Abstract
Neurotoxic viral proteins released from HIV-infected cells are believed to play a major role in the pathogenesis of the dementia displayed in a significant number of AIDS patients. HIV-1 associated neuropathology severely affects dopaminergic regions of the brain. Growing evidence indicates that HIV-1 neurotoxic proteins, such as Tat may affect the function of the dopamine transmission system. In turn, molecular components of dopamine neurotransmission may participate in a complex network of Tat-induced cell responses which result in neurodegeneration. In this study we investigated whether D1 dopamine receptors are involved in the mechanism of Tat neurotoxicity in primary rat neuronal cell cultures. We found that in rat midbrain cell cultures, which express significant levels of D1 dopamine receptors, the specific D1 antagonist SCH 23390 attenuates the cell death caused by HIV-1 Tat. In rat hippocampal cell cultures, where the expression of D1 receptors is low, SCH 23390 did not change the toxicity of Tat. Thus, the protective effect of SCH 23390 in rat primary neuronal cell cultures correlates with the level of D1 receptor protein expression. Our results provide further evidence for the involvement of the dopaminergic transmission system in the mechanism of HIV-1 Tat neurotoxicity.
Keywords: viral proteins, dopamine receptors, cell culture
INTRODUCTION
In the era of potent antiretroviral therapy, neurological disorders represent one common disturbance accompanying HIV infection. The signs of HIV-associated dementia (HAD) are characteristic of subcortical dementia: apathy, bradykinesia, psychomotor slowing and an altered posture and gait. These features are similar to those observed in advanced Parkinson’s disease and suggest a profound abnormality of the nigro-striatal dopaminergic system (Berger & Arendt, 2000). Indeed, human PET imaging has confirmed specific dopaminergic alterations in HAD (Wang et al., 2004) and decreased tyrosine hydroxylase immunoreactivity has been detected in HIV-positive human substantia nigra (Silvers at al, 2006).
A unique feature of the neurodegenerative pathology associated with HIV is that neuronal cell loss occurs in the absence of neuronal infection. The neuropathology associated with HIV appears to be related to the neurotoxicity of viral proteins released into the extracellular environment. The HIV-1 trans-activator (Tat) protein is one of the viral proteins capable of causing neuronal degeneration, both in vivo and in vitro (Aksenov et al., 2001; 2003; 2006; Aksenova et al., 2006; Nath et al., 2002). Exposure to Tat has been shown to negatively affect cognitive processes in neonatal and adult rats (Fitting et al., 2006a; b). Along with other viral proteins, Tat has been detected in the brains of patients with HIV-1-associated brain pathology (Valle et al., 2000) and in the brains of HIV-1-infected primates (Hudson et al., 2000). Tat is a nonstructural viral protein of 86–101 amino acid residues and a product of two exons. The first exon contributes the initial 72 amino acids to the protein. Tat is a transactivating nuclear regulatory protein that is essential for viral replication. This protein may be secreted by HIV-infected cells and taken up by neighboring cells. In this way, Tat can affect both infected and uninfected cells (Nath, 2002). Tat causes apoptosis in neurons. Tat is known to trigger oxidative stress-dependent apoptotic cascades in neurons in vivo and in vitro (Kruman et al., 1998; Iacovitti et al., 1999) and neurotoxic effects of Tat depend on the binding to low density lipoprotein receptor (LRP) as well as activation of N-methyl-D-aspartate (NMDA) receptors (King et al., 2006).
Previous studies suggested that dopaminergic neurons are vulnerable to neurotoxic HIV-1 proteins (Nath et al., 2000). Evidence is accumulating that HIV-1 virotoxins may cause dysfunction of dopaminergic transmission in the brain (Aksenov et al., 2006; Aksenova et al., 2006; Silvers et al., 2006; Koutsilieri et al., 2002). Neurotoxic HIV-1 proteins Tat and/or gp120 were shown to inhibit dopamine uptake (Wallace et al., 2006a; b; Aksenova et al., 2006) and to suppress the expression of tyrosine hydroxylase-specific mRNA (Zauli et al., 2000) in neural cell lines and primary neuronal cell cultures. Through changes in dopamine uptake and metabolism, neurotoxic HIV-1 proteins Tat and/or gp120 may modulate the activity of different types of dopamine receptors.
Dopamine-glutamate interactions have been shown in different brain regions. Numerous studies have demonstrated that NMDA receptor function may be regulated by dopamine D1-like receptors, composed of D1 and D5 like receptors (Cepeda and Levine, 1998; Lee and Liu, 2004; Lezcano and Bergson, 2002; Pei et al., 2004; Cepeda and Levine, 2006; Missale et al., 2006). However, it is not known whether D1 dopamine receptors are involved in neurotoxicity of HIV-1 proteins. In the current study we investigated whether the selective antagonist of D1 dopamine receptors, SCH 23390, affects the toxicity of Tat 1–72 in cultured rat fetal neurons.
