Abstract
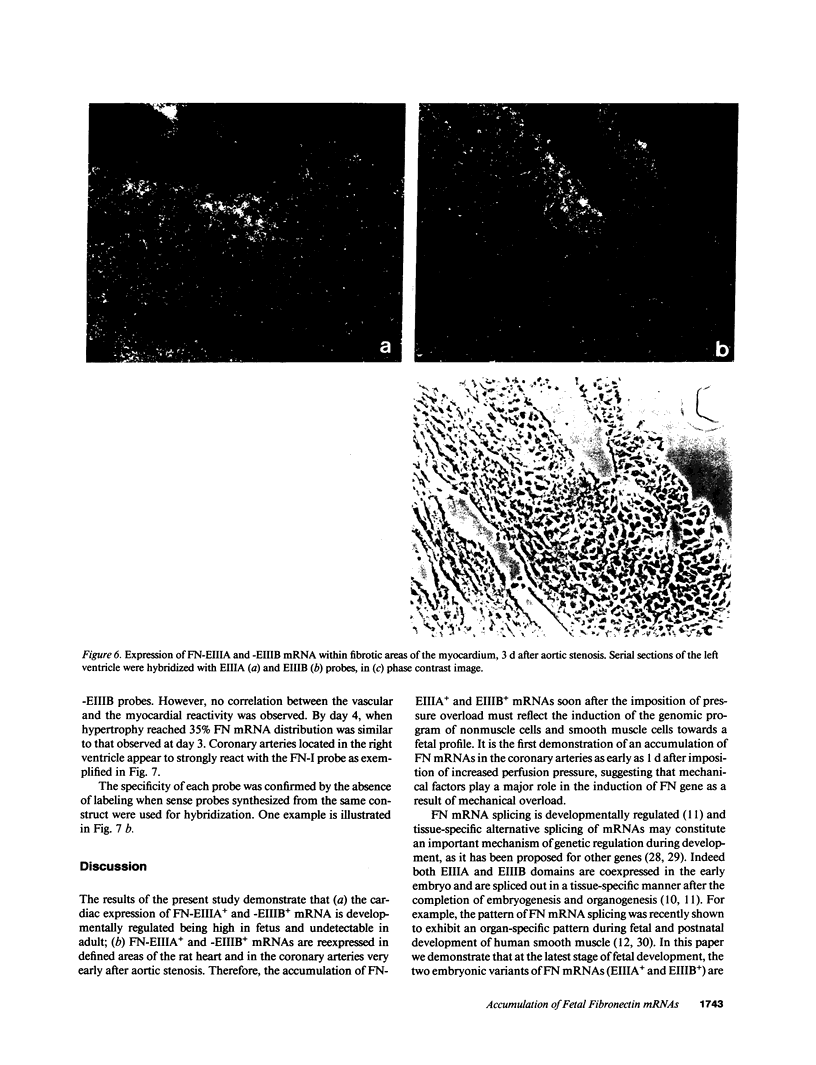
Cardiac pressure overload induces a shift towards the fetal form of major proteins expressed by the myocytes, and an accumulation of extracellular matrix proteins. One of them, fibronectin (FN), accumulates soon after the imposition of pressure overload. Because FN exists both as cellular FN (c-FN) locally synthesized by nonmuscle cells and as "plasma-FN" (p-FN) synthesized by the hepatocytes, the first issue of this study was to determine whether FN accumulation within the myocardium in response to pressure overload is paralleled by a local increase in mRNA. The expression of c-FN isoforms being developmentally regulated in a tissue-specific manner, the types of FN exons expressed by cardiac cells were analyzed. Pressure overload was induced in 25-d-old rats by stenosis of the thoracic aorta. Using in situ hybridization, we show that the mRNAs encoding the fetal forms of c-FN are accumulated in the interstitial tissue of fetal rat hearts but are absent in adult. 1-3 d after aortic stenosis, the fetal forms of c-FN mRNAs were found in the wall of coronary arteries and in focal areas of the myocardium. Thus nonmuscle cells and smooth muscle cells, like myocytes, do respond to pressure overload by reexpressing fetal gene transcripts.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahumada G. G., Saffitz J. E. Fibronectin in rat heart: a link between cardiac myocytes and collagen. J Histochem Cytochem. 1984 Apr;32(4):383–388. doi: 10.1177/32.4.6707462. [DOI] [PubMed] [Google Scholar]
- Andreadis A., Gallego M. E., Nadal-Ginard B. Generation of protein isoform diversity by alternative splicing: mechanistic and biological implications. Annu Rev Cell Biol. 1987;3:207–242. doi: 10.1146/annurev.cb.03.110187.001231. [DOI] [PubMed] [Google Scholar]
- Balza E., Borsi L., Allemanni G., Zardi L. Transforming growth factor beta regulates the levels of different fibronectin isoforms in normal human cultured fibroblasts. FEBS Lett. 1988 Feb 8;228(1):42–44. doi: 10.1016/0014-5793(88)80580-5. [DOI] [PubMed] [Google Scholar]
- Borg T. K., Rubin K., Lundgren E., Borg K., Obrink B. Recognition of extracellular matrix components by neonatal and adult cardiac myocytes. Dev Biol. 1984 Jul;104(1):86–96. doi: 10.1016/0012-1606(84)90038-1. [DOI] [PubMed] [Google Scholar]
- Borsi L., Carnemolla B., Castellani P., Rosellini C., Vecchio D., Allemanni G., Chang S. E., Taylor-Papadimitriou J., Pande H., Zardi L. Monoclonal antibodies in the analysis of fibronectin isoforms generated by alternative splicing of mRNA precursors in normal and transformed human cells. J Cell Biol. 1987 Mar;104(3):595–600. doi: 10.1083/jcb.104.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbart R. E., Nadal-Ginard B. Developmentally induced, muscle-specific trans factors control the differential splicing of alternative and constitutive troponin T exons. Cell. 1987 Jun 19;49(6):793–803. doi: 10.1016/0092-8674(87)90617-9. [DOI] [PubMed] [Google Scholar]
- Brilla C. G., Pick R., Tan L. B., Janicki J. S., Weber K. T. Remodeling of the rat right and left ventricles in experimental hypertension. Circ Res. 1990 Dec;67(6):1355–1364. doi: 10.1161/01.res.67.6.1355. [DOI] [PubMed] [Google Scholar]
- Bugaisky L. B., Siegel E., Whalen R. G. Myosin isozyme changes in the heart following constriction of the ascending aorta of a 25-day old rat. FEBS Lett. 1983 Sep 19;161(2):230–234. doi: 10.1016/0014-5793(83)81014-x. [DOI] [PubMed] [Google Scholar]
- Chapman D., Weber K. T., Eghbali M. Regulation of fibrillar collagen types I and III and basement membrane type IV collagen gene expression in pressure overloaded rat myocardium. Circ Res. 1990 Oct;67(4):787–794. doi: 10.1161/01.res.67.4.787. [DOI] [PubMed] [Google Scholar]
- Contard F., Koteliansky V., Marotte F., Dubus I., Rappaport L., Samuel J. L. Specific alterations in the distribution of extracellular matrix components within rat myocardium during the development of pressure overload. Lab Invest. 1991 Jan;64(1):65–75. [PubMed] [Google Scholar]
- Delcayre C., Samuel J. L., Marotte F., Best-Belpomme M., Mercadier J. J., Rappaport L. Synthesis of stress proteins in rat cardiac myocytes 2-4 days after imposition of hemodynamic overload. J Clin Invest. 1988 Aug;82(2):460–468. doi: 10.1172/JCI113619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour S., Duband J. L., Kornblihtt A. R., Thiery J. P. The role of fibronectins in embryonic cell migrations. Trends Genet. 1988 Jul;4(7):198–203. doi: 10.1016/0168-9525(88)90076-5. [DOI] [PubMed] [Google Scholar]
- Dufour S., Gutman A., Bois F., Lamb N., Thiery J. P., Kornblihtt A. R. Generation of full-length cDNA recombinant vectors for the transient expression of human fibronectin in mammalian cell lines. Exp Cell Res. 1991 Apr;193(2):331–338. doi: 10.1016/0014-4827(91)90104-3. [DOI] [PubMed] [Google Scholar]
- Eghbali M., Blumenfeld O. O., Seifter S., Buttrick P. M., Leinwand L. A., Robinson T. F., Zern M. A., Giambrone M. A. Localization of types I, III and IV collagen mRNAs in rat heart cells by in situ hybridization. J Mol Cell Cardiol. 1989 Jan;21(1):103–113. doi: 10.1016/0022-2828(89)91498-3. [DOI] [PubMed] [Google Scholar]
- Eghbali M., Czaja M. J., Zeydel M., Weiner F. R., Zern M. A., Seifter S., Blumenfeld O. O. Collagen chain mRNAs in isolated heart cells from young and adult rats. J Mol Cell Cardiol. 1988 Mar;20(3):267–276. doi: 10.1016/s0022-2828(88)80059-2. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Hynes R. O. Alternative splicing of fibronectin is temporally and spatially regulated in the chicken embryo. Development. 1989 Jun;106(2):375–388. doi: 10.1242/dev.106.2.375. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Hynes R. O. Patterns of fibronectin gene expression and splicing during cell migration in chicken embryos. Development. 1988 Nov;104(3):369–382. doi: 10.1242/dev.104.3.369. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Van de Water L., Dvorak H. F., Hynes R. O. Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J Cell Biol. 1989 Aug;109(2):903–914. doi: 10.1083/jcb.109.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glukhova M. A., Frid M. G., Shekhonin B. V., Balabanov Y. V., Koteliansky V. E. Expression of fibronectin variants in vascular and visceral smooth muscle cells in development. Dev Biol. 1990 Sep;141(1):193–202. doi: 10.1016/0012-1606(90)90114-x. [DOI] [PubMed] [Google Scholar]
- Glukhova M. A., Frid M. G., Shekhonin B. V., Vasilevskaya T. D., Grunwald J., Saginati M., Koteliansky V. E. Expression of extra domain A fibronectin sequence in vascular smooth muscle cells is phenotype dependent. J Cell Biol. 1989 Jul;109(1):357–366. doi: 10.1083/jcb.109.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman A., Kornblihtt A. R. Identification of a third region of cell-specific alternative splicing in human fibronectin mRNA. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7179–7182. doi: 10.1073/pnas.84.20.7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. Molecular biology of fibronectin. Annu Rev Cell Biol. 1985;1:67–90. doi: 10.1146/annurev.cb.01.110185.000435. [DOI] [PubMed] [Google Scholar]
- Kornblihtt A. R., Vibe-Pedersen K., Baralle F. E. Human fibronectin: molecular cloning evidence for two mRNA species differing by an internal segment coding for a structural domain. EMBO J. 1984 Jan;3(1):221–226. doi: 10.1002/j.1460-2075.1984.tb01787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn C., 3rd, Boldt J., King T. E., Jr, Crouch E., Vartio T., McDonald J. A. An immunohistochemical study of architectural remodeling and connective tissue synthesis in pulmonary fibrosis. Am Rev Respir Dis. 1989 Dec;140(6):1693–1703. doi: 10.1164/ajrccm/140.6.1693. [DOI] [PubMed] [Google Scholar]
- Lompré A. M., Mercadier J. J., Schwartz K. Changes in gene expression during cardiac growth. Int Rev Cytol. 1991;124:137–186. doi: 10.1016/s0074-7696(08)61526-0. [DOI] [PubMed] [Google Scholar]
- Nadal-Ginard B., Mahdavi V. Molecular basis of cardiac performance. Plasticity of the myocardium generated through protein isoform switches. J Clin Invest. 1989 Dec;84(6):1693–1700. doi: 10.1172/JCI114351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton P. A., Hynes R. O. Alternative splicing of chicken fibronectin in embryos and in normal and transformed cells. Mol Cell Biol. 1987 Dec;7(12):4297–4307. doi: 10.1128/mcb.7.12.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E., Pierschbacher M., Ruoslahti E. Deposition of plasma fibronectin in tissues. Proc Natl Acad Sci U S A. 1981 May;78(5):3218–3221. doi: 10.1073/pnas.78.5.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker T. G., Packer S. E., Schneider M. D. Peptide growth factors can provoke "fetal" contractile protein gene expression in rat cardiac myocytes. J Clin Invest. 1990 Feb;85(2):507–514. doi: 10.1172/JCI114466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel J. L., Dubus I., Contard F., Schwartz K., Rappaport L. Biological signals of cardiac hypertrophy. Eur Heart J. 1990 Nov;11 (Suppl G):1–7. doi: 10.1093/eurheartj/11.suppl_g.1. [DOI] [PubMed] [Google Scholar]
- Schiaffino S., Samuel J. L., Sassoon D., Lompré A. M., Garner I., Marotte F., Buckingham M., Rappaport L., Schwartz K. Nonsynchronous accumulation of alpha-skeletal actin and beta-myosin heavy chain mRNAs during early stages of pressure-overload--induced cardiac hypertrophy demonstrated by in situ hybridization. Circ Res. 1989 May;64(5):937–948. doi: 10.1161/01.res.64.5.937. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Patel R. S., Fonda D., Hynes R. O. Multiple sites of alternative splicing of the rat fibronectin gene transcript. EMBO J. 1987 Sep;6(9):2573–2580. doi: 10.1002/j.1460-2075.1987.tb02547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhonin B. V., Guriev S. B., Irgashev S. B., Koteliansky V. E. Immunofluorescent identification of fibronectin and fibrinogen/fibrin in experimental myocardial infarction. J Mol Cell Cardiol. 1990 May;22(5):533–541. doi: 10.1016/0022-2828(90)90955-2. [DOI] [PubMed] [Google Scholar]
- Tamkun J. W., Hynes R. O. Plasma fibronectin is synthesized and secreted by hepatocytes. J Biol Chem. 1983 Apr 10;258(7):4641–4647. [PubMed] [Google Scholar]
- Thompson N. L., Bazoberry F., Speir E. H., Casscells W., Ferrans V. J., Flanders K. C., Kondaiah P., Geiser A. G., Sporn M. B. Transforming growth factor beta-1 in acute myocardial infarction in rats. Growth Factors. 1988;1(1):91–99. doi: 10.3109/08977198809000251. [DOI] [PubMed] [Google Scholar]
- Vane J. Endothelins come home to roost. Nature. 1990 Dec 20;348(6303):673–673. doi: 10.1038/348673a0. [DOI] [PubMed] [Google Scholar]
- Vartio T., Laitinen L., Närvänen O., Cutolo M., Thornell L. E., Zardi L., Virtanen I. Differential expression of the ED sequence-containing form of cellular fibronectin in embryonic and adult human tissues. J Cell Sci. 1987 Nov;88(Pt 4):419–430. doi: 10.1242/jcs.88.4.419. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bailes J. A., McMahon A. P. Expression of the proto-oncogene int-1 is restricted to specific neural cells in the developing mouse embryo. Cell. 1987 Jul 3;50(1):79–88. doi: 10.1016/0092-8674(87)90664-7. [DOI] [PubMed] [Google Scholar]