Abstract
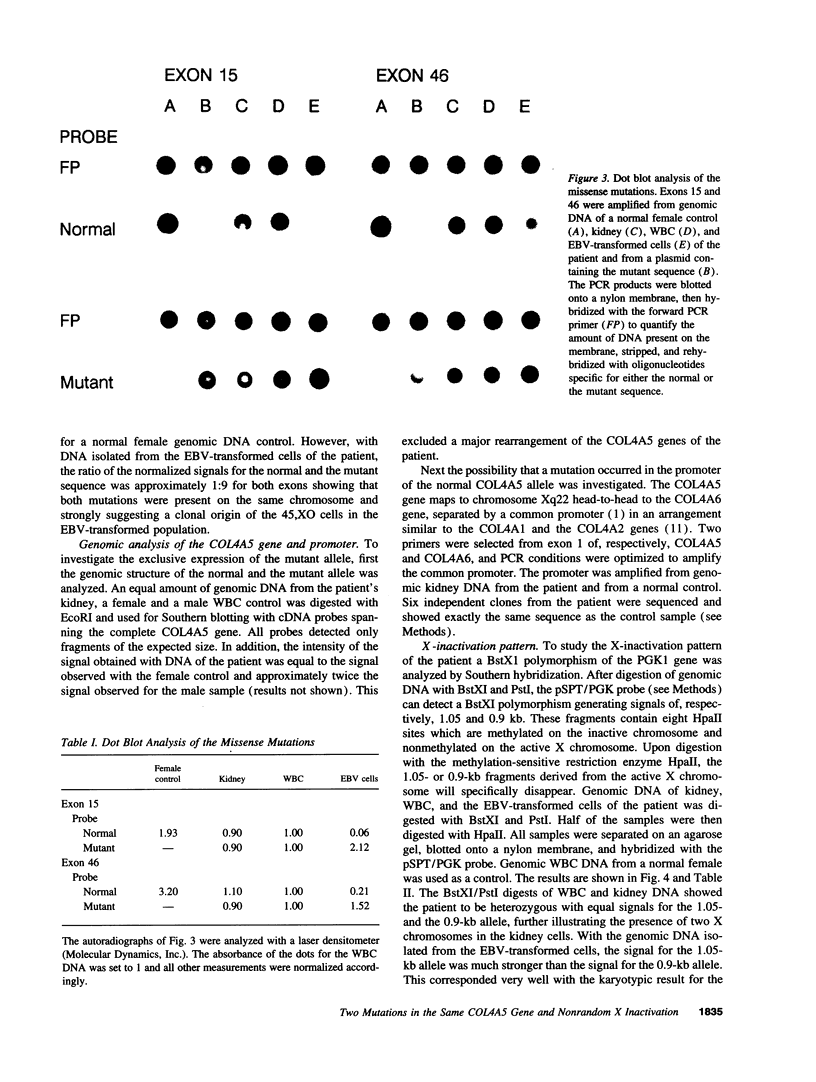
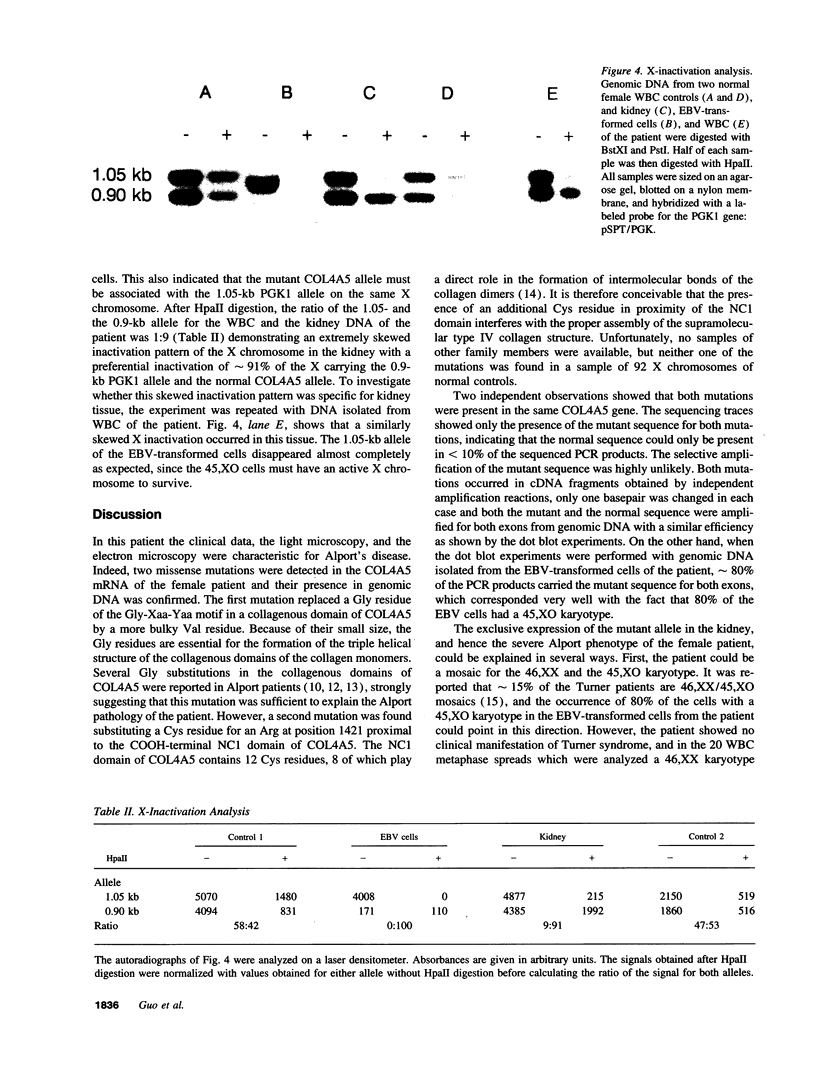
The X-linked form of Alport disease, caused by mutations in the COL4A5 or the COL4A6 gene, usually leads to terminal renal failure in males, while affected females have a more variable and moderate phenotype. We detected in a female patient, with a severe Alport phenotype, two new missense mutations. One mutation (G289V) occurred in exon 15 and converted a glycine in a collagenous domain of COL4A5 to a valine. The second mutation, located in exon 46, substituted a cysteine proximal to the NC1 domain of COL4A5 for an arginine. In white blood cells and kidney both mutations were present on > 90% of the mRNA, while at the genomic level the patient was heterozygous for both mutations. The two mutations therefore occurred in the same COL4A5 allele. No mutation was found in the COL4A5 promoter region by sequencing nor was a major rearrangement of the normal allele detected. A skewed pattern of X inactivation was demonstrated in DNA isolated from the patient's kidney and white blood cells: > 90% of the X chromosomes with the normal COL4A5 allele was inactivated. It is suggested that this skewed inactivation pattern is responsible for the absence of detectable normal COL4A5 mRNA and hence the severe phenotype in this woman.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown R. M., Brown G. K. X chromosome inactivation and the diagnosis of X linked disease in females. J Med Genet. 1993 Mar;30(3):177–184. doi: 10.1136/jmg.30.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale R. E., Wheadon H., Boulos P., Linch D. C. Tissue specificity of X-chromosome inactivation patterns. Blood. 1994 May 15;83(10):2899–2905. [PubMed] [Google Scholar]
- Grünfeld J. P., Noël L. H., Hafez S., Droz D. Renal prognosis in women with hereditary nephritis. Clin Nephrol. 1985 Jun;23(6):267–271. [PubMed] [Google Scholar]