MATERIALS AND METHODS
Neuronal Cell Culture
Neuronal cultures were prepared from 18-day-old Sprague-Dawley rat fetuses. Rat midbrain and/or hippocampus were dissected and incubated for 15 min in a solution of 2 mg/mL trypsin in Ca2+- and Mg2+ - free Hanks’ balanced salt solution (HBSS) buffered with 10 mM HEPES (GIBCO Life Technologies, Paisley, Scotland). The tissue was then exposed for 2 min to soybean trypsin inhibitor (1 mg/mL in HBSS) and rinsed three times in HBSS. Cells were dissociated by trituration and distributed to 96-well or 24-well poly-L-lysine-coated plastic culture plates (Costar, Cambridge, MA). Initial plating densities were approximately 160–180 cells/mm2. At the time of plating, each well contained 0.2 ml of DMEM/F12 medium (GIBCO LifeTechnologies, NY) supplemented with 100 mL/L fetal bovine serum (Sigma Chemicals, St. Louis, MO). After a 24-hr period, the DMEM/F12 medium was replaced with 0.15 mL of 2% v/v B-27 Neurobasal medium supplemented with 2 mM GlutaMAX and 0.5% w/v D-(+) glucose (GIBCO LifeTechnologies). Two-thirds of the Neurobasal medium was replaced with freshly prepared medium of the same composition twice a week. Cultures were used for neurotoxicity experiments after 12 days in culture and were >95% neuronal as observed by anti-MAP-2 immunostaining. The reminder (approximately 5%) of the cells were astrocytes as determined by anti-GFAP/Hoechst staining
Experimental Treatment of Cultures
Recombinant biologically active Tat 1–72 and its biologically inactive analog Tat δ 31–61 were produced as previously described (Nath et al., 2000). Stock solutions of Tat 1–72 and Tat Δ31–61 were prepared in phosphate buffered saline (PBS) (5000 nM) and stored at −20°C until use.
The day before Tat treatment, B-27-supplemented Neurobasal medium was replaced with Neurobasal medium without antioxidants (-AO supplement replaced B-27 supplement). Groups of cultures growing in 96-well or 24-well plates were subjected to 50 nM Tat 1–72. Appropriate vehicle controls (same volume of solvent added) were included for each group. The biologically inactive analog of HIV-1 Tat, Tat Δ31–61 (50 nM dose) served as a control for specificity of Tat neurotoxicity in our cell cultures. The number of sister cultures (wells) per each treatment/control group was between 8 and 16. A 10 µM dose of the D1 specific antagonist, SCH 23390 was used to determine whether Tat neurotoxicity could be modified by inhibition of D1 dopamine receptors. Preliminary tests determined that final concentrations of SCH 23390 within the range of 1–100 µM did not affect the viability of rat brain cell cultures. Groups of cell cultures were treated with 50 nM Tat or 50 nM Tat + 10 µM SCH 23390. In addition to control cultures that received equivolume vehicle, in each experiment a group of sister cultures (wells) was treated with 10 µM SCH 23390 alone. When cell cultures were treated with Tat + SCH 23390, the specific D1 antagonist was added 5 minutes before Tat 1–72. Following the addition of treatment components to the growth medium, all cell cultures were incubated for 48 hours prior to analyses.
Cell viability test
Neuronal survival was determined using a Live/Dead viability/cytotoxicity kit from Molecular Probes (Eugene, OR). In accordance with the manufacturer's protocol, neurons were exposed to cell-permeant calcein AM (2 µM), which is hydrolyzed by intracellular esterases, and to ethidium homodimer-1 (4 µM), which binds to nucleic acids. The cleavage product of calcein AM produces a green fluorescence (F530nm) when exposed to 494-nm light and is used to identify live cells. Bound ethidium homodimer-1 produces a red fluorescence (F645nm) when exposed to 528-nm light, allowing the identification of dead cells.). Fluorescence was measured using a Bio-Tek Synergy HT microplate reader (Bio-Tek Instruments, Inc., Winooski, VT). Each individual F530nm and F645nm value on a plate were corrected for background fluorescence (readings obtained from cell cultures (wells) that were not exposed to calcein AM and ethidium bromide) by the microplate reader KC4 software package (Bio-Tek Instruments, Inc., Winooski, VT). For each individual cell culture (well) on a plate ratios between corrected green and red fluorescence (F530nm/ F645nm, Live/Dead ratios) were calculated. All individual relative numbers of live and dead cells were expressed in terms of percentages of average maximum Live/Dead ratio determined for the set of non-treated control cell cultures (8–16 wells) from the same plate: [F530nm/ F645nm]well n/[ F530nm/ F645nm]average max × 100%.
Immunocytochemistry
For the determination of D1 dopamine receptor immunoreactivity in rat fetal brain cell cultures, cells growing in 96-well plates or in glass-bottom culture dishes were fixed in 4% paraformaldehyde as described elsewhere (Basarsky et al., 1994; Vincent et al., 2005; Farnie et al., 2007). Following the fixation, plates were blocked with 10% normal goat serum (NGS) in PBS. Rabbit polyclonal anti-Dopamine D1A receptor antibody (Chemicon, Temecula, CA) was used to determine D1 immunoreactivity in cell cultures. Primary antibodies were diluted 1:200 in 1% NGS/PBS. Plates were incubated with primary antibodies overnight at 4°C. In each plate 4 wells were left without primary antibody. These wells served as controls for non-specific binding of secondary antibodies. After the incubation with primary antibodies was complete, plates were rinsed once and washed three times (5 min per wash) with PBS. For the immunofluorescent detection of D1 immunoreactivity goat anti-rabbit IgG conjugated with Alexa 594 dye (Molecular Probes) diluted 1:500 in 1%NGS/PBS was used. Plates were incubated with secondary antibodies for 1 hour at room temperature and then washed 3 times with PBS.
The detection of immunofluorescent signal was performed in a Bio-Tek Synergy HT microplate reader using 590 excitation/645 emission filter set. Primary cultures of midbrain and hippocampal neurons were prepared in the same 96-well plate. Wells without antibodies (4 cell cultures) served as “blanks” for KC4 software to correct all individual readings from wells on the plate. The immunofluorescent signal from “no primary antibody” controls (average of 8 cell cultures from the same plate) was used to determine the specific anti-D1 immunofluorescence in each individual culture (well): [F645nm ]total in well n - [F645nm ]average no prim.
Microscopic images of D1 immunoreactivity in midbrain and hippocampal rat fetal cell cultures prepared in glass-bottom culture dishes were captured using computer-controlled inverted fluorescent microscope (Nikon Eclipse TE2000-E) under 20X magnification. Neurons were counterstained with Hoechst (0.4 µg/ml) for 10 min. The results are presented as merged images of red D1 immunofluorescence and blue (Hoechst) staining of intact cell nuclei.
Western blotting
Western blotting was performed on cell lysates of midbrain and hippocampal cell cultures. Rabbit polyclonal anti-D1 antibodies were used which recognize the CP domain of the receptor (Alpha Diagnostic Intl. Inc., San Antonio, TX). Working dilution of the primary antibody was 1:1000. Bovine anti-rabbit alkaline-phosphatase-conjugated IgG were used as the secondary antibody (Santa Cruz, Santa Cruz, Ca, 1:2500). Polyclonal antibodies against neuron-specific enolase (NSE, 1:200 dilution) (Chemicon, Temecula, CA) were used to measure this neuronal cell marker protein. Cell lysates were prepared from cultures grown in 6-well plates. At 12–14th day in vitro (DIV) growth medium was removed, cells were rinsed with Dulbecco phosphate-buffered saline, D-PBS, (8 mM Na2HPO4, 1.5 mM KH2PO4, 0.137 M NaCl, and 2.7 mM KCl at pH 7.4) and lysed with CelLytic ™-M mammalian cell lysis buffer (Sigma Chemicals) containing protease inhibitors (proteinase inhibitors cocktail, Sigma Chemicals). All samples in a group (3 sister culture lysates) were pooled together and protein concentration was determined by BCA method (Pierce). Fifteen micrograms of total cell protein from midbrain cell cultures and fifty micrograms of total cell protein from hippocampal cell cultures were used for immunoblotting analysis of D1 protein levels. The same samples of cell lysates were diluted 3 times and than used for anti-NSE immunoblotting.
D1- specific ligand binding
To determine the binding of [3H]SCH 23390 to functional D1 receptors, rat fetal brain cell cultures (midbrain or hippocampal) were prepared in 24-well plates. Cultures were rinsed and preincubated for 5 min in Dulbecco phosphate-buffered saline (D-PBS): 8 mM Na2HPO4, 1.5 mM KH2PO4, 0.137 M NaCl, and 2.7 mM KCl at pH 7.4. Cultures were then incubated with buffer containing 1 mM ascorbic acid, 1uM ketanserin, and 1.0 nM of [3H]SCH 23390 (specific activity, 80 Ci/mmol; PerkinElmer, Boston, MA) for 1 h at room temperature (25 C°). Non-specific binding was determined in the presence of 5.0 µM butaclamol. Binding was performed with intact cells, and was terminated by removal of incubation buffer and 3×5 min wash with gentle agitation. Cultures were then lyzed with 