1 orthologue) but not to rhp14 or rad2 for N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) toxicity [40]. Analyses of the spontaneous mutation rate of rad13 and rad13 atl mutants suggested that ATL-DNA complexes block an alternative repair pathway probably because ATL proteins form a highly stable complex with DNA in the absence of Rad13 or other NER proteins [40]. However, the mechanism by which ATL proteins repair lesions in collaboration with NER proteins is not well understood.
The protein Ada repairs alkylated lesions in the same manner as AGTs in E. coli (Figure 2(b)) [27]. The amino acid sequence and the molecular function of the C-terminal domain of Ada (C-Ada) show similarity to those of AGTs. The N-terminal domain of Ada (N-Ada) can repair a methyl phosphotriester lesion in DNA in vitro [44]. Methylated N-Ada specifically binds to the promoter region of the ada-alkB operon and the alkA and aidB genes and C-Ada can bind to RNA polymerase [45, 46]. Thus, the methylated Ada acts as a transcriptional activator.
2.3. AlkB
AlkB homologues are conserved in many organisms including humans and E. coli. As described above, alkB is one of the genes regulated by Ada. AlkB requires α-ketoglutarate and Fe(II) as cofactors to repair N 1-methyladenine or N 3-methylcytosine via an oxidative demethylation mechanism [46]. These properties are consistent with the fact that AlkB has sequence motifs in common with 2-oxoglutarate and iron-dependent dioxygenases (Figure 2(d)) [47]. AlkB oxidizes the methyl group using nonheme Fe2+, O2, and α-ketoglutarate to restore undamaged bases with subsequent release of succinate, CO2, and formaldehyde. The detailed mechanisms of substrate recognition and catalysis were identified by structural and mutational analyses.
Eight AlkB homologues are known in humans, [48] and, of these, ALKBH1, ALKBH2, and ALKBH3 have been identified as repair enzymes, each of which has a different substrate specificity [49, 50]. E. coli AlkB can repair a lesion in both single-stranded DNA (ssDNA) and dsDNA, whereas ALKBH3 repairs lesions only in ssDNA. ALKBH1 and ALKBH2 can act only on DNA whereas E. coli AlkB and ALKBH3 can act on both DNA and RNA [51]. The crystal structures of AlkB-dsDNA and ALKBH2-dsDNA complexes explain distinct preferences of AlkB homologues for substrates [51]. Cell cycle-dependent subcellular localization experiments suggested that ALKBH2 and ALKBH3 repair mainly newly synthesized DNA and mRNA, respectively, and withhold demethylation of modified rRNA or tRNA.
3. Base Excision Repair
DNA is altered and damaged by various endogenous and exogenous reactions [52]. With regard to endogenous reactions, deamination of cytosine, adenine and guanine produce uracil, hypoxanthine, and xanthine, respectively. Depurination and depyrimidination result in the formation of an apurinic/apyrimidinic site (AP site). Reactive oxygen species (ROSs) convert guanine to 7,8-dihydro-8-oxoguanine (8-oxoguanine, 8-oxoG, or its isomeric form 8-hydroxyguanine) and purine bases to 2,6-diamino-4-hydroxy-5-formamidopyrimidine (FaPyG) and 4,6-diamino-5-formamidopyrimidine (FaPyA). Thymine glycol, cytosine hydrates, and etheno adducts of adenine, cytosine, and guanine are also generated as a result of oxygen damage. DNA replication errors also introduce lesions into the DNA. For example, DNA polymerases sometimes incorporate mismatched bases or damaged nucleotides (such as dUMP and 8-oxo-dGMP) [53–55]. With regard to exogenous reactions, DNA is susceptible to damage by agents such as UV radiation and alkylating compounds. The lesions caused by endogenous and exogenous reactive species can be repaired through the base excision repair (BER) pathway described below.
3.1. General Mechanism of BER
BER is probably the most frequently used DNA repair pathway in the cell (Figure 3, Table 1) [56, 57]. Bases damaged as described above are specifically recognized by various DNA glycosylases to initiate BER [58]. Monofunctional DNA glycosylases catalyze the hydrolysis of N-glycosyl bonds and generate an AP site. Bi- and trifunctional DNA glycosylases have AP lyase activity via a β- or β/δ-elimination mechanism using an ε amino group of a lysine residue or α-imino group in addition to DNA glycosylase activity [59]. However, it is still unclear whether this lyase activity is the primary in vivo mechanism. AP sites are targeted by both AP endonuclease and AP lyase. AP endonuclease nicks an AP site through a hydrolytic reaction to generate a 3′-OH and 5′-deoxyribosephosphate (dRP) [60–62]. This 5′ block is removed by deoxyribophosphodiesterase (dRPase) or dRP lyase using hydrolytic or lyase (β-elimination) mechanisms, respectively [63–65]. When the AP lyase incises an AP site, it produces 3′-α,β-unsaturated aldehyde (by β-elimination) or 3′-phosphate (by β/δ-elimination) and 5′-phosphate [66]. These 3′-blocking groups must be removed by 3′-phosphoesterase to allow DNA polymerase activity. A one-nucleotide gap typically remains after AP site processing. When repair synthesis is performed by incorporation of a single nucleotide, this pathway is called single nucleotide-BER (SN-BER) [67]. Some DNA polymerases can synthesize DNA of more than 2 bases by strand displacement activity, followed by cleaving flap DNA via flap endonuclease activity. This pathway is called long-patch BER (LP-BER) [67]. In both pathways, the resulting nick is sealed by DNA ligase.
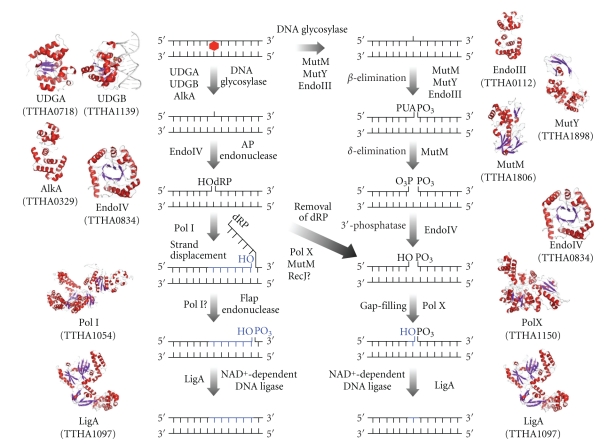
Figure 3.
General mechanism of the BER pathway in T. thermophilus. UDGA, UDGB, and AlkA are monofunctional DNA glycosylases. UDGA (PDB ID: 1UI0) and UDGB (PDB ID: 2DDG) remove uracil from DNA. AlkA removes 3-methyladenine in E. coli. MutY and EndoIII are bifunctional DNA glycosylases and have both DNA glycosylase and AP lyase activities. MutY removes adenine opposite 8-oxoG, and EndoIII removes pyrimidine residues damaged by ring saturation, fragmentation, and contraction [41], by which 3′-phospho α,β-unsaturated aldehyde (3′-PUA) remains. MutM (PDB ID: 1EE8) is a trifunctional DNA glycosylase that removes 8-oxoG from oxidatively damaged DNA and 3′-phosphate remains. An AP site resulting from DNA glycosylase activity is processed by EndoIV or multifunctional DNA glycosylases. EndoIV has both AP endonuclease activity and 3′-esterase activity in E. coli [42, 43]. PolX or MutM removes 5′-dRP by dRP lyase activity. In addition, 5′-3′ exonuclease (RecJ) may have dRPase activity. The resulting gap is filled by PolI or PolX followed by sealing of the nick by LigA. The structures of AlkA, EndoIII, MutY, PolI, PolX, and LigA were obtained using SWISS-MODEL [19, 20] (PDB ID: 2H56, 2ABK, 3FSP, 1TAU, 2W9M, and 1V9P, resp.) based on amino acids sequences of T. thermophilus HB8.
3.2. BER in T. thermophilus
The T. thermophilus HB8 genome contains the genes for all the fundamental BER enzymes. The genome includes the following monofunctional DNA glycosylases: 3-methyl-adenine DNA glycosylase, TTHA0329 (ttAlkA); uracil DNA glycosylase A, TTHA0718 (ttUDGA); uracil DNA glycosylase B, TTHA1149 (ttUDGB). It also includes the following bifunctional DNA glycosylases: endonuclease III (Nth), TTHA0112 (ttEndoIII); adenine DNA glycosylase, TTHA1898 (ttMutY); formamidopyrimidine DNA glycosylase, TTHA1806 (ttMutM). AP endonucleases are classified on the basis of their structure as members of either the exonuclease III family or the endonuclease IV (Nfo) family. The only AP endonuclease in T. thermophilus is the EndoIV, TTHA0834 (ttEndoIV); a similar restriction occurs in other bacterial and archaeal species. T. thermophilus has been found to have two DNA polymerases, TTHA1054 (ttPolI) and TTHA1150 (ttPolX), and an NAD+-dependent DNA ligase, TTHA1097 (ttLigA). The crystal structures of ttUDGA [68], ttUDGB [69], ttMutM [70], and ttEndoIV (unpublished data) have been determined.
Uracil-DNA glycosylases (Ungs or UDGs) remove uracil from DNA by cleaving the N-glycosylic bond. These enzymes are classified into several families on the basis of similarities in their amino acid sequences [71, 72]. T. thermophilus HB8 has two Ungs that belong to families 4 (ttUDGA) and 5 (ttUDGB). ttUDGA removes uracil from not only U : G but also U : C, U : A, and U : T and can also remove uracil from ssDNA. Moreover, the crystal structure of ttUDGA with uracil indicates that the mechanism by which family 4 Ungs remove uracils from DNA is similar to that of family 1 enzymes [68]. The crystal structures of apo-form ttUDGB and ttUDGB complexed with AP site containing DNA have been solved [69]. The active site structures suggest that this enzyme uses both steric force and water activation for its excision reaction. Based on the absence of a significant open-closed conformational change upon binding to DNA, it was proposed that Ungs recognize the damaged base by sliding along the target-containing strand [69].
MutM is a trifunctional DNA glycosylase which removes 8-oxoG from oxidatively damaged DNA [73]. ttMutM was cloned, characterized, and crystallized. Based on crystal structure and biochemical experiments of ttMutM, DNA-binding mode and catalytic mechanism of MutM were proposed [70].
In mammalian cells, SN-BER is the principal BER sub-pathway and is catalyzed mainly by Polβ [74, 75]. Nevertheless, LP-BER also occurs in vivo [76]. The selection of which sub-pathway to use is dependent on the nature of the damaged base, the 5′-blocking structure, and the enzymes involved [74, 77–82]. Bacteria have both SN- and LP-BER pathways [83]. Bacterial PolIs, including ttPolI, have strand displacement [84] and flap endonuclease-like activities (structure-specific 5′-nuclease activity) [85–89]. Therefore, PolI is probably the main DNA polymerase in bacterial LP-BER. Furthermore, the fact that the β-clamp, the β subunit of DNA polymerase III holoenzyme, interacts with some DNA repair enzymes, such as PolI and LigA [90], indicates that it is possibly involved in bacterial LP-BER in a similar manner to mammalian PCNA clamp [77].
Many bacteria have PolX, which belongs to the X-family DNA polymerases; the mammalian homologues of this enzyme are Polβ, Polλ, Polμ, TdT, and Polσ [91]. PolXs can efficiently fill a short DNA gap in mammals [79, 92] and bacteria [93] and are therefore thought to be the main DNA polymerases in the SN-BER pathway [74, 75, 94]. Although PolX is conserved in many bacteria, including T. thermophilus, E. coli does not have this enzyme. Therefore, T. thermophilus has an advantage as a model organism in understanding human and bacterial BER. ttPolX has two principal active regions, the N-terminal POLX core (POLXc) domain and the C-terminal polymerase and histidinol phosphatase (PHP) domain. These domains are conserved in many bacteria, but eukaryotic PolXs lack the PHP domain. Furthermore, it is thought that only PHP domain-containing PolXs have 3′-5′ exonuclease activity [95, 96]. The PHP domain has nine catalytic residues and is mainly responsible for the nuclease activity; however, the POLXc domain is also needed for this activity [97]. Although the PHP domain is thought to have a phosphoesterase activity, details of the function of the PHP domain remain to be clarified. Bacterial PolXs may play more than two roles in the BER pathway whereas these functions might be performed in eukaryotes by two or more separate enzymes. Identifying the role of the PHP domain of bacterial PolXs in BER will be important for understanding both bacterial and eukaryotic BERs.
3.3. Eukaryotic-Specific BER Enzymes
Eukaryotes have many functional homologues of bacterial BER enzymes, and the mechanism of BER is similar to that of prokaryotes. However, eukaryotes also have specific BER enzymes. To date, poly(ADP-ribose) polymerase (PARP) and X-ray cross-complementing group 1 (XRCC1) have been identified as eukaryotic-specific enzymes. PARP1 uses NAD to add branched ADP-ribose chains to proteins. PARP1 functions as a DNA nick-sensor in DNA repair and as a negative regulator of the activity of Polβ in LP-BER [98]. XRCC1 interacts with DNA ligase III and PARP through its two BRCT domains and with Polβ through an N-terminal domain. XRCC1 also interacts with many other proteins and forms a large DNA repair complex [99, 100].
4. Nucleotide Excision Repair
Nucleotide excision repair (NER) is one of the most important repair systems and is conserved from prokaryotes to higher eukaryotes [101, 102]. The most important feature of the NER system is its broad substrate specificity: NER can excise DNA lesions such as UV-induced pyrimidine dimers or more bulky adducts [103].
In the prokaryotic NER system, recognition and excision of DNA lesions are mediated by UvrABC excinucleases (Figure 4) [101, 102]. After the incision event, UvrD helicase removes the nucleotide fragment, PolI synthesizes the complementary strand, and then DNA ligase completes the repair process. NER has two sub-pathways, global genomic repair (GGR) and transcription-coupled repair (TCR) [104, 105]. In GGR, recognition of DNA lesions by UvrAB initiates the initiation of the repair reaction, whereas, in TCR, stalling of the RNA polymerase is responsible for the initiation of repair [106]. When a transcribing RNA polymerase meets a bulky DNA lesion, the polymerase stalls. Transcription-repair coupling factor (TRCF) releases the stalled RNA polymerase from the template DNA and then recruits UvrA. After UvrA has bound to the DNA, the subsequent reactions proceed in the same fashion as in GGR.
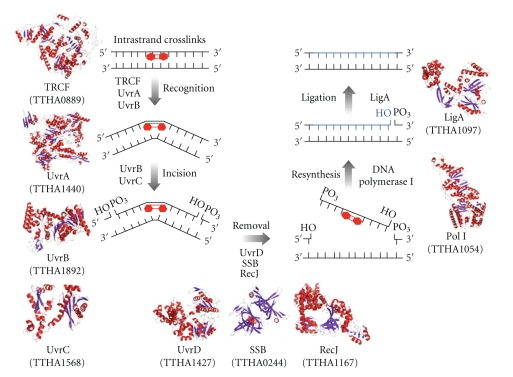
Figure 4.
A schematic representation of models for the nucleotide excision repair pathway controlled by Uvr proteins. All of the predicted protein structures were modeled using SWISS-MODEL. The template structures used in the model building were Geobacillus stearothermophilus UvrA, the N- and C-terminal domain of Thermotoga maritime UvrC, G. stearothermophilus UvrD, Thermus aquaticus DNA polymerase I, Thermus filiformis DNA ligase, and E. coli TRCF. UvrA (TTHA1440; predicted model) and UvrB (TTHA1892; PDB ID: 1D2M) recognize the DNA lesion. In transcribing strand, TRCF (TTHA0889; predicted model) is also involved in recognition of the lesion. UvrC (TTHA1568; predicted model) incises both sides of the lesion. The DNA fragment containing the lesion is excised by UvrD (TTHA1427; predicted model), SSB (TTHA0244; 2CWA), and exonuclease RecJ (TTHA1167; PDB ID: 2ZXO). A new strand is resynthesized by DNA polymerase I (TTHA1054; predicted model) and ligated by DNA ligase (TTHA1097; predicted model).
Most eukaryote species, including humans, possess an NER system. The amino acid sequences of the proteins that act in eukaryotic NER are very different from those of bacterial proteins, but the functions of these proteins are nevertheless similar [101]. The molecular mechanism of NER is more complicated in eukaryotes than bacteria. The eukaryotic NER pathway involves more than ten proteins, including some that are functional homologues of those required for bacterial NER [107].
4.1. Global Genomic Repair (GGR)
Bacterial GGR is a multistep process that removes a wide variety of DNA lesions. In solution, UvrA and UvrB form UvrA2B or UvrA2B2 that can recognize lesions in DNA and can make a stable complex with the DNA [108, 109]. When UvrB detects a lesion, it hydrolyzes ATP to form the pro-preincision complex. After UvrA is released, UvrB binds tightly to DNA and makes a stable UvrB-DNA complex, that is, a pre-incision complex. In this state, UvrB hydrolyzes ATP and can then specifically recognize damage in the absence of UvrA [110]. In E. coli, UvrB can hydrolyze ATP in this step with UvrA but not without UvrA [111]. In T. thermophilus HB8, the UvrB protein (ttUvrB; TTHA1892) shows ATPase activity at its physiological temperature even in the absence of UvrA (ttUvrA; TTHA1440) [112, 113]. Finally, a new pre-incision complex is formed by binding new ATP [110]. UvrC can bind to the pre-incision complex to incise both sides of a DNA lesion. The first incision is made at the fourth or fifth phosphodiester bond on the 3′ side of the lesion and is immediately followed by incision at the eighth phosphodiester bond on the 5′ side [114, 115]. The catalytic sites for 3′ and 5′ incisions are located in different domains of UvrC. It has been reported that the expression levels of uvrA and uvrB are approximately three times higher than that of uvrC (ttha1548) in T. thermophilus [116].
UvrD is a DNA helicase that releases lesion-containing DNA fragments from dsDNA. The purification and characterization of UvrD from T. thermophilus (ttUvrD; TTHA1427) have been reported [117]. After removing the nucleotide fragment, PolI synthesizes a new strand with the same sequence as the removed nucleotide fragment. The newly synthesized sequence is ligated to the adjacent strand by DNA ligase, and all of the repair steps are completed.
4.2. Transcription-Coupled Repair (TCR)
Bacterial TCR is a highly efficient NER system. In 1985, it became apparent that the DNA lesion in the transcribed strand is preferentially repaired [118]. The first consequence of this mechanism is that a stalled RNA polymerase interacts with UvrA with high affinity. Interestingly, however, a stalled RNA polymerase interrupts the NER repair system in vitro [119]. Hence, it was suspected that an unknown factor must release the stalled RNA polymerase and recruit NER proteins. Selby et al. showed in E. coli that the gene product (transcription-repair coupling factor, TRCF) of the mfd gene is the factor involved [106, 120].
TRCF can release a stalled elongation complex but not an initiation complex [106]. The activity for releasing an elongation complex is dependent on ATP hydrolysis. After the complex is released, TRCF can recruit UvrA to the DNA lesion. TRCF has a UvrB homology module, which interacts with UvrA [106, 121]. After recruiting UvrA to the DNA lesion, the subsequent reactions are the same as in GGR. UvrB and DNA form a pre-incision complex, and then UvrC incises both sides of the DNA strand.
The broad substrate specificity of TCR is similar to that of GGR, but TCR repairs lesions with a higher efficiency [106]. In TCR, UvrA can be more rapidly directed to the DNA lesion because the stalled RNA polymerase and TRCF mediate binding of UvrA, whereas, in GGR, UvrA needs to search for DNA lesions across the whole genome without the aid of cofactors. An increased efficiency in finding the substrate also increases the efficiency of the repair system.
4.3. Crystal Structures and Functions of Key Enzymes
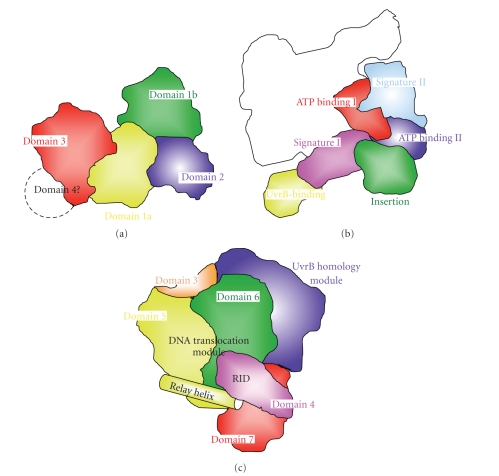
The overall crystal structures of UvrA, UvrB, and TRCF and the two domains of UvrC were determined some years ago [122–128]. In 1999, UvrB was the first of the proteins involved in NER to have its crystal structure established [124, 125, 127]. Later, in 2006, the 3D structure of the UvrB-DNA complex was reported [129]. It was suggested by limited proteolysis that ttUvrB is comprised of four domains, whereas analysis of the 3D structure identified five domains, 1a, 1b, 2, 3, and 4 (Figure 5(a)) [125, 130]. Domain 2 interacts with UvrA, and domain 4 interacts with both UvrA and UvrC. Domains 1a and 3 contain helicase motifs and share high structural similarity to the DNA helicases NS3, PcrA, and Rep. The flexible β-hairpin-connecting domains 1a and 1b are predicted to play important roles in DNA binding. The structure of the UvrB-DNA complex shows that the nucleotide directly behind the β-hairpin is flipped out and inserted into a small pocket in UvrB [129].
Figure 5.
The domain architectures of UvrB, UvrA, and TRCF. (a) UvrB is comprised of five domains. Domains 1a (yellow) and 3 (red) contain helicase motifs. Domain 1b (green) has the flexible β-hairpin invosved in substrate recognition. Domain 2 (blue) interacts with UvrA. Domain 4 is disordered in the crystal structures. (b) UvrA is comprised of six domains: ATP-binding I (red), ATP-binding II (blue), signature I (pink), signature II (cyan), UvrB-binding (yellow), and insertion (green) domain. The white region is the other subunit of the dimer. (c) TRCF is comprised of seven domains. Domains 1 and 2 (not separated in the figure) comprise UvrB homology module (blue). Domains 5 (yellow) and 6 (green) comprise DNA translocation module. Relay helix (yellow) interacts with domain 4 (pink), RNA polymerase interaction domain (RID). The functions of domain 3 (orange) and domain 7 (red) are unclear.
The crystal structures of the N-terminal and C-terminal domains of UvrC were reported in 2005 and 2007, respectively, but the 3D structure of the interdomain loop and of full-length UvrC is still unclear [123, 128]. The N-terminal domain of UvrC catalyzes the 3′ incision reaction and shares homology with the catalytic domain of GIY-YIG family endonucleases. The C-terminal domain of UvrC is responsible for the 5′ incision [123]. It includes an endonuclease domain and an (HhH)2 domain. Despite the lack of sequence homology, the endonuclease domain has an RNase H-like fold. We established the methods of purification of UvrC from T. thermophilus (ttUvrC; TTHA1568), and Hori et al. developed an in vitro reconstitution system of NER using purified ttUvrA, ttUvrB, and ttUvrC [131]. The ttUvrABC system can recognize a (6-4) thymine dimer and excise the affected strand; however, it does not excise a strand containing 8-hydroxy-2′-deoxyguanine or 2-hydroxy-2′-deoxyadenine [131].
The overall structure of UvrA was reported in 2008 [126]. UvrA is comprised of six domains: ATP-binding I, signature I, ATP-binding II, signature II, UvrB-binding, and insertion domains (Figure 5(b)). UvrA has two ATPase modules: one is divided into an ATP-binding domain I and a signature domain I, the other is divided into an ATP-binding domain II and a signature domain II. UvrA contains three zinc ions. It has been reported that ttUvrA and ttUvrB can recognize bulky adducts, such as tetramethylrhodamine and tetramethylrhodamine ethyl ester, and (6-4) pyrimidine dimer [113, 131]. Furthermore, it has been shown that ttUvrA can interact with the ATL protein, but the physiological significance of this interaction remains unclear [37].
The overall structure of TRCF was reported in 2006 [122]. Domains 1a, 2, and 1b comprise a UvrB homology module, which interacts with UvrA (Figure 5(c)). Domains 5 and 6 comprise a DNA translocation module. Domain 4 is an RNA polymerase interaction domain (RID). The RID and the DNA translocation modules are linked by a long helix called the relay helix. The functions of domains 3 and 7 are unclear. The mfd gene from T. thermophilus (the gene product name is ttTRCF; TTHA0889) is listed in the genome annotation but no formal report has yet been published.
The 3D structures of these proteins show that they all contain several enzymatic domains. The NER pathways involve multi-step processes; therefore, almost all the proteins can interact in order to advance the process to the next repair step. TRCF has a UvrA-binding domain whose amino acid sequence and 3D structure are similar to those of the UvrB domain 2 [122]. Therefore, it might be expected that TRCF would bind to UvrA in the same manner as UvrB. The mechanisms of interaction of TRCF with UvrA and other proteins, such as the ATL protein, are not yet well defined.
5. Mismatch Repair
The DNA mismatch repair (MMR) machinery recognizes and corrects mismatched or unpaired bases that principally result from errors by DNA polymerases during DNA replication. MMR increases the accuracy of DNA replication by at least 3 orders of magnitude [132]. Mutations in the genes involved in MMR are associated with increased predisposition to human hereditary nonpolyposis colorectal cancers [133]. Postreplication MMR is achieved by removal of a relatively long tract of mismatch-containing oligonucleotides, a process called long-patch MMR. Here, we refer to long-patch MMR simply as MMR.
5.1. Methyl-Directed MMR in E. coli
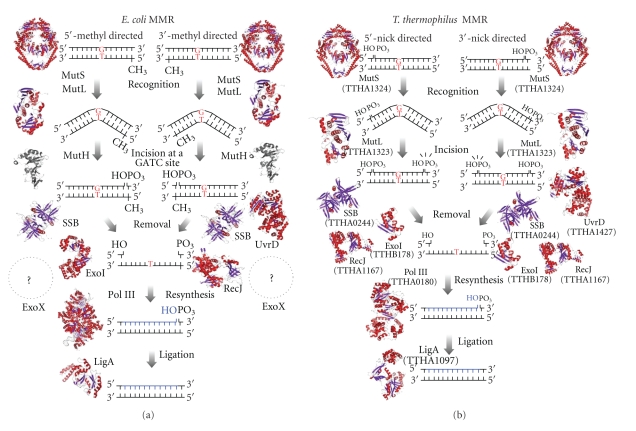
In E. coli, the first steps in MMR are performed by the MutHLS system, which consists of three proteins, MutS, MutL, and MutH (Figure 6(a)) [134, 135]. In this system, a MutS homodimer recognizes and attaches to a mismatched base in the dsDNA [136–138]. A MutL homodimer then interacts with and stabilizes the MutS-mismatch complex and activates a MutH restriction endonuclease [139]. The MMR system needs to discriminate the newly synthesized DNA strand in order to remove the incorrect base of the mismatched pair. However the mismatch itself contains no signal for such discrimination. The E. coli MMR system utilizes the absence of methylation at a restriction site to direct repair to the newly synthesized strand [135]. Immediately after replication, the restriction sites in the newly synthesized strand remain unmethylated. At the site of a mismatch, the MutH endonuclease nicks the unmethylated strand at a hemimethylated GATC site to introduce an entry point for the excision reaction. The error-containing region is excised by a DNA helicase [140] and an ssDNA-specific exonuclease [141–143]. The excised tract of oligonucleotides is then replaced by DNA synthesis directed by DNA polymerase III and a ligase. Since the absence or presence of methylation provides the signal for strand discrimination, E. coli MMR is termed methyl-directed MMR [135]. Homologues of E. coli MutS and MutL are found in almost all organisms; however, no homologue of E. coli MutH has been identified in the majority of eukaryotes or most bacteria.
Figure 6.
A schematic representation of models for MMR pathways in E. coli and mutH-less bacteria. (a) 5′- and 3′-methyl-directed MMR in E. coli. DNA mismatches principally result from misincorporation of bases during DNA replication. The MutS (PDB ID: 1E3M)/MutL (PDB ID: 1NHJ) complex recognizes a mismatch and activates the MutH endonuclease (PDB ID: 1AZO). MutH nicks the unmethylated strand of the duplex to introduce an entry point for the excision reaction. In 3′-methyl-directed MMR, one of the 5′-3′ exonucleases (RecJ and exonuclease VII (ExoVII)) removes the error-containing DNA strand in cooperation with UvrD helicase (PDB ID: 2IS4) and single-stranded DNA-binding protein (SSB; PDB ID: 1EYG). By contrast, one of the 3′-5′ exonucleases (exonuclease I (ExoI; PDB ID: 1FXX) and exonuclease X (ExoX)) is responsible for the 3′-5′ excision reaction. DNA polymerase III (PDB ID: 2HNH) and DNA ligase (PDB ID: 2OWO) synthesize a new strand to complete the repair. (b) A predicted model for 5′- and 3′-nick-directed MMR in T. thermophilus. After recognition of a mismatch by MutS (TTHA1324), MutL (TTHA1323) incises the discontinuous strand of the mismatched duplex to direct the excision reaction to the newly synthesized strand. The error-containing DNA segment is excised by UvrD helicase (TTHA1427), SSB (TTHA0244), and an exonuclease (either RecJ (TTHA1167; PDB ID: 2ZXR) or ExoI (TTHB178)) followed by the resynthesis of a new strand by DNA polymerase III (TTHA0180) and DNA ligase (TTHA1097). The modeled structures of T. thermophilus MutS, MutL (amino acid residues 1–316), ExoI, DNA polymerase III α subunit, DNA ligase, and E. coli RecJ were modeled using SWISS-MODEL. The template structures used for model building were E. coli MutS, the N-terminal domain of MutL, ExoI, UvrD, DNA polymerase III α subunit, DNA ligase, and T. thermophilus RecJ.
5.2. Nick-Directed MMR in Eukaryotes
In eukaryotes, it has been demonstrated that strand discontinuity serves as a signal for directing MMR to a particular strand of the mismatched duplex in vitro. In living cells, newly synthesized strands contain discontinuities as 3′-ends or termini of Okazaki fragments. Since the presence or absence of a nick can be a strand discrimination signal, eukaryotic MMR is termed nick-directed MMR. It has also been reported that the shorter path from a nick to the mismatch is removed by the excision reaction, indicating that 5′- and 3′-nick-directed MMR are distinct [144–147]. Surprisingly, both 5′- and 3′-nick-directed strand removal requires the 5′-3′ exonuclease activity of exonuclease 1 (EXO1) [148, 149]. This apparently contradictory requirement for 5′-3′ exonuclease activity in 3′-nick-directed MMR was explained by the breakthrough discovery that the human MutL homologue MutLα (MLH1-PMS2 heterodimer) and the yeast homologue MutLα (MLH1-PMS1 heterodimer) harbor latent endonuclease activity, which nicks the discontinuous strand of the mismatched duplex [147, 150, 151]. In eukaryotic 5′-nick-directed and 3′-nick-directed MMR, MutLα incises the 3′- and 5′- sides of a mismatch, respectively, to yield a DNA segment spanning the mismatch. Then, the 5′-3′ exonuclease activity of EXO1 removes the segment.
5.3. MMR in mutH-Less Bacteria
The DQHA(X)2E(X)4 motif in the C-terminal domain of the PMS2 subunit of human MutLα comprises the metal-binding site, which is essential for endonuclease activity [150]. In mutH-less bacteria, the C-terminal domains of MutL homologues contain this metal-binding motif and exhibit endonuclease activity [150, 152]; moreover, in T. thermophilus, Aquifex aeolicus, and Neisseria gonorrhoeae, this activity is abolished by mutations in the motif [152–154]. The endonuclease activity of T. thermophilus MutL has been shown to be essential for in vivo DNA repair activity [152]. Thus, the molecular mechanism of MMR in mutH-less bacteria appears to resemble that of eukaryotic MMR (Figure 6(b)).
MutS homologues from mutH-less bacteria show fundamentally similar properties to E. coli MutS and eukaryotic MutSα. First, T. thermophilus MutS exhibits a high affinity for mismatched heteroduplexes [138, 155], and the mismatch-MutS complex seems to be stabilized by MutL [152]. Second, similar ATP binding-dependent conformational changes have been observed in MutS homologues from T. thermophilus [156], E. coli [157, 158], and humans [159, 160]. Third, the crystal structures of Thermus aquaticus MutS [137], E. coli MutS [136, 161], and human MutSα [162] exhibit a common mismatch recognition mode in which the mismatched base is recognized by the intercalated phenylalanine residue from one of the two subunits. Finally, T. thermophilus mutS gene complements the hypermutability of the E. coli mutS-deleted null mutant [138]. These results indicate that interspecies variations in MMR machinery may principally derive from differences in the functions of the MutL homologues.
The biochemical properties of MutL endonucleases have been studied using MutL homologues from mutH-less thermophilic bacteria such as T. thermophilus and A. aeolicus. The endonuclease activity of T. thermophilus MutL is suppressed by the binding of ATP [152]. MutL homologues belong to the GHKL ATPase superfamily that also includes homologues of DNA gyrase, Hsp90, and histidine kinase [163]. GHKL superfamily proteins undergo large conformational changes upon ATP binding and/or hydrolysis. Such conformational changes are expected to affect the molecular functions of the MutL homologues [164, 165]. The endonuclease activities of MutL homologues exhibit no sequence or structure specificity [150, 152]; hence, it is thought that living cells may have mechanisms for regulating these activities. Cells may employ ATP binding-induced suppression of MutL endonuclease activity in order to ensure mismatch-specific incision. It has also been suggested that the ATP binding form of T. thermophilus MutL preferably interacts with a MutS-mismatch complex [152]. Since the ATPase activity of MutL is activated by interaction with MutS, it could be speculated that the ATP binding-dependent suppression of the endonuclease activity of MutL is canceled by the interaction with a MutS-mismatch complex. Recently, it was reported that the endonuclease activity of A. aeolicus MutL in response to ATP depends on the concentration of the protein and that when A. aeolicus MutL is present at relatively high concentrations activity is stimulated, not suppressed, by ATP [154]. This result indicates that ATP is required not only for suppression but also for active enhancement of the endonuclease activity of MutL.
5.4. Strand Discrimination in Nick-Directed MMR
As mentioned above, a pre-existing strand break serves as a signal to direct the excision reaction in eukaryotic nick-directed MMR [146, 150]. Since daughter strands always contain 3′- or 5′- termini during replication, these ends may act as strand discrimination signals in vivo. In eukaryotic nick-directed MMR, MutLα is responsible for strand discrimination by incising the discontinuous strand [150]. Interestingly, MutLα has been found to incise the discontinuous strand at a distal site from the pre-existing strand break. It remains to be elucidated how MutLα discriminates the discontinuous strand of the duplex at a site far removed from the strand discrimination signal. One possible explanation may lie in the association of MutS and MutL homologues with replication machinery. MSH6 and MSH3 subunits contain a PCNA-interacting motif [166], and this interaction between MutSα and PCNA is now well characterized [167]. Furthermore, both PCNA and replication factor C (RFC) are required for stimulation of the latent endonuclease activity of MutLα in eukaryotic MMR [150]. These results suggest that MutSα (or MutSβ) and MutLα are loaded onto the substrate DNA through an interaction with PCNA in the presence of RFC to produce binding to the newly synthesized strand in the catalytic site of the MutLα endonuclease domain [168–170]. In mutH-less bacteria, it has been also demonstrated that mismatch-provoked localization of MutS and MutL is controlled through an association with β-clamp, a bacterial counterpart of eukaryotic PCNA [171]. These interactions may also be responsible for strand discrimination in bacterial nick-directed MMR.
5.5. Downstream Events in Nick-Directed MMR
EXO1 is responsible for the excision reaction in eukaryotic MMR in vitro. To date, EXO1 is the only ssDNA-specific exonuclease that has been reported to be involved in the reaction [150, 172]. In addition, no MMR-related eukaryotic DNA helicase has yet been identified. The exonuclease activity of eukaryotic EXO1 is enhanced by a direct interaction with MutSα in a mismatch- and ATP-dependent manner [173]. MutSα is known to form a sliding clamp that diffuses along the DNA after mismatch recognition. The diffusion of MutSα from the mismatch may be required for the activation of EXO1 at the 5′-terminus of the error-containing DNA segments. In contrast to eukaryotes, the MutL of A. aeolicus stimulates DNA helicase activity in UvrD, an enzyme that shows high conservation of amino acid sequence among bacteria [174]. Furthermore, in T. thermophilus, genetic analyses have indicated that 5′-3′ exonuclease RecJ and 3′-5′ exonuclease ExoI are involved in parallel pathways of MMR [175]. It is possible that mutH-less bacteria employ the cooperative function of multiple exonucleases and helicases to remove error-containing DNA segments.
Termination of the EXO1-dependent excision reaction in eukaryotic 3′-nick-directed and MutLα-dependent 5′-nick-directed MMR is expected to be determined by pre-existing and newly introduced 3′-termini, respectively. In mutH-less bacteria, the mechanism for termination of the excision-reaction remains unknown. Since not only 5′-3′ exonuclease but also 3′-5′ exonuclease can be involved in the repair [175], termination of an excision reaction in 5′- and 3′-nick-directed MMR might be achieved by the 3′- and 5′-termini that are introduced by MutL.
Further biochemical and structural studies on exonucleases are required to achieve a deeper understanding of the excision reaction. Recently, the crystal structure of intact RecJ, a 5′-3′ exonuclease, from T. thermophilus was reported [176]. The entire structure of RecJ consists of four domains that form a ring-like structure with the catalytic site in the center of the ring. One of these four domains contains an oligonucleotides/oligosaccharide-binding fold that is known as a nucleic acid-binding fold. Knowledge of these structural features increases our understanding of the molecular basis for the high processivity and specificity of this enzyme. Furthermore, two Mn2+ ions in the catalytic site suggest that RecJ utilizes a two-metal ion mechanism [177] for the exonuclease activity. The understanding of a 3′-5′ exonuclease in MMR has been also enhanced by the ongoing biochemical studies on T. thermophilus ExoI [175]. The study revealed that ExoI has extremely high K M value compared with other exonucleases. The interactions with other MMR proteins might stimulate the DNA-binding activity of ExoI. Especially, it would be intriguing to examine the interaction between ExoI and MutS.
6. Recombination Repair
DNA double-strand breaks (DSBs) are the most crucial lesions in DNA for inducing loss of genetic information and chromosomal instabilities. DSBs can be caused by ionizing radiation, ROS, nuclease dysfunction, or replication fork collapse [178]. Defects in the repair of DSBs lead to cancer or other severe diseases [179–181]. There are two different pathways for repair of DSBs, homologous recombination (HR) and nonhomologous end-joining [178]. HR is the accurate pathway and makes use of undamaged homologous DNA as a template for repair. Nonhomologous end-joining directly ligates two DSB ends together, and although it is efficient, it is prone to loss of genetic information at the ligation sites. In most bacteria, the HR pathway is thought to be the major route for repair of DSBs [182–184].
Recombination repair of DSBs consists of various steps: end resection, strand invasion, DNA repair synthesis, branch migration, and Holliday junction (HJ) resolution (Figure 7). Although the repair-related components and details of each step show variations among organisms, these steps are conserved in all organisms, and there are many evolutionarily conserved functional homologues involved in recombination repair [182, 184]. The first step of recombination repair, end resection, is initiated by a 5′ to 3′ degradation of DSB ends to generate 3′-ssDNA tails. Next, mediator proteins bind to the 3′-tailed ssDNA and load the recombinase to promote formation of a nucleoprotein filament. The recombinase searches for a homologous DNA sequence and catalyzes strand invasion to yield a D-loop structure. After strand invasion, DNA synthesis occurs using the homologous DNA as the template, and the intermediates are processed through a branch migration reaction to form HJs, stable four-stranded DNA structures. Finally, HJs are endonucleolytically resolved into linear duplexes, and the nicks at cleavage site are sealed by DNA ligase to complete the repair. HR significantly contributes to retention of genome integrity; however, this mechanism is also utilized for the rearrangement of genome, such as incorporation of foreign DNAs or intrachromosomal gene conversion [185, 186]. There are various anti-recombination mechanisms to suppress excessive recombination that might cause genomic instabilities [187, 188]. These sub-pathways interact with each other to regulate the HR system.
Figure 7.
A schematic pathway of recombination repair and structures of the proteins involved in T. thermophilus. Recombination repair of DSBs is initiated by an end resection step in which DSB ends are processed by the concerted action of RecJ nuclease (TTHA1167; PDB ID: 2ZXR) and SSB (TTHA0244; PDB ID: 2CWA) to form 3′-ssDNA tails. After end resection, the SSB-ssDNA complex is disassembled and RecA recombinase (TTHA1818) is loaded onto ssDNA by “mediators”, RecF (TTHA0264), RecO (TTHA0623), and RecR (TTHA1600), to promote strand invasion. DNA repair synthesis is primed by PolI (TTHA1054) and PolIII (TTHA0180) from the invaded strand of the D-loop structure. Alternatively, second-end capture is mediated by RecO and SSB and branch migration mediated by the RuvA-RuvB complex (TTHA0291-TTHA0406; PDB ID: 1IXR) and RecG (TTHA1266) to yield HJs. HJs are cleaved by RuvC resolvase (TTHA1090) and the nicks sealed by LigA (TTHA1097). Newly synthesized DNA is colored in blue. The model structures of T. thermophilus RecA, RecF, RecO, RecR, PolI, PolIII α subunit, RecG, RuvC, and LigA were generated using SWISS-MODEL. The models were based on the structures of Mycobacterium smegmatis RecA (PDB ID: 2OE2), D. radiodurans RecF (PDB ID: 2O5V), RecO (PDB ID: 1U5K), RecR (PDB ID: 1VDD), E. coli PolI (PDB ID: 1TAU), PolIII α subunit (PDB ID: 2HNH), RuvC (PDB ID: 1HJR), LigA (PDB ID: 2OWO), and Thermotoga maritima RecG (PDB ID: 1GM5).
6.1. End Resection and Loading of Recombinase
Recombination repair is initiated by an end resection step that processes DSB ends to generate 3′-ssDNA tails. In mammals, various nucleases and helicases have been implicated in this step, such as the MRN complex, CTIP, EXO1, DNA2, and RECQ paralogues [189]. By contrast, most bacteria have two major sub-pathways, the RecF pathway and the RecBCD/AddAB pathway [183, 190, 191]. The RecF pathway is highly conserved in many bacteria and is similar to the eukaryotic end resection pathway whereas the RecBCD/AddAB pathway differs from that of eukaryotes and also shows diversity in bacteria. In the RecF pathway, RecJ nuclease, RecQ helicase, and SSB act in concert in the processing of DSB ends. After DNA unwinding by RecQ helicase and 5′ to 3′ exonucleolytic degradation by RecJ nuclease, the generated 3′-ssDNA tails are coated and stabilized with SSB [192]. Interestingly, there is no RecQ homologue in T. thermophilus HB8 [193]. However, a recent in vitro reconstitution study of the E. coli RecF pathway showed that RecJ nuclease degrades dsDNA exonucleolytically in the absence of RecQ helicase [190]. Another study also showed that Haemophilus influenzae SSB directly interacts with RecJ nuclease and stimulates exonuclease activity [194]. Based on these results, it could be speculated that in T. thermophilus HB8, RecJ nuclease and SSB might synergistically perform the end resection step without involvement of a helicase. Recently, the crystal structures of T. thermophilus RecJ and SSB were solved [176]. By combining these structural data with biochemical analyses, it should soon be feasible to elucidate the molecular mechanism of the end resection step.
In the RecF pathway, after generation of 3′-ssDNA tails, recombination mediators, RecFOR or RecOR, disassemble the SSB-ssDNA complex and load RecA recombinase onto ssDNA to form nucleoprotein filaments [190, 195]. Structural and biochemical analyses of T. thermophilus RecF, RecO, and RecR proteins showed that RecR forms a tetrameric ring-like structure and acts as a DNA clamp and also binds to RecF and RecO; on the other hand, RecO can also bind to RecR, SSB, and ssDNA [196–198]. These studies found that SSB is displaced from ssDNA by RecO and that RecA loading is mediated by RecR [198]. Based on these results, there appear to be two distinct ways for SSB displacement and RecA loading [190]. The RecFOR complex binds at the ssDNA-dsDNA junction on the resected DNA and loads RecA onto ssDNA in a 5′ to 3′ direction. The RecOR complex binds to SSB-ssDNA complex and promotes the exchange of SSB by RecA. These processes are very similar to the eukaryotic recombination repair pathway mediated by RAD52, RAD54, BRCA2, and RAD51 paralogues [199–202]. Recombinase loading by “mediators” is thought to be a common system of recombination repair in all three kingdoms of life.
6.2. Strand Invasion by Recombinase
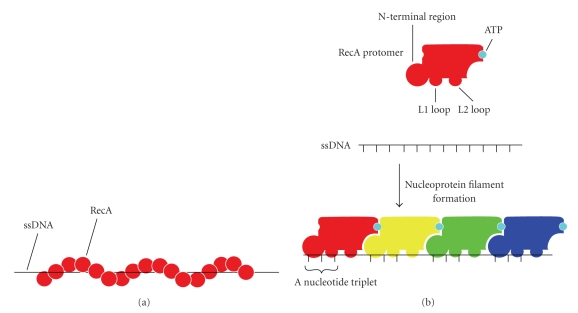
The DNA strand exchange between homologous segments of chromosomes is catalyzed by the RecA-family recombinases, which include RecA in bacteria, RAD51 in eukaryotes, and RadA in archaea [203]. The processes catalyzed by these recombinases have been studied in detail [204–206]. In bacteria, RecA binds to ssDNA, forming helical nucleoprotein filament (Figure 8(a)). Contact between the RecA-coated ssDNA and dsDNA allows ssDNA to search sequence homology. Strand exchange is initiated by local denaturation of dsDNA in a region of homology. The invading strand forms a paranemic joint, which is an unstable intermediate. When the free end of the strand invades, the paranemic joint is converted into a plectonemic joint, in which the two strands are intertwined. Then heteroduplex formation is extended by branch migration.
Figure 8.
A schematic illustration of RecA-ssDNA interaction in the nucleoprotein filament. (a) A schematic representation of a RecA-ssDNA nucleoprotein filament. The filament comprises a helical structure. RecA molecules are shown as red spheres and the ssDNA as a black line. (b) A schematic model of RecA-ssDNA interaction. The RecA protomer has the L1 and L2 loops and the N-terminal region to make contact with the ssDNA. The bound ssDNA comprises a nucleotide triplet with a nearly normal B-form distance between bases followed by a long internucleotide stretch before the next triplet. The ATP binds to RecA-RecA interfaces. The schematic model was prepared from the crystal structure of RecA-ssDNA complex (PDB ID: 3CMW).
The crystal structure of RecA filament determined in 1992 [207] revealed six subunits in each helical turn, but this structure contained no DNA. In 2008, Chen et al. determined the structures of both RecA complexed with ssDNA and with dsDNA [208], which are the substrate and product forms of DNA strand exchange, respectively. The RecA-ssDNA filament is different from the RecA filament primarily in the orientation of the subunit relative to the filament axis. The bound ssDNA makes contact with the L1 and L2 loops, which had been suggested to be DNA binding sites and the N-terminal region (Figure 8(b)). It had been previously assumed that in the nucleoprotein filament ssDNA is uniformly stretched by about 1.5-fold [209]. However, unexpectedly, the DNA comprises a nucleotide triplet (three-nucleotide segment) with a nearly normal B-form distance between bases followed by a long untwisted internucleotide stretch before the next triplet. In addition, ATP binds to RecA-RecA interfaces, which can couple RecA-ATP interaction to RecA-DNA interaction.
6.3. Postsynaptic Phase
After strand invasion, HJs are formed through DNA repair synthesis, second-end capture, and branch migration during the postsynaptic phase. In most organisms, a range of DNA polymerases deal with the various DNA processes, and several of these DNA polymerases are involved in recombination-associated DNA repair synthesis [210]. It has been shown that the translesion synthesis (TLS) polymerase, Polη, and replicative polymerase, Polδ, are involved in mammalian recombination-associated DNA synthesis [211–214]. In addition, a recent genetic study suggested the possible involvement of human Polν, prokaryotic PolI-like enzyme, in HR [215]. However, it is still unclear whether other DNA polymerases can synthesize the DNA strand during recombination. Interestingly, bacterial TLS polymerases, PolII, PolIV, and PolV, are also able to synthesize the DNA strand in recombination processes as well as PolI and PolIII in E. coli; however, the details of the relationship between TLS and HR remain to be elucidated [216]. The Deinococcus-Thermus group of bacteria has only two processive DNA polymerases, PolI and PolIII, and, therefore, it should be relatively straightforward to analyze the involvement of DNA polymerases in recombination-associated DNA synthesis [217, 218]. A recent study on genome repair after ionizing radiation in Deinococcus radiodurans showed that PolI and PolIII had distinct roles in the extensive synthesis-dependent strand annealing repair pathway [219]; therefore, it might be expected that in T. thermophilus, PolI and PolIII will also act in concert in recombination-associated DNA synthesis.
Second-end capture and branch migration also occur at the same time as DNA repair synthesis in the postsynaptic phase. In eukaryotes, second-end capture appears to be mediated by RAD52 and RPA, whereas their functional homologues in bacteria are RecO and SSB, respectively [220–222]. Interestingly, it has been shown that E. coli RecO cannot form joint molecules with the S. cerevisiae RPA-ssDNA complex nor can S. cerevisiae RAD52 promote second-end capture with either the human RPA-ssDNA complex or the E. coli SSB-ssDNA complex [222]. These results indicate that the second-end capture event can be performed in a species-specific manner. Various DNA translocases are involved in branch migration. There is evidence that RAD54 and RECQ paralogues process the joint molecules to generate HJs in eukaryotes. By contrast, RuvAB, RecG and RadA/Sms promote branch migration in bacteria [201, 223–225]. To date, there is no satisfactory explanation as to why a single organism might redundantly possess multiple branch migration activities. In bacteria, RuvAB are believed to be the main branch migration proteins based on their genetic properties [223, 226]. Currently, the crystal structure of the RuvAB-HJ complex is not available. However, various crystal structures involving T. thermophilus RuvA and RuvB proteins have been solved and their biochemical properties determined [227–232]. In addition, an atomic model of the RuvAB-HJ complex has been proposed based on data from electron microscopic analyses [229, 233]. These structural and functional analyses of RuvAB provide insights into its molecular properties with regard to branch migration. Two RuvA tetramers sandwich an HJ forming a planar conformation while two RuvB hexameric rings are bound to the arms of the junction symmetrically via RuvA and promote branch migration using energy from ATP hydrolysis [224]. Furthermore, by combining structural and biochemical data on RuvC resolvase, it is possible to suggest a model for HJ resolution that involves the formation of a RuvABC resolvasome [224, 234–237].
Recombination repair is completed by HJ resolution and sealing of its cleavage sites. In mammals, members of a structure-specific endonuclease family, including GEN1, SLX1, MUS81-EME1, and ERCC4-ERCC1, are involved in the resolution of HJs and recombination intermediates [238]. Recent work showed that GEN1 can act as an HJ resolvase. Other studies have suggested that the SLX4 protein can form a complex with SLX1, MUS81-EME1, or ERCC4-ERCC1 and control their activities [239–243]. It has been shown that the SLX1-SLX4 complex can resolve HJs symmetrically. In bacteria, RuvC and RusA have HJ resolvase activity. RuvC forms a dimeric structure and cleaves HJs symmetrically in a sequence-specific manner [234, 244]. Biochemical analyses of RuvC in the presence of RuvAB suggest that RuvC forms a complex with RuvAB and that the HJ resolution event is coupled with the branch migration reaction [235, 236]. In E. coli, there is another resolvase, RusA, which has cleaved HJs symmetrically at specific sites [245, 246]. It has also been suggested that topoisomerase III can resolve HJs in E. coli as an alternative to the RuvABC pathway [247]. T. thermophilus does not have either RusA or topoisomerase III [217]. Thus, this organism will be a suitable model for analyzing this step of HJ resolution because of its simple and minimal systems.
6.4. Anti-Recombination
Since excessive recombination events lead to the alteration of the genetic information, various anti-recombination mechanisms are employed by organisms to regulate the frequency of recombination [188]. For example, the MMR system is present in a wide range of organisms and serves particularly to prevent homeologous recombination [187]. In bacteria, RecX acts as an anti-recombinase that inhibits RecA recombinase in both direct and indirect manners [248]. Direct interaction with RecX inhibits the recombinase activity of RecA and destabilizes the nucleoprotein filament [249, 250]. RecX also suppresses recA induction at the transcription level [248]. The UvrD helicase is suspected to be an anti-recombinase because of its activity to disassemble the RecA nucleoprotein filament in vitro [251, 252].
Recently, a novel anti-recombination mechanism was identified in Helicobacter pylori and T. thermophilus. It was found that disruption of mutS2, a bacterial paralogue of the MMR gene mutS, significantly increased the frequency of recombination events, indicating that mutS2 had an anti-recombination function [253, 254]. It has also been shown that MutS2 is not involved in MMR, that is, MutS2 prevents recombination in an MMR-independent manner. Detailed biochemical investigation showed that T. thermophilus MutS2 possesses an endonuclease activity that preferably incises the D-loop structure, the primary intermediate in HR [253, 255–257]. MutS2 might suppress HR through the resolution of early intermediates.
Acknowledgments
This work was supported in part by Grant-in-Aid for Scientific Research 20570131 (to R. Masui), 19770083 (to N. Nakagawa) and 20870042 (to K. Fukui) from the Ministry of Education, Science, Sports and Culture of Japan. R. Morita and S. Nakane are the recipients of a Research Fellowship of the Japan Society for the Promotion of Science for Young Scientists (no. 21-1019 and 22-37, resp.).
References
- 1.Schärer OD. Chemistry and biology of DNA repair. Angewandte Chemie. 2003;42(26):2946–2974. doi: 10.1002/anie.200200523. [DOI] [PubMed] [Google Scholar]
- 2.Altieri F, Grillo C, Maceroni M, Chichiarelli S. DNA damage and repair: from molecular mechanisms to health implications. Antioxidants and Redox Signaling. 2008;10(5):891–937. doi: 10.1089/ars.2007.1830. [DOI] [PubMed] [Google Scholar]
- 3.Friedberg EC, Walker GC, Siede W. DNA Repair and Mutagenesis. Washington, DC, USA: ASM Press; 2006. [Google Scholar]
- 4.Oshima T, Imahori K. Description of Thermus thermophilus (Yoshida and Oshima) comb. nov., a nonsporulating thermophilic bacterium from a Japanese thermal spa. International Journal of Systematic Bacteriology. 1974;24(1):102–112. [Google Scholar]
- 5.Iino H, Naitow H, Nakamura Y, et al. Crystallization screening test for the whole-cell project on Thermus thermophilus HB8. Acta Crystallographica Section F. 2008;64(6):487–491. doi: 10.1107/S1744309108013572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yokoyama S, Hirota H, Kigawa T, et al. Structural genomics projects in Japan. Nature Structural Biology. 2000;7, supplement:943–945. doi: 10.1038/80712. [DOI] [PubMed] [Google Scholar]
- 7.Payne G, Heelis PF, Rohrs BR, Sancar A. The active form of Escherichia coli DNA photolyase contains a fully reduced flavin and not a flavin radical, both in vivo and in vitro. Biochemistry. 1987;26(22):7121–7127. doi: 10.1021/bi00396a038. [DOI] [PubMed] [Google Scholar]
- 8.Sancar A, Sancar GB. DNA repair enzymes. Annual Review of Biochemistry. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- 9.Aubert C, Vos MH, Mathis P, Eker APM, Brettel K. Intraprotein radical transfer during photoactivation of DNA photolyase. Nature. 2000;405(6786):586–590. doi: 10.1038/35014644. [DOI] [PubMed] [Google Scholar]
- 10.Kato R, Hasegawa K, Hidaka Y, Kuramitsu S, Hoshino T. Characterization of a thermostable DNA photolyase from an extremely thermophilic bacterium, Thermus thermophilus HB27. Journal of Bacteriology. 1997;179(20):6499–6503. doi: 10.1128/jb.179.20.6499-6503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park H-W, Kim S-T, Sancar A, Deisenhofer J. Crystal structure of DNA photolyase from Escherichia coli . Science. 1995;268(5219):1866–1872. doi: 10.1126/science.7604260. [DOI] [PubMed] [Google Scholar]
- 12.Tamada T, Kitadokoro K, Higuchi Y, et al. Crystal structure of DNA photolyase from Anacystis nidulans . Nature Structural Biology. 1997;4(11):887–891. doi: 10.1038/nsb1197-887. [DOI] [PubMed] [Google Scholar]
- 13.Komori H, Masui R, Kuramitsu S, et al. Crystal structure of thermostable DNA photolyase: pyrimidine-dimer recognition mechanism. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(24):13560–13565. doi: 10.1073/pnas.241371398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torizawa T, Ueda T, Kuramitsu S, et al. Investigation of the cyclobutane pyrimidine dimer (CPD) photolyase DNA recognition mechanism by NMR analyses. Journal of Biological Chemistry. 2004;279(31):32950–32956. doi: 10.1074/jbc.M404536200. [DOI] [PubMed] [Google Scholar]
- 15.Mees A, Klar T, Gnau P, et al. Crystal structure of a photolyase bound to a CPD-like DNA lesion after in situ repair. Science. 2004;306(5702):1789–1793. doi: 10.1126/science.1101598. [DOI] [PubMed] [Google Scholar]
- 16.Ueda T, Kato A, Ogawa Y, et al. NMR study of repair mechanism of DNA photolyase by FAD-induced paramagnetic relaxation enhancement. Journal of Biological Chemistry. 2004;279(50):52574–52579. doi: 10.1074/jbc.M409942200. [DOI] [PubMed] [Google Scholar]
- 17.Ueda T, Kato A, Kuramitsu S, Terasawa H, Shimada I. Identification and characterization of a second chromophore of DNA photolyase from Thermus thermophilus HB27. Journal of Biological Chemistry. 2005;280(43):36237–36243. doi: 10.1074/jbc.M507972200. [DOI] [PubMed] [Google Scholar]
- 18.Klar T, Kaiser G, Hennecke U, Carell T, Batschauer A, Essen L-O. Natural and non-natural antenna chromophores in the DNA photolyase from Thermus thermophilus . ChemBioChem. 2006;7(11):1798–1806. doi: 10.1002/cbic.200600206. [DOI] [PubMed] [Google Scholar]
- 19.Kiefer F, Arnold K, Künzli M, Bordoli L, Schwede T. The SWISS-MODEL repository and associated resources. Nucleic Acids Research. 2009;37(supplement 1):D387–D392. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwede T, Kopp J, Guex N, Peitsch MC. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Research. 2003;31(13):3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Der Horst GTJ, Muijtjens M, Kobayashi K, et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398(6728):627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- 22.Müller M, Carell T. Structural biology of DNA photolyases and cryptochromes. Current Opinion in Structural Biology. 2009;19(3):277–285. doi: 10.1016/j.sbi.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Loechler EL, Green CL, Essigmann JM. In vivo mutagenesis by O 6-methylguanine built into a unique site in a viral genome. Proceedings of the National Academy of Sciences of the United States of America. 1984;81(20):6271–6275. doi: 10.1073/pnas.81.20.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969;223(5202):206–207. doi: 10.1038/223206a0. [DOI] [PubMed] [Google Scholar]
- 25.Snow ET, Foote RS, Mitra S. Base-pairing properties of O 6-methylguanine in template DNA during in vitro DNA replication. Journal of Biological Chemistry. 1984;259(13):8095–8100. [PubMed] [Google Scholar]
- 26.Lindahl T, Demple B, Robins P. Suicide inactivation of the E. coli O 6-methylguanine-DNA methyltransferase. EMBO Journal. 1982;1(11):1359–1363. doi: 10.1002/j.1460-2075.1982.tb01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsson M, Lindahl T. Repair of alkylated DNA in Escherichia coli. Methyl group transfer from O 6-methylguanine to a protein cysteine residue. Journal of Biological Chemistry. 1980;255(22):10569–10571. [PubMed] [Google Scholar]
- 28.Wiestler O, Kleihues P, Pegg AE. O 6-alkylguanine-DNA alkyltransferase activity in human brain and brain tumors. Carcinogenesis. 1984;5(1):121–124. doi: 10.1093/carcin/5.1.121. [DOI] [PubMed] [Google Scholar]
- 29.Bhattacharyya D, Foote RS, Boulden AM, Mitra S. Physicochemical studies of human O 6-methylguanine-DNA methyltransferase. European Journal of Biochemistry. 1990;193(2):337–343. doi: 10.1111/j.1432-1033.1990.tb19343.x. [DOI] [PubMed] [Google Scholar]
- 30.Boulden AM, Foote RS, Fleming GS, Mitra S. Purification and some properties of human DNA-O 6-methylguanine methyltransferase. Journal of Biosciences. 1987;11(1–4):215–224. [Google Scholar]
- 31.Demple B, Jacobsson A, Olsson M. Repair of alkylated DNA in Escherichia coli. Physical properties of O 6-methylguanine-DNA methyltransferase. Journal of Biological Chemistry. 1982;257(22):13776–13780. [PubMed] [Google Scholar]
- 32.Daniels DS, Tainer JA. Conserved structural motifs governing the stoichiometric repair of alkylated DNA by O 6-alkylguanine-DNA alkyltransferase. Mutation Research. 2000;460(3-4):151–163. doi: 10.1016/s0921-8777(00)00024-0. [DOI] [PubMed] [Google Scholar]
- 33.Daniels DS, Woo TT, Luu KX, et al. DNA binding and nucleotide flipping by the human DNA repair protein AGT. Nature Structural and Molecular Biology. 2004;11(8):714–720. doi: 10.1038/nsmb791. [DOI] [PubMed] [Google Scholar]
- 34.Aramini JM, Tubbs JL, Kanugula S, et al. Structural basis of O 6-alkylguanine recognition by a bacterial alkyltransferase-like DNA repair protein. Journal of Biological Chemistry. 2010;285(18):13736–13741. doi: 10.1074/jbc.M109.093591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Margison GP, Butt A, Pearson SJ, et al. Alkyltransferase-like proteins. DNA Repair. 2007;6(8):1222–1228. doi: 10.1016/j.dnarep.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 36.Mazon G, Philippin G, Cadet J, Gasparutto D, Fuchs RP. The alkyltransferase-like ybaZ gene product enhances nucleotide excision repair of O 6-alkylguanine adducts in E. coli . DNA Repair. 2009;8(6):697–703. doi: 10.1016/j.dnarep.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 37.Morita R, Nakagawa N, Kuramitsu S, Masui R. An O6-methylguanine-DNA methyltransferase-like protein from Thermus thermophilus interacts with a nucleotide excision repair protein. Journal of Biochemistry. 2008;144(2):267–277. doi: 10.1093/jb/mvn065. [DOI] [PubMed] [Google Scholar]
- 38.Pearson SJ, Ferguson J, Santibanez-Koref M, Margison GP. Inhibition of O6-methylguanine-DNA methyltransferase by an alkyltransferase-like protein from Escherichia coli . Nucleic Acids Research. 2005;33(12):3837–3844. doi: 10.1093/nar/gki696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pearson SJ, Wharton S, Watson AJ, et al. A novel DNA damage recognition protein in Schizosaccharomyces pombe . Nucleic Acids Research. 2006;34(8):2347–2354. doi: 10.1093/nar/gkl270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tubbs JL, Latypov V, Kanugula S, et al. Flipping of alkylated DNA damage bridges base and nucleotide excision repair. Nature. 2009;459(7248):808–813. doi: 10.1038/nature08076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Breimer LH, Lindahl T. DNA glycosylase activities for thymine residues damaged by ring saturation, fragmentation, or ring contraction are functions of endonuclease III in Escherichia coli . Journal of Biological Chemistry. 1984;259(9):5543–5548. [PubMed] [Google Scholar]
- 42.Demple B, Johnson A, Fung D. Exonuclease III and endonuclease IV remove 3′ blocks from DNA synthesis primers in H2O2-damaged Escherichia coli . Proceedings of the National Academy of Sciences of the United States of America. 1986;83(20):7731–7735. doi: 10.1073/pnas.83.20.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levin JD, Johnson AW, Demple B. Homogeneous Escherichia coli endonuclease IV. Characterization of an enzyme that recognizes oxidative damage in DNA. Journal of Biological Chemistry. 1988;263(17):8066–8071. [PubMed] [Google Scholar]
- 44.McCarthy TV, Lindahl T. Methyl phosphotriesters in alkylated DNA are repaired by the Ada regulatory protein of E. coli . Nucleic Acids Research. 1985;13(8):2683–2698. doi: 10.1093/nar/13.8.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Landini P, Volkert MR. Regulatory responses of the adaptive response to alkylation damage: a simple regulon with complex regulatory features. Journal of Bacteriology. 2000;182(23):6543–6549. doi: 10.1128/jb.182.23.6543-6549.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sedgwick B, Lindahl T. Recent progress on the Ada response for inducible repair of DNA alkylation damage. Oncogene. 2002;21(58):8886–8894. doi: 10.1038/sj.onc.1205998. [DOI] [PubMed] [Google Scholar]
- 47.Trewick SC, Henshaw TF, Hausinger RP, Lindahl T, Sedgwick B. Oxidative demethylation by Escherichia coli AlkB directly reverts DNA base damage. Nature. 2002;419(6903):174–178. doi: 10.1038/nature00908. [DOI] [PubMed] [Google Scholar]
- 48.Kurowski MA, Bhagwat AS, Papaj G, Bujnicki JM. Phylogenomic identification of five new human homologs of the DNA repair enzyme AlkB. BMC Genomics. 2003;4(1, article no. 48) doi: 10.1186/1471-2164-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duncan T, Trewick SC, Koivisto P, Bates PA, Lindahl T, Sedgwick B. Reversal of DNA alkylation damage by two human dioxygenases. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(26):16660–16665. doi: 10.1073/pnas.262589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei Y-F, Carter KC, Wang R-P, Shell BK. Molecular cloning functional analysis of a human cDNA encoding an Escherichia coli AlkB homolog, a protein involved in DNA alkylation damage repair. Nucleic Acids Research. 1996;24(5):931–937. doi: 10.1093/nar/24.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aas PA, Otterlei M, Falnes PØ, et al. Human and bacterial oxidative demethylases repair alkylation damage in both RNA and DNA. Nature. 2003;421(6925):859–863. doi: 10.1038/nature01363. [DOI] [PubMed] [Google Scholar]
- 52.Gates KS. An overview of chemical processes that damage cellular DNA: spontaneous hydrolysis, alkylation, and reactions with radicals. Chemical Research in Toxicology. 2009;22(11):1747–1760. doi: 10.1021/tx900242k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gibbs MD, Reeves RA, Mandelman D, Mi Q, Lee J, Bergquist PL. Molecular diversity and catalytic activity of Thermus DNA polymerases. Extremophiles. 2009;13(5):817–826. doi: 10.1007/s00792-009-0269-8. [DOI] [PubMed] [Google Scholar]
- 54.Makiela-Dzbenska K, Jaszczur M, Banach-Orlowska M, Jonczyk P, Schaaper RM, Fijalkowska IJ. Role of Escherichia coli DNA polymerase I in chromosomal DNA replication fidelity. Molecular Microbiology. 2009;74(5):1114–1127. doi: 10.1111/j.1365-2958.2009.06921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshiyama K, Higuchi K, Matsumura H, Maki H. Directionality of DNA replication fork movement strongly affects the generation of spontaneous mutations in Escherichia coli . Journal of Molecular Biology. 2001;307(5):1195–1206. doi: 10.1006/jmbi.2001.4557. [DOI] [PubMed] [Google Scholar]
- 56.Dalhus B, Laerdahl JK, Backe PH, Bjørås M. DNA base repair—recognition and initiation of catalysis. FEMS Microbiology Reviews. 2009;33(6):1044–1078. doi: 10.1111/j.1574-6976.2009.00188.x. [DOI] [PubMed] [Google Scholar]
- 57.Zharkov DO. Base excision DNA repair. Cellular and Molecular Life Sciences. 2008;65(10):1544–1565. doi: 10.1007/s00018-008-7543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Visnes T, Doseth B, Pettersen HS, et al. Uracil in DNA and its processing by different DNA glycosylases. Philosophical Transactions of the Royal Society B. 2009;364(1517):563–568. doi: 10.1098/rstb.2008.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fromme JC, Banerjee A, Verdine GL. DNA glycosylase recognition and catalysis. Current Opinion in Structural Biology. 2004;14(1):43–49. doi: 10.1016/j.sbi.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 60.Ischenko AA, Saparbaev MK. Alternative nucleotide incision repair pathway for oxidative DNA damage. Nature. 2002;415(6868):183–187. doi: 10.1038/415183a. [DOI] [PubMed] [Google Scholar]
- 61.Motta ES, Souza-Santos PT, Cassiano TR, Dantas FJS, Caldeira-De-Araujo A, De Mattos JCP. Endonuclease IV is the main base excision repair enzyme involved in DNA damage induced by UVA radiation and stannous chloride. Journal of Biomedicine and Biotechnology. 2010;2010:9 pages. doi: 10.1155/2010/376218. Article ID 376218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mundle ST, Delaney JC, Essigmann JM, Strauss PR. Enzymatic mechanism of human apurinic/apyrimidinic endonuclease against a THF AP site model substrate. Biochemistry. 2009;48(1):19–26. doi: 10.1021/bi8016137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dianov G, Sedgwick B, Daly G, Olsson M, Lovett S, Lindahl T. Release of 5′-terminal deoxyribose-phosphate residues from incised abasic sites in DNA by the Escherichia coli RecJ protein. Nucleic Acids Research. 1994;22(6):993–998. doi: 10.1093/nar/22.6.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Piersen CE, McCullough AK, Lloyd RS. AP lyases and dRPases: commonality of mechanism. Mutation Research. 2000;459(1):43–53. doi: 10.1016/s0921-8777(99)00054-3. [DOI] [PubMed] [Google Scholar]
- 65.Prasad R, Batra VK, Yang X-P, et al. Structural insight into the DNA polymerase β deoxyribose phosphate lyase mechanism. DNA Repair. 2005;4(12):1347–1357. doi: 10.1016/j.dnarep.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 66.McCullough AK, Sanchez A, Dodson ML, Marapaka P, Taylor J-S, Stephen Lloyd R. The reaction mechanism of DNA glycosylase/AP lyases at abasic sites. Biochemistry. 2001;40(2):561–568. doi: 10.1021/bi002404+. [DOI] [PubMed] [Google Scholar]
- 67.Robertson AB, Klungland A, Rognes T, Leiros I. DNA repair in mammalian cells. Cellular and Molecular Life Sciences. 2009;66(6):981–993. doi: 10.1007/s00018-009-8736-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoseki J, Okamoto A, Masui R, et al. Crystal structure of a family 4 uracil-DNA glycosylase from Thermus thermophilus HB8. Journal of Molecular Biology. 2003;333(3):515–526. doi: 10.1016/j.jmb.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 69.Kosaka H, Hoseki J, Nakagawa N, Kuramitsu S, Masui R. Crystal structure of family 5 uracil-DNA glycosylase bound to DNA. Journal of Molecular Biology. 2007;373(4):839–850. doi: 10.1016/j.jmb.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 70.Sugahara M, Mikawa T, Kato R, et al. Crystallization and preliminary X-ray crystallographic studies of Thermus thermophilus HB8 MutM protein involved in repairs of oxidative DNA damage. Journal of Biochemistry. 2000;127(1):9–11. doi: 10.1093/oxfordjournals.jbchem.a022588. [DOI] [PubMed] [Google Scholar]
- 71.Pearl LH. Structure and function in the uracil-DNA glycosylase superfamily. Mutation Research. 2000;460(3-4):165–181. doi: 10.1016/s0921-8777(00)00025-2. [DOI] [PubMed] [Google Scholar]
- 72.Schärer OD, Jiricny J. Recent progress in the biology, chemistry and structural biology of DNA glycosylases. BioEssays. 2001;23(3):270–281. doi: 10.1002/1521-1878(200103)23:3<270::AID-BIES1037>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 73.Mikawa T, Kato R, Sugahara M, Kuramitsu S. Thermostable repair enzyme for oxidative DNA damage from extremely thermophilic bacterium, Thermus thermophilus HB8. Nucleic Acids Research. 1998;26(4):903–910. doi: 10.1093/nar/26.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fortini P, Pascucci B, Parlanti E, Sobol RW, Wilson SH, Dogliotti E. Different DNA polymerases are involved in the short- and long-patch base excision repair in mammalian cells. Biochemistry. 1998;37(11):3575–3580. doi: 10.1021/bi972999h. [DOI] [PubMed] [Google Scholar]
- 75.Podlutsky AJ, Dianova II, Wilson SH, Bohr VA, Dianov GL. DNA synthesis and dRPase activities of polymerase β are both essential for single-nucleotide patch base excision repair in mammalian cell extracts. Biochemistry. 2001;40(3):809–813. doi: 10.1021/bi002064s. [DOI] [PubMed] [Google Scholar]
- 76.Sattler U, Frit P, Salles B, Calsou P. Long-patch DNA repair synthesis during base excision repair in mammalian cells. EMBO Reports. 2003;4(4):363–367. doi: 10.1038/sj.embor.embor796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gary R, Kim K, Cornelius HL, Park MS, Matsumoto Y. Proliferating cell nuclear antigen facilitates excision in long-patch base excision repair. Journal of Biological Chemistry. 1999;274(7):4354–4363. doi: 10.1074/jbc.274.7.4354. [DOI] [PubMed] [Google Scholar]
- 78.Jaiswal AS, Balusu R, Armas ML, Kundu CN, Narayan S. Mechanism of adenomatous polyposis coli (APC)-mediated blockage of long-patch base excision repair. Biochemistry. 2006;45(51):15903–15914. doi: 10.1021/bi0607958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lebedeva NA, Rechkunova NI, Dezhurov SV, et al. Comparison of functional properties of mammalian DNA polymerase λ and DNA polymerase β in reactions of DNA synthesis related to DNA repair. Biochimica et Biophysica Acta. 2005;1751(2):150–158. doi: 10.1016/j.bbapap.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 80.Lin Y, Beard WA, Shock DD, Prasad R, Hou EW, Wilson SH. DNA polymerase β and flap endonuclease 1 enzymatic specificities sustain DNA synthesis for long patch base excision repair. Journal of Biological Chemistry. 2005;280(5):3665–3674. doi: 10.1074/jbc.M412922200. [DOI] [PubMed] [Google Scholar]
- 81.Prasad R, Longley MJ, Sharief FS, Hou EW, Copeland WC, Wilson SH. Human DNA polymerase θ possesses 5′-dRP lyase activity and functions in single-nucleotide base excision repair in vitro . Nucleic Acids Research. 2009;37(6):1868–1877. doi: 10.1093/nar/gkp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stucki M, Pascucci B, Parlanti E, et al. Mammalian base excision repair by DNA polymerases δ and ε . Oncogene. 1998;17(7):835–843. doi: 10.1038/sj.onc.1202001. [DOI] [PubMed] [Google Scholar]
- 83.Sung J-S, Mosbaugh DW. Escherichia coli uracil- and ethenocytosine-initiated base excision DNA repair: rate-limiting step and patch size distribution. Biochemistry. 2003;42(16):4613–4625. doi: 10.1021/bi027115v. [DOI] [PubMed] [Google Scholar]
- 84.Singh K, Srivastava A, Patel SS, Modak MJ. Participation of the fingers subdomain of Escherichia coli DNA polymerase I in the strand displacement synthesis of DNA. Journal of Biological Chemistry. 2007;282(14):10594–10604. doi: 10.1074/jbc.M611242200. [DOI] [PubMed] [Google Scholar]
- 85.Ho DL, Byrnes WM, Ma W-P, Shi Y, Callaway DJE, Bu Z. Structure-specific DNA-induced conformational changes in Taq polymerase revealed by small angle neutron scattering. Journal of Biological Chemistry. 2004;279(37):39146–39154. doi: 10.1074/jbc.M404565200. [DOI] [PubMed] [Google Scholar]
- 86.Kaiser MW, Lyamicheva N, Ma W, et al. A comparison of eubacterial and archaeal structure-specific 5′- exonucleases. Journal of Biological Chemistry. 1999;274(30):21387–21394. doi: 10.1074/jbc.274.30.21387. [DOI] [PubMed] [Google Scholar]
- 87.Lyamichev V, Brow MAD, Dahlberg JE. Structure-specific endonucleolytic cleavage of nucleic acids by eubacterial DNA polymerases. Science. 1993;260(5109):778–783. doi: 10.1126/science.7683443. [DOI] [PubMed] [Google Scholar]
- 88.Ma W-P, Kaiser MW, Lyamicheva N, et al. RNA template-dependent 5′ nuclease activity of Thermus aquaticus and Thermus thermophilus DNA polymerases. Journal of Biological Chemistry. 2000;275(32):24693–24700. doi: 10.1074/jbc.M002268200. [DOI] [PubMed] [Google Scholar]
- 89.Xu Y, Grindley NDF, Joyce CM. Coordination between the polymerase and 5′-nuclease components of DNA polymerase I of Escherichia coli . Journal of Biological Chemistry. 2000;275(27):20949–20955. doi: 10.1074/jbc.M909135199. [DOI] [PubMed] [Google Scholar]
- 90.López de Saro FJ, O’Donnell M. Interaction of the β sliding clamp with MutS, ligase, and DNA polymerase I. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(15):8376–8380. doi: 10.1073/pnas.121009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yamtich J, Sweasy JB. DNA polymerase Family X: function, structure, and cellular roles. Biochimica et Biophysica Acta. 2010;1804(5):1136–1150. doi: 10.1016/j.bbapap.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Duym WW, Fiala KA, Bhatt N, Suo Z. Kinetic effect of a downstream strand and its 5′-terminal moieties on single nucleotide gap-filling synthesis catalyzed by human DNA polymerase λ . Journal of Biological Chemistry. 2006;281(47):35649–35655. doi: 10.1074/jbc.M607479200. [DOI] [PubMed] [Google Scholar]
- 93.Baños B, Lázaro JM, Villar L, Salas M, de Vega M. Characterization of a Bacillus subtilis 64-kDa DNA Polymerase X Potentially Involved in DNA Repair. Journal of Molecular Biology. 2008;384(5):1019–1028. doi: 10.1016/j.jmb.2008.09.081. [DOI] [PubMed] [Google Scholar]
- 94.Khairnar NP, Misra HS. DNA polymerase X from Deinococcus radiodurans implicated in bacterial tolerance to DNA damage is characterized as a short patch base excision repair polymerase. Microbiology. 2009;155(9):3005–3014. doi: 10.1099/mic.0.029223-0. [DOI] [PubMed] [Google Scholar]
- 95.Baños B, Lázaro JM, Villar L, Salas M, de Vega M. Editing of misaligned 3′-termini by an intrinsic 3′–5′ exonuclease activity residing in the PHP domain of a family X DNA polymerase. Nucleic Acids Research. 2008;36(18):5736–5749. doi: 10.1093/nar/gkn526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Blasius M, Shevelev I, Jolivet E, Sommer S, Hübscher U. DNA polymerase X from Deinococcus radiodurans possesses a structure-modulated 3′ > 5′ exonuclease activity involved in radioresistance. Molecular Microbiology. 2006;60(1):165–176. doi: 10.1111/j.1365-2958.2006.05077.x. [DOI] [PubMed] [Google Scholar]
- 97.Nakane S, Nakagawa N, Kuramitsu S, Masui R. Characterization of DNA polymerase X from Thermus thermophilus HB8 reveals the POLXc and PHP domains are both required for 3′ − 5′ exonuclease activity. Nucleic Acids Research. 2009;37(6):2037–2052. doi: 10.1093/nar/gkp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sukhanova M, Khodyreva S, Lavrik O. Poly(ADP-ribose) polymerase 1 regulates activity of DNA polymerase β in long patch base excision repair. Mutation Research. 2009;685(1-2):80–89. doi: 10.1016/j.mrfmmm.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 99.Cuneo MJ, London RE. Oxidation state of the XRCC1 N-terminal domain regulates DNA polymerase β binding affinity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(15):6805–6810. doi: 10.1073/pnas.0914077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nazarkina ZHK, Khodyreva SN, Marsin S, Radicella JP, Lavrik OI. Study of interaction of XRCC1 with DNA and proteins of base excision repair by photoaffinity labeling technique. Biochemistry. 2007;72(8):878–886. doi: 10.1134/s000629790708010x. [DOI] [PubMed] [Google Scholar]
- 101.Sancar A. DNA excision repair. Annual Review of Biochemistry. 1996;65:43–81. doi: 10.1146/annurev.bi.65.070196.000355. [DOI] [PubMed] [Google Scholar]
- 102.Van Houten B. Nucleotide excision repair in Escherichia coli . Microbiological Reviews. 1990;54(1):18–51. doi: 10.1128/mr.54.1.18-51.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Truglio JJ, Croteau DL, van Houten B, Kisker C. Prokaryotic nucleotide excision repair: the UvrABC system. Chemical Reviews. 2006;106(2):233–252. doi: 10.1021/cr040471u. [DOI] [PubMed] [Google Scholar]
- 104.Selby CP, Sancar A. Mechanisms of transcription-repair coupling and mutation frequency decline. Microbiological Reviews. 1994;58(3):317–329. doi: 10.1128/mr.58.3.317-329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Svejstrup JQ. Mechanisms of transcription-coupled DNA repair. Nature Reviews Molecular Cell Biology. 2002;3(1):21–29. doi: 10.1038/nrm703. [DOI] [PubMed] [Google Scholar]
- 106.Selby CP, Sancar A. Molecular mechanism of transcription-repair coupling. Science. 1993;259(5104):53–58. doi: 10.1126/science.8465200. [DOI] [PubMed] [Google Scholar]
- 107.Gillet LCJ, Schärer OD. Molecular mechanisms of mammalian global genome nucleotide excision repair. Chemical Reviews. 2006;106(2):253–276. doi: 10.1021/cr040483f. [DOI] [PubMed] [Google Scholar]
- 108.Orren DK, Sancar A. The (A)BC excinuclease of Escherichia coli has only the UvrB and UvrC subunits in the incision complex. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(14):5237–5241. doi: 10.1073/pnas.86.14.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Verhoeven EEA, Wyman C, Moolenaar GF, Goosen N. The presence of two UvrB subunits in the UvrAB complex ensures damage detection in both DNA strands. EMBO Journal. 2002;21(15):4196–4205. doi: 10.1093/emboj/cdf396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moolenaar GF, Herron MF, Monaco V, et al. The role of ATP binding and hydrolysis by UvrB during nucleotide excision repair. Journal of Biological Chemistry. 2000;275(11):8044–8050. doi: 10.1074/jbc.275.11.8044. [DOI] [PubMed] [Google Scholar]
- 111.Caron PR, Grossman L. Involvement of a cryptic ATPase activity of UvrB and its proteolysis product, UvrB* in DNA repair. Nucleic Acids Research. 1988;16(20):9651–9662. doi: 10.1093/nar/16.20.9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kato R, Yamamoto N, Kito K, Kuramitsu S. ATPase activity of UvrB protein from Thermus thermophilus HB8 and its interaction with DNA. Journal of Biological Chemistry. 1996;271(16):9612–9618. doi: 10.1074/jbc.271.16.9612. [DOI] [PubMed] [Google Scholar]
- 113.Yamagata A, Masui R, Kato R, et al. Interaction of UvrA and UvrB proteins with a fluorescent single-stranded DNA. Implication for slow conformational change upon interaction of UvrB with DNA. Journal of Biological Chemistry. 2000;275(18):13235–13242. doi: 10.1074/jbc.275.18.13235. [DOI] [PubMed] [Google Scholar]
- 114.Lin J-J, Sancar A. Active site of (A)BC excinuclease. I. Evidence for 5′ incision by UvrC through a catalytic site involving Asp399, Asp438, Asp466, and His538 residues. Journal of Biological Chemistry. 1992;267(25):17688–17692. [PubMed] [Google Scholar]
- 115.Verhoeven EEA, Van Kesteren M, Moolenaar GF, Visse R, Goosen N. Catalytic sites for 3′ and 5′ incision of Escherichia coli nucleotide excision repair are both located in UvrC. Journal of Biological Chemistry. 2000;275(7):5120–5123. doi: 10.1074/jbc.275.7.5120. [DOI] [PubMed] [Google Scholar]
- 116.Ohta T, Tokishita S-I, Imazuka R, Mori I, Okamura J, Yamagata H. β-Glucosidase as a reporter for the gene expression studies in Thermus thermophilus and constitutive expression of DNA repair genes. Mutagenesis. 2006;21(4):255–260. doi: 10.1093/mutage/gel025. [DOI] [PubMed] [Google Scholar]
- 117.Collins R, McCarthy TV. Purification and characterization of Thermus thermophilus UvrD. Extremophiles. 2003;7(1):35–41. doi: 10.1007/s00792-002-0293-4. [DOI] [PubMed] [Google Scholar]
- 118.Bohr VA, Smith CA, Okumoto DS, Hanawalt PC. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985;40(2):359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- 119.Selby CP, Sancar A. Transcription preferentially inhibits nucleotide excision repair of the template DNA strand in vitro . Journal of Biological Chemistry. 1990;265(34):21330–21336. [PubMed] [Google Scholar]
- 120.Selby CP, Witkin EM, Sancar A. Escherichia coli mfd mutant deficient in “mutation frequency decline” lacks strand-specific repair: in vitro complementation with purified coupling factor. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(24):11574–11578. doi: 10.1073/pnas.88.24.11574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Selby CP, Sancar A. Structure and function of transcription-repair coupling factor. I. Structural domains and binding properties. Journal of Biological Chemistry. 1995;270(9):4882–4889. doi: 10.1074/jbc.270.9.4882. [DOI] [PubMed] [Google Scholar]
- 122.Deaconescu AM, Chambers AL, Smith AJ, et al. Structural basis for bacterial transcription-coupled DNA repair. Cell. 2006;124(3):507–520. doi: 10.1016/j.cell.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 123.Karakas E, Truglio JJ, Croteau D, et al. Structure of the C-terminal half of UvrC reveals an RNase H endonuclease domain with an Argonaute-like catalytic triad. EMBO Journal. 2007;26(2):613–622. doi: 10.1038/sj.emboj.7601497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Machius M, Henry L, Palnitkar M, Deisenhofer J. Crystal structure of the DNA nucleotide excision repair enzyme UvrB from Thermus thermophilus . Proceedings of the National Academy of Sciences of the United States of America. 1999;96(21):11717–11722. doi: 10.1073/pnas.96.21.11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nakagawa N, Sugahara M, Masui R, Kato R, Fukuyama K, Kuramitsu S. Crystal structure of Thermus thermophilus HB8 UvrB protein, a key enzyme of nucleotide excision repair. Journal of Biochemistry. 1999;126(6):986–990. doi: 10.1093/oxfordjournals.jbchem.a022566. [DOI] [PubMed] [Google Scholar]
- 126.Pakotiprapha D, Inuzuka Y, Bowman BR, et al. Crystal Structure of Bacillus stearothermophilus UvrA provides insight into ATP-modulated dimerization, UvrB interaction, and DNA binding. Molecular Cell. 2008;29(1):122–133. doi: 10.1016/j.molcel.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Theis K, Chen PJ, Skorvaga M, Van Houten B, Kisker C. Crystal structure of UvrB, a DNA helicase adapted for nucleotide excision repair. EMBO Journal. 1999;18(24):6899–6907. doi: 10.1093/emboj/18.24.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Truglio JJ, Rhau B, Croteau DL, et al. Structural insights into the first incision reaction during nucleotide excision repair. EMBO Journal. 2005;24(5):885–894. doi: 10.1038/sj.emboj.7600568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Truglio JJ, Karakas E, Rhau B, et al. Structural basis for DNA recognition and processing by UvrB. Nature Structural and Molecular Biology. 2006;13(4):360–364. doi: 10.1038/nsmb1072. [DOI] [PubMed] [Google Scholar]
- 130.Nakagawa N, Masui R, Kato R, Kuramitsu S. Domain structure of Thermus thermophilus UvrB protein. Similarity in domain structure to a helicase. Journal of Biological Chemistry. 1997;272(36):22703–22713. doi: 10.1074/jbc.272.36.22703. [DOI] [PubMed] [Google Scholar]
- 131.Hori M, Ishiguro C, Suzuki T, et al. UvrA and UvrB enhance mutations induced by oxidized deoxyribonucleotides. DNA Repair. 2007;6(12):1786–1793. doi: 10.1016/j.dnarep.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 132.Schaaper RM. Base selection, proofreading, and mismatch repair during DNA replication in Escherichia coli . Journal of Biological Chemistry. 1993;268(32):23762–23765. [PubMed] [Google Scholar]
- 133.Fishel R, Kolodner RD. Identification of mismatch repair genes and their role in the development of cancer. Current Opinion in Genetics and Development. 1995;5(3):382–395. doi: 10.1016/0959-437x(95)80055-7. [DOI] [PubMed] [Google Scholar]
- 134.Lyer RR, Pluciennik A, Burdett V, Modrich PL. DNA mismatch repair: functions and mechanisms. Chemical Reviews. 2006;106(2):302–323. doi: 10.1021/cr0404794. [DOI] [PubMed] [Google Scholar]
- 135.Modrich P. Methyl-directed DNA mismatch correction. Journal of Biological Chemistry. 1989;264(12):6597–6600. [PubMed] [Google Scholar]
- 136.Lamers MH, Perrakis A, Enzlin JH, Winterwerp HHK, De Wind N, Sixma TK. The crystal structure of DNA mismatch repair protein MutS binding to a G·T mismatch. Nature. 2000;407(6805):711–717. doi: 10.1038/35037523. [DOI] [PubMed] [Google Scholar]
- 137.Obmolova G, Ban C, Hsieh P, Yang W. Crystal structures of mismatch repair protein MutS and its complex with a substrate DNA. Nature. 2000;407(6805):703–710. doi: 10.1038/35037509. [DOI] [PubMed] [Google Scholar]
- 138.Takamatsu S, Kato R, Kuramitsu S. Mismatch DNA recognition protein from an extremely thermophilic bacterium, Thermus thermophilus HB8. Nucleic Acids Research. 1996;24(4):640–647. doi: 10.1093/nar/24.4.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ban C, Yang W. Structural basis for MutH activation in E. coli mismatch repair and relationship of MutH to restriction endonucleases. EMBO Journal. 1998;17(5):1526–1534. doi: 10.1093/emboj/17.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mechanic LE, Frankel BA, Matson SW. Escherichia coli MutL loads DNA helicase II onto DNA. Journal of Biological Chemistry. 2000;275(49):38337–38346. doi: 10.1074/jbc.M006268200. [DOI] [PubMed] [Google Scholar]
- 141.Burdett V, Baitinger C, Viswanathan M, Lovett ST, Modrich P. In vivo requirement for RecJ, ExoVII, ExoI, and ExoX in methyl-directed mismatch repair. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(12):6765–6770. doi: 10.1073/pnas.121183298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yamagata A, Kakuta Y, Masui R, Fukuyama K. The crystal structure of exonuclease RecJ bound to Mn2+ ion suggests how its characteristic motifs are involved in exonuclease activity. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(9):5908–5912. doi: 10.1073/pnas.092547099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yamagata A, Masui R, Kakuta Y, Kuramitsu S, Fukuyama K. Overexpression, purification and characterization of RecJ protein from Thermus thermophilus HB8 and its core domain. Nucleic Acids Research. 2001;29(22):4617–4624. doi: 10.1093/nar/29.22.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fang W-H, Modrich P. Human strand-specific mismatch repair occurs by a bidirectional mechanism similar to that of the bacterial reaction. Journal of Biological Chemistry. 1993;268(16):11838–11844. [PubMed] [Google Scholar]
- 145.Dzantiev L, Constantin N, Genschel J, Iyer RR, Burgers PM, Modrich P. A defined human system that supports bidirectional mismatch-provoked excision. Molecular Cell. 2004;15(1):31–41. doi: 10.1016/j.molcel.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 146.Constantin N, Dzantiev L, Kadyrov FA, Modrich P. Human mismatch repair: reconstitution of a nick-directed bidirectional reaction. Journal of Biological Chemistry. 2005;280(48):39752–39761. doi: 10.1074/jbc.M509701200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Modrich P. Mechanisms in eukaryotic mismatch repair. Journal of Biological Chemistry. 2006;281(41):30305–30309. doi: 10.1074/jbc.R600022200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Genschel J, Bazemore LR, Modrich P. Human exonuclease I is required for 5′ and 3′ mismatch repair. Journal of Biological Chemistry. 2002;277(15):13302–13311. doi: 10.1074/jbc.M111854200. [DOI] [PubMed] [Google Scholar]
- 149.Wei K, Clark AB, Wong E, et al. Inactivation of exonuclease I in mice results in DNA mismatch repair defects, increased cancer susceptibility, and male and female sterility. Genes and Development. 2003;17(5):603–614. doi: 10.1101/gad.1060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kadyrov FA, Dzantiev L, Constantin N, Modrich P. Endonucleolytic function of MutLα in human mismatch repair. Cell. 2006;126(2):297–308. doi: 10.1016/j.cell.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 151.Kadyrov FA, Holmes SF, Arana ME, et al. Saccharomyces cerevisiae MutLα is a mismatch repair endonuclease. Journal of Biological Chemistry. 2007;282(51):37181–37190. doi: 10.1074/jbc.M707617200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fukui K, Nishida M, Nakagawa N, Masui R, Kuramitsu S. Bound nucleotide controls the endonuclease activity of mismatch repair enzyme MutL. Journal of Biological Chemistry. 2008;283(18):12136–12145. doi: 10.1074/jbc.M800110200. [DOI] [PubMed] [Google Scholar]
- 153.Duppatla V, Bodda C, Urbanke C, Friedhoff P, Rao DN. The C-terminal domain is sufficient for endonuclease activity of Neisseria gonorrhoeae MutL. Biochemical Journal. 2009;423(2):265–277. doi: 10.1042/BJ20090626. [DOI] [PubMed] [Google Scholar]
- 154.Mauris J, Evans TC., Jr. Adenosine triphosphate stimulates Aquifex aeolicus MutL endonuclease activity. PLoS ONE. 2009;4(9, article no. e7175) doi: 10.1371/journal.pone.0007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tachiki H, Kato R, Kuramitsu S. DNA binding and protein-protein interaction sites in MutS, a mismatched DNA recognition protein from Thermus thermophilus HB8. Journal of Biological Chemistry. 2000;275(52):40703–40709. doi: 10.1074/jbc.M007124200. [DOI] [PubMed] [Google Scholar]
- 156.Kato R, Kataoka M, Kamikubo H, Kuramitsu S. Direct observation of three conformations of MutS protein regulated by adenine nucleotides. Journal of Molecular Biology. 2001;309(1):227–238. doi: 10.1006/jmbi.2001.4752. [DOI] [PubMed] [Google Scholar]
- 157.Acharya S, Foster PL, Brooks P, Fishel R. The coordinated functions of the E. coli MutS and MutL proteins in mismatch repair. Molecular Cell. 2003;12(1):233–246. doi: 10.1016/s1097-2765(03)00219-3. [DOI] [PubMed] [Google Scholar]
- 158.Mendillo ML, Putnam CD, Mo AO, et al. Probing DNA- and ATP-mediated conformational changes in the MutS family of mispair recognition proteins using deuterium exchange mass spectrometry. Journal of Biological Chemistry. 2010;285(17):13170–13182. doi: 10.1074/jbc.M110.108894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Blackwell LJ, Martik D, Bjornson KP, Bjornson ES, Modrich P. Nucleotide-promoted release of hMutSa from heteroduplex DNA is consistent with an ATP-dependent translocation mechanism. The Journal of Biological Chemistry. 1998;273(48):32055–32062. doi: 10.1074/jbc.273.48.32055. [DOI] [PubMed] [Google Scholar]
- 160.Blackwell LJ, Bjornson KP, Allen DJ, Modrich P. Distinct MutS DNA-binding modes that are differentially modulated by ATP binding and hydrolysis. Journal of Biological Chemistry. 2001;276(36):34339–34347. doi: 10.1074/jbc.M104256200. [DOI] [PubMed] [Google Scholar]
- 161.Natrajan G, Lamers MH, Enzlin JH, Winterwerp HHK, Perrakis A, Sixma TK. Structures of Escherichia coli DNA mismatch repair enzyme MutS in complex with different mismatches: a common recognition mode for diverse substrates. Nucleic Acids Research. 2003;31(16):4814–4821. doi: 10.1093/nar/gkg677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Warren JJ, Pohlhaus TJ, Changela A, et al. Structure of the human MutSa DNA lesion recognition complex. Molecular Cell. 2007;26(4):579–592. doi: 10.1016/j.molcel.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 163.Dutta R, Inouye M. GHKL, an emergent ATPase/kinase superfamily. Trends in Biochemical Sciences. 2000;25(1):24–28. doi: 10.1016/s0968-0004(99)01503-0. [DOI] [PubMed] [Google Scholar]
- 164.Ban C, Junop M, Yang W. Transformation of MutL by ATP binding and hydrolysis: a switch in DNA mismatch repair. Cell. 1999;97(1):85–97. doi: 10.1016/s0092-8674(00)80717-5. [DOI] [PubMed] [Google Scholar]
- 165.Sacho EJ, Kadyrov FA, Modrich P, Kunkel TA, Erie DA. Direct visualization of asymmetric adenine nucleotide-induced conformational changes in MutLα . Molecular Cell. 2008;29(1):112–121. doi: 10.1016/j.molcel.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Moldovan G-L, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129(4):665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 167.Shell SS, Putnam CD, Kolodner RD. The N terminus of Saccharomyces cerevisiae Msh6 is an unstructured tether to PCNA. Molecular Cell. 2007;26(4):565–578. doi: 10.1016/j.molcel.2007.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kunkel TA, Erie DA. DNA mismatch repair. Annual Review of Biochemistry. 2005;74:681–710. doi: 10.1146/annurev.biochem.74.082803.133243. [DOI] [PubMed] [Google Scholar]
- 169.Masih PJ, Kunnev D, Melendy T. Mismatch repair proteins are recruited to replicating DNA through interaction with Proliferating Cell Nuclear Antigen (PCNA) Nucleic Acids Research. 2008;36(1):67–75. doi: 10.1093/nar/gkm943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Iyer RR, Pohlhaus TJ, Chen S, et al. The MutSα-proliferating cell nuclear antigen interaction in human DNA mismatch repair. Journal of Biological Chemistry. 2008;283(19):13310–13319. doi: 10.1074/jbc.M800606200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Simmons LA, Davies BW, Grossman AD, Walker GC. β clamp directs localization of mismatch repair in Bacillus subtilis . Molecular Cell. 2008;29(3):291–301. doi: 10.1016/j.molcel.2007.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Genschel J, Modrich P. Analysis of the excision step in human DNA mismatch repair. Methods in Enzymology. 2006;408:273–284. doi: 10.1016/S0076-6879(06)08017-7. [DOI] [PubMed] [Google Scholar]
- 173.Genschel J, Modrich P. Mechanism of 5′-directed excision in human mismatch repair. Molecular Cell. 2003;12(5):1077–1086. doi: 10.1016/s1097-2765(03)00428-3. [DOI] [PubMed] [Google Scholar]
- 174.Mauris J, Evans Jr. TC. A human PMS2 homologue from Aquifex aeolicus stimulates an ATP-dependent DNA helicase. Journal of Biological Chemistry. 2010;285(15):11087–11092. doi: 10.1074/jbc.M109.050955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shimada A, Masui R, Nakagawa N, et al. A novel single-stranded DNA-specific 3′–5′ exonuclease, Thermus thermophilus exonuclease I, is involved in several DNA repair pathways. Nucleic Acids Research. 2010;38(17):5792–5705. doi: 10.1093/nar/gkq350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wakamatsu T, Kitamura Y, Kotera Y, Nakagawa N, Kuramitsu S, Masui R. Structure of RecJ exonuclease defines its specificity for single-stranded DNA. Journal of Biological Chemistry. 2010;285(13):9762–9769. doi: 10.1074/jbc.M109.096487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yang W. An equivalent metal ion in one- and two-metal-ion catalysis. Nature Structural and Molecular Biology. 2008;15(11):1228–1231. doi: 10.1038/nsmb.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shrivastav M, De Haro LP, Nickoloff JA. Regulation of DNA double-strand break repair pathway choice. Cell Research. 2008;18(1):134–147. doi: 10.1038/cr.2007.111. [DOI] [PubMed] [Google Scholar]
- 179.Moynahan ME, Jasin M. Mitotic homologous recombination maintains genomic stability and suppresses tumorigenesis. Nature Reviews Molecular Cell Biology. 2010;11(3):196–207. doi: 10.1038/nrm2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Helleday T, Lo J, van Gent DC, Engelward BP. DNA double-strand break repair: from mechanistic understanding to cancer treatment. DNA Repair. 2007;6(7):923–935. doi: 10.1016/j.dnarep.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 181.Thompson LH, Schild D. Recombinational DNA repair and human disease. Mutation Research. 2002;509(1-2):49–78. doi: 10.1016/s0027-5107(02)00224-5. [DOI] [PubMed] [Google Scholar]
- 182.Nowosielska A. Bacterial DNA repair genes and their eukaryotic homologues: 5. The role of recombination in DNA repair and genome stability. Acta Biochimica Polonica. 2007;54(3):483–494. [PubMed] [Google Scholar]
- 183.Rocha EPC, Cornet E, Michel B. Comparative and evolutionary analysis of the bacterial homologous recombination systems. PLoS Genetics. 2005;1(2, article no. e15):0247–0259. doi: 10.1371/journal.pgen.0010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cromie GA, Connelly JC, Leach DRF. Recombination at double-strand breaks and DNA ends: conserved mechanisms from phage to humans. Molecular Cell. 2001;8(6):1163–1174. doi: 10.1016/s1097-2765(01)00419-1. [DOI] [PubMed] [Google Scholar]
- 185.Sasaki M, Lange J, Keeney S. Genome destabilization by homologous recombination in the germ line. Nature Reviews Molecular Cell Biology. 2010;11(3):182–195. doi: 10.1038/nrm2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Thomas CM, Nielsen KM. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nature Reviews Microbiology. 2005;3(9):711–721. doi: 10.1038/nrmicro1234. [DOI] [PubMed] [Google Scholar]
- 187.Li G-M. Mechanisms and functions of DNA mismatch repair. Cell Research. 2008;18(1):85–98. doi: 10.1038/cr.2007.115. [DOI] [PubMed] [Google Scholar]
- 188.Sung P, Klein H. Mechanism of homologous recombination: mediators and helicases take on regulatory functions. Nature Reviews Molecular Cell Biology. 2006;7(10):739–750. doi: 10.1038/nrm2008. [DOI] [PubMed] [Google Scholar]
- 189.Huertas P. DNA resection in eukaryotes: deciding how to fix the break. Nature Structural and Molecular Biology. 2010;17(1):11–16. doi: 10.1038/nsmb.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Handa N, Morimatsu K, Lovett ST, Kowalczykowski SC. Reconstitution of initial steps of dsDNA break repair by the RecF pathway of E. coli . Genes and Development. 2009;23(10):1234–1245. doi: 10.1101/gad.1780709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yeeles JTP, Dillingham MS. The processing of double-stranded DNA breaks for recombinational repair by helicase-nuclease complexes. DNA Repair. 2010;9(3):276–285. doi: 10.1016/j.dnarep.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 192.Shereda RD, Bernstein DA, Keck JL. A central role for SSB in Escherichia coli RecQ DNA helicase function. Journal of Biological Chemistry. 2007;282(26):19247–19258. doi: 10.1074/jbc.M608011200. [DOI] [PubMed] [Google Scholar]
- 193.Brüggemann H, Chen C. Comparative genomics of Thermus thermophilus: plasticity of the megaplasmid and its contribution to a thermophilic lifestyle. Journal of Biotechnology. 2006;124(4):654–661. doi: 10.1016/j.jbiotec.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 194.Sharma R, Rao DN. Orchestration of Haemophilus influenzae RecJ exonuclease by interaction with single-stranded DNA-binding protein. Journal of Molecular Biology. 2009;385(5):1375–1396. doi: 10.1016/j.jmb.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 195.Morimatsu K, Kowalczykowski SC. RecFOR proteins load RecA protein onto gapped DNA to accelerate DNA strand exchange: a universal step of recombinational repair. Molecular Cell. 2003;11(5):1337–1347. doi: 10.1016/s1097-2765(03)00188-6. [DOI] [PubMed] [Google Scholar]
- 196.Honda M, Fujisawa T, Shibata T, Mikawa T. RecR forms a ring-like tetramer that encircles dsDNA by forming a complex with RecF. Nucleic Acids Research. 2008;36(15):5013–5020. doi: 10.1093/nar/gkn471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Honda M, Inoue J, Yoshimasu M, Ito Y, Shibata T, Mikawa T. Identification of the RecR Toprim domain as the binding site for both recF and recO: A role of recR in recFOR assembly at double-stranded DNA-single-stranded DNA junctions. Journal of Biological Chemistry. 2006;281(27):18549–18559. doi: 10.1074/jbc.M512658200. [DOI] [PubMed] [Google Scholar]
- 198.Inoue J, Honda M, Ikawa S, Shibata T, Mikawa T. The process of displacing the single-stranded DNA-binding protein from single-stranded DNA by RecO and RecR proteins. Nucleic Acids Research. 2008;36(1):94–109. doi: 10.1093/nar/gkm1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Carreira A, Kowalczykowski SC. BRCA2: shining light on the regulation of DNA-binding selectivity by RAD51. Cell Cycle. 2009;8(21):3445–3447. doi: 10.4161/cc.8.21.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gasior SL, Olivares H, Ear U, Hari DM, Weichselbaum R, Bishop DK. Assembly of RecA-like recombinases: distinct roles for mediator proteins in mitosis and meiosis. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(15):8411–8418. doi: 10.1073/pnas.121046198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mazin AV, Mazina OM, Bugreev DV, Rossi MJ. Rad54, the motor of homologous recombination. DNA Repair. 2010;9(3):286–302. doi: 10.1016/j.dnarep.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.McIlwraith MJ, Van Dyck E, Masson J-Y, Stasiak AZ, Stasiak A, West SC. Reconstitution of the strand invasion step of double-strand break repair using human Rad51 Rad52 and RPA proteins. Journal of Molecular Biology. 2000;304(2):151–164. doi: 10.1006/jmbi.2000.4180. [DOI] [PubMed] [Google Scholar]
- 203.Lin Z, Kong H, Nei M, Ma H. Origins and evolution of the recA/RAD51 gene family: evidence for ancient gene duplication and endosymbiotic gene transfer. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(27):10328–10333. doi: 10.1073/pnas.0604232103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.McGrew DA, Knight KL. Molecular design and functional organization of the RecA protein. Critical Reviews in Biochemistry and Molecular Biology. 2003;38(5):385–432. doi: 10.1080/10409230390242489. [DOI] [PubMed] [Google Scholar]
- 205.Cox MM. Motoring along with the bacterial RecA protein. Nature Reviews Molecular Cell Biology. 2007;8(2):127–138. doi: 10.1038/nrm2099. [DOI] [PubMed] [Google Scholar]
- 206.San Filippo J, Sung P, Klein H. Mechanism of eukaryotic homologous recombination. Annual Review of Biochemistry. 2008;77:229–257. doi: 10.1146/annurev.biochem.77.061306.125255. [DOI] [PubMed] [Google Scholar]
- 207.Story RM, Weber IT, Steitz TA. The structure of the E. coli recA protein monomer and polymer. Nature. 1992;355(6358):318–325. doi: 10.1038/355318a0. [DOI] [PubMed] [Google Scholar]
- 208.Chen Z, Yang H, Pavletich NP. Mechanism of homologous recombination from the RecA-ssDNA/dsDNA structures. Nature. 2008;453(7194):489–494. doi: 10.1038/nature06971. [DOI] [PubMed] [Google Scholar]
- 209.Nishinaka T, Ito Y, Yokoyama S, Shibata T. An extended DNA structure through deoxyribose-base stacking induced by RecA protein. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(13):6623–6628. doi: 10.1073/pnas.94.13.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sutton MD, Walker GC. Managing DNA polymerases: coordinating DNA replication, DNA repair, and DNA recombination. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(15):8342–8349. doi: 10.1073/pnas.111036998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kawamoto T, Araki K, Sonoda E, et al. Dual roles for DNA polymerase η in homologous DNA recombination and translesion DNA synthesis. Molecular Cell. 2005;20(5):793–799. doi: 10.1016/j.molcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 212.Li X, Stith CM, Burgers PM, Heyer W-D. PCNA is required for initiation of recombination-associated DNA synthesis by DNA polymerase δ . Molecular Cell. 2009;36(4):704–713. doi: 10.1016/j.molcel.2009.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.McIlwraith MJ, West SC. DNA repair synthesis facilitates RAD52-mediated second-end capture during DSB repair. Molecular Cell. 2008;29(4):510–516. doi: 10.1016/j.molcel.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 214.McIlwraith MJ, Vaisman A, Liu Y, et al. Human DNA polymerase η promotes DNA synthesis from strand invasion intermediates of homologous recombination. Molecular Cell. 2005;20(5):783–792. doi: 10.1016/j.molcel.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 215.Moldovan G-L, Madhavan MV, Mirchandani KD, McCaffrey RM, Vinciguerra P, D'Andrea AD. DNA polymerase POLN participates in cross-link repair and homologous recombination. Molecular and Cellular Biology. 2010;30(4):1088–1096. doi: 10.1128/MCB.01124-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Delmas S, Matic I. Interplay between replication and recombination in Escherichia coli: impact of the alternative DNA polymerases. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(12):4564–4569. doi: 10.1073/pnas.0509012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Henne A, Brüggemann H, Raasch C, et al. The genome sequence of the extreme thermophile Thermus thermophilus . Nature Biotechnology. 2004;22(5):547–553. doi: 10.1038/nbt956. [DOI] [PubMed] [Google Scholar]
- 218.White O, Eisen JA, Heidelberg JF, et al. Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1. Science. 1999;286(5444):1571–1577. doi: 10.1126/science.286.5444.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Slade D, Lindner AB, Paul G, Radman M. Recombination and replication in DNA repair of heavily irradiated Deinococcus radiodurans . Cell. 2009;136(6):1044–1055. doi: 10.1016/j.cell.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 220.Kantake N, Madiraju MVVM, Sugiyama T, Kowalczykowski SC. Escherichia coli RecO protein anneals ssDNA complexed with its cognate ssDNA-binding protein: a common step in genetic recombination. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(24):15327–15332. doi: 10.1073/pnas.252633399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sugiyama T, Kantake N, Wu Y, Kowalczykowski SC. Rad52-mediated DNA annealing after Rad51-mediated DNA strand exchange promotes second ssDNA capture. EMBO Journal. 2006;25(23):5539–5548. doi: 10.1038/sj.emboj.7601412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Nimonkar AV, Sica RA, Kowalczykowski SC. Rad52 promotes second-end DNA capture in double-stranded break repair to form complement-stabilized joint molecules. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(9):3077–3082. doi: 10.1073/pnas.0813247106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Martínez-Salazar JM, Zuñiga-Castillo J, Romero D. Differential roles of proteins involved in migration of Holliday junctions on recombination and tolerance to DNA damaging agents in Rhizobium etli . Gene. 2009;432(1-2):26–32. doi: 10.1016/j.gene.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 224.Yamada K, Ariyoshi M, Morikawa K. Three-dimensional structural views of branch migration and resolution in DNA homologous recombination. Current Opinion in Structural Biology. 2004;14(2):130–137. doi: 10.1016/j.sbi.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 225.Rudolph CJ, Upton AL, Briggs GS, Lloyd RG. Is RecG a general guardian of the bacterial genome? DNA Repair. 2010;9(3):210–223. doi: 10.1016/j.dnarep.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 226.Beam CE, Saveson CJ, Lovett ST. Role for radA/sms in recombination intermediate processing in Escherichia coli . Journal of Bacteriology. 2002;184(24):6836–6844. doi: 10.1128/JB.184.24.6836-6844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ariyoshi M, Nishino T, Iwasaki H, Shinagawa H, Morikawa K. Crystal structure of the holliday junction DNA in complex with a single RuvA tetramer. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(15):8257–8262. doi: 10.1073/pnas.140212997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Fujiwara Y, Mayanagi K, Morikawa K. Functional significance of octameric RuvA for a branch migration complex from Thermus thermophilus . Biochemical and Biophysical Research Communications. 2008;366(2):426–431. doi: 10.1016/j.bbrc.2007.11.149. [DOI] [PubMed] [Google Scholar]
- 229.Yamada K, Miyata T, Tsuchiya D, et al. Crystal structure of the RuvA-RuvB complex: a structural basis for the holliday junction migrating motor machinery. Molecular Cell. 2002;10(3):671–681. doi: 10.1016/s1097-2765(02)00641-x. [DOI] [PubMed] [Google Scholar]
- 230.Yamada K, Kunishima N, Mayanagi K, et al. Crystal structure of the Holliday junction migration motor protein RuvB from Thermus thermophilus HB8. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(4):1442–1447. doi: 10.1073/pnas.031470598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ohnishi T, Hishida T, Harada Y, Iwasaki H, Shinagawa H. Structure-function analysis of the three domains of RuvB DNA motor protein. Journal of Biological Chemistry. 2005;280(34):30504–30510. doi: 10.1074/jbc.M502400200. [DOI] [PubMed] [Google Scholar]
- 232.Ohnishi T, Iwasaki H, Ishino Y, Kuramitsu S, Nakata A, Shinagawa H. Identification and characterization of Thermus thermophilus HB8 RuvA protein, the subunit of the RuvAB protein complex that promotes branch migration of Holliday junctions. Genes and Genetic Systems. 2000;75(5):233–243. doi: 10.1266/ggs.75.233. [DOI] [PubMed] [Google Scholar]
- 233.Mayanagi K, Fujiwara Y, Miyata T, Morikawa K. Electron microscopic single particle analysis of a tetrameric RuvA/RuvB/Holliday junction DNA complex. Biochemical and Biophysical Research Communications. 2008;365(2):273–278. doi: 10.1016/j.bbrc.2007.10.165. [DOI] [PubMed] [Google Scholar]
- 234.Ariyoshi M, Vassylyev DG, Iwasaki H, Nakamura H, Shinagawa H, Morikawa K. Atomic structure of the RuvC resolvase: a Holliday junction-specific endonuclease from E. coli . Cell. 1994;78(6):1063–1072. doi: 10.1016/0092-8674(94)90280-1. [DOI] [PubMed] [Google Scholar]
- 235.Eggleston AK, West SC. Cleavage of holliday junctions by the Escherichia coli RuvABC complex. Journal of Biological Chemistry. 2000;275(34):26467–26476. doi: 10.1074/jbc.M001496200. [DOI] [PubMed] [Google Scholar]
- 236.Van Gool AJ, Hajibagheri NMA, Stasiak A, West SC. Assembly of the Escherichia coli RuvABC resolvasome directs the orientation of Holliday junction resolution. Genes and Development. 1999;13(14):1861–1870. doi: 10.1101/gad.13.14.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zerbib D, Mézard C, George H, West SC. Coordinated actions of RuvABC in Holliday junction processing. Journal of Molecular Biology. 1998;281(4):621–630. doi: 10.1006/jmbi.1998.1959. [DOI] [PubMed] [Google Scholar]
- 238.Svendsen JM, Harper JW. GEN1/Yen1 and the SLX4 complex: solutions to the problem of Holliday junction resolution. Genes and Development. 2010;24(6):521–536. doi: 10.1101/gad.1903510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Andersen SL, Bergstralh DT, Kohl KP, LaRocque JR, Moore CB, Sekelsky J. Drosophila MUS312 and the vertebrate ortholog BTBD12 interact with DNA structure-specific endonucleases in DNA repair and recombination. Molecular Cell. 2009;35(1):128–135. doi: 10.1016/j.molcel.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Fekairi S, Scaglione S, Chahwan C, et al. Human SLX4 is a holliday junction resolvase subunit that binds multiple DNA repair/recombination endonucleases. Cell. 2009;138(1):78–89. doi: 10.1016/j.cell.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Muñoz IM, Hain K, Déclais A-C, et al. Coordination of structure-specific nucleases by human SLX4/BTBD12 is required for DNA repair. Molecular Cell. 2009;35(1):116–127. doi: 10.1016/j.molcel.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 242.Saito TT, Youds JL, Boulton SJ, Colaiácovo MP. Caenorhabditis elegans HIM-18/SLX-4 interacts with SLX-1 and XPF-1 and maintains genomic integrity in the germline by processing recombination intermediates. PLoS Genetics. 2009;5(11, article no. e1000735) doi: 10.1371/journal.pgen.1000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Svendsen JM, Smogorzewska A, Sowa ME, et al. Mammalian BTBD12/SLX4 assembles a Holliday junction resolvase and is required for DNA repair. Cell. 2009;138(1):63–77. doi: 10.1016/j.cell.2009.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Iwasaki H, Takahagi M, Shiba T, Nakata A, Shinagawa H. Escherichia coli RuvC protein is an endonuclease that resolves the Holliday structure. EMBO Journal. 1991;10(13):4381–4389. doi: 10.1002/j.1460-2075.1991.tb05016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Bolt EL, Lloyd RG. Substrate specificity of RusA resolvase reveals the DNA structures targeted by RuvAB and RecG in vivo. Molecular Cell. 2002;10(1):187–198. doi: 10.1016/s1097-2765(02)00560-9. [DOI] [PubMed] [Google Scholar]
- 246.Chan SN, Harris L, Bolt EL, Whitby MC, Lloyd RG. Sequence specificity and biochemical characterization of the RusA Holliday junction resolvase of Escherichia coli . Journal of Biological Chemistry. 1997;272(23):14873–14882. doi: 10.1074/jbc.272.23.14873. [DOI] [PubMed] [Google Scholar]
- 247.Lopez CR, Yang S, Deibler RW, et al. A role for topoisomerase III in a recombination pathway alternative to RuvABC. Molecular Microbiology. 2005;58(1):80–101. doi: 10.1111/j.1365-2958.2005.04812.x. [DOI] [PubMed] [Google Scholar]
- 248.Sheng D, Liu R, Xu Z, Singh P, Shen B, Hua Y. Dual negative regulatory mechanisms of RecX on RecA functions in radiation resistance, DNA recombination and consequent genome instability in Deinococcus radiodurans . DNA Repair. 2005;4(6):671–678. doi: 10.1016/j.dnarep.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 249.Jimbo K, Inoue J, Masuda T, Shibata T, Mikawa T. Purification and characterization of the Thermus thermophilus HB8 RecX protein. Protein Expression and Purification. 2007;51(2):320–323. doi: 10.1016/j.pep.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 250.Ragone S, Maman JD, Furnham N, Pellegrini L. Structural basis for inhibition of homologous recombination by the RecX protein. EMBO Journal. 2008;27(16):2259–2269. doi: 10.1038/emboj.2008.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lestini R, Michel B. UvrD controls the access of recombination proteins to blocked replication forks. EMBO Journal. 2007;26(16):3804–3814. doi: 10.1038/sj.emboj.7601804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Veaute X, Delmas S, Selva M, et al. UvrD helicase, unlike Rep helicase, dismantles RecA nucleoprotein filaments in Escherichia coli . EMBO Journal. 2005;24(1):180–189. doi: 10.1038/sj.emboj.7600485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Fukui K, Nakagawa N, Kitamura Y, Nishida Y, Masui R, Kuramitsu S. Crystal structure of Muts2 endonuclease domain and the mechanism of homologous recombination suppression. Journal of Biological Chemistry. 2008;283(48):33417–33427. doi: 10.1074/jbc.M806755200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Pinto AV, Mathieu A, Marsin S, et al. Suppression of homologous and homeologous recombination by the bacterial MutS2 protein. Molecular Cell. 2005;17(1):113–120. doi: 10.1016/j.molcel.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 255.Fukui K, Takahata Y, Nakagawa N, Kuramitsu S, Masui R. Analysis of a nuclease activity of catalytic domain of Thermus thermophilus MutS2 by high-accuracy mass spectrometry. Nucleic Acids Research. 2007;35(15, article no. e100) doi: 10.1093/nar/gkm575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Fukui K, Kosaka H, Kuramitsu S, Masui R. Nuclease activity of the MutS homologue MutS2 from Thermus thermophilus is confined to the Smr domain. Nucleic Acids Research. 2007;35(3):850–860. doi: 10.1093/nar/gkl735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Fukui K, Masui R, Kuramitsu S. Thermus thermophilus MutS2, a MutS paralogue, possesses an endonuclease activity promoted by MutL. Journal of Biochemistry. 2004;135(3):375–384. doi: 10.1093/jb/mvh045. [DOI] [PubMed] [Google Scholar]