- Guo C., Van Damme B., Van Damme-Lombaerts R., Van den Berghe H., Cassiman J. J., Marynen P. Differential splicing of COL4A5 mRNA in kidney and white blood cells: a complex mutation in the COL4A5 gene of an Alport patient deletes the NC1 domain. Kidney Int. 1993 Dec;44(6):1316–1321. doi: 10.1038/ki.1993.384. [DOI] [PubMed] [Google Scholar]
- Hook E. B., Warburton D. The distribution of chromosomal genotypes associated with Turner's syndrome: livebirth prevalence rates and evidence for diminished fetal mortality and severity in genotypes associated with structural X abnormalities or mosaicism. Hum Genet. 1983;64(1):24–27. doi: 10.1007/BF00289473. [DOI] [PubMed] [Google Scholar]
- Hudson B. G., Reeders S. T., Tryggvason K. Type IV collagen: structure, gene organization, and role in human diseases. Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. J Biol Chem. 1993 Dec 15;268(35):26033–26036. [PubMed] [Google Scholar]
- Jørgensen A. L., Philip J., Raskind W. H., Matsushita M., Christensen B., Dreyer V., Motulsky A. G. Different patterns of X inactivation in MZ twins discordant for red-green color-vision deficiency. Am J Hum Genet. 1992 Aug;51(2):291–298. [PMC free article] [PubMed] [Google Scholar]
- Knebelmann B., Deschenes G., Gros F., Hors M. C., Grünfeld J. P., Zhou J., Tryggvason K., Gubler M. C., Antignac C. Substitution of arginine for glycine 325 in the collagen alpha 5 (IV) chain associated with X-linked Alport syndrome: characterization of the mutation by direct sequencing of PCR-amplified lymphoblast cDNA fragments. Am J Hum Genet. 1992 Jul;51(1):135–142. [PMC free article] [PubMed] [Google Scholar]
- Müllenbach R., Lagoda P. J., Welter C. An efficient salt-chloroform extraction of DNA from blood and tissues. Trends Genet. 1989 Dec;5(12):391–391. [PubMed] [Google Scholar]
- Siebold B., Deutzmann R., Kühn K. The arrangement of intra- and intermolecular disulfide bonds in the carboxyterminal, non-collagenous aggregation and cross-linking domain of basement-membrane type IV collagen. Eur J Biochem. 1988 Oct 1;176(3):617–624. doi: 10.1111/j.1432-1033.1988.tb14321.x. [DOI] [PubMed] [Google Scholar]
- Soininen R., Huotari M., Hostikka S. L., Prockop D. J., Tryggvason K. The structural genes for alpha 1 and alpha 2 chains of human type IV collagen are divergently encoded on opposite DNA strands and have an overlapping promoter region. J Biol Chem. 1988 Nov 25;263(33):17217–17220. [PubMed] [Google Scholar]
- Tryggvason K., Zhou J., Hostikka S. L., Shows T. B. Molecular genetics of Alport syndrome. Kidney Int. 1993 Jan;43(1):38–44. doi: 10.1038/ki.1993.8. [DOI] [PubMed] [Google Scholar]
- Vetrie D., Flinter F., Bobrow M., Harris A. X inactivation patterns in females with Alport's syndrome: a means of selecting against a deleterious gene? J Med Genet. 1992 Sep;29(9):663–666. doi: 10.1136/jmg.29.9.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Preisinger A. C., Willard H. F., Michelson A. M., Riggs A. D., Orkin S. H. Clonal analysis using recombinant DNA probes from the X-chromosome. Cancer Res. 1987 Sep 15;47(18):4806–4813. [PubMed] [Google Scholar]
- Zhou J., Hertz J. M., Leinonen A., Tryggvason K. Complete amino acid sequence of the human alpha 5 (IV) collagen chain and identification of a single-base mutation in exon 23 converting glycine 521 in the collagenous domain to cysteine in an Alport syndrome patient. J Biol Chem. 1992 Jun 25;267(18):12475–12481. [PubMed] [Google Scholar]
- Zhou J., Hertz J. M., Tryggvason K. Mutation in the alpha 5(IV) collagen chain in juvenile-onset Alport syndrome without hearing loss or ocular lesions: detection by denaturing gradient gel electrophoresis of a PCR product. Am J Hum Genet. 1992 Jun;50(6):1291–1300. [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Hostikka S. L., Chow L. T., Tryggvason K. Characterization of the 3' half of the human type IV collagen alpha 5 gene that is affected in the Alport syndrome. Genomics. 1991 Jan;9(1):1–9. doi: 10.1016/0888-7543(91)90214-y. [DOI] [PubMed] [Google Scholar]
- Zhou J., Mochizuki T., Smeets H., Antignac C., Laurila P., de Paepe A., Tryggvason K., Reeders S. T. Deletion of the paired alpha 5(IV) and alpha 6(IV) collagen genes in inherited smooth muscle tumors. Science. 1993 Aug 27;261(5125):1167–1169. doi: 10.1126/science.8356449. [DOI] [PubMed] [Google Scholar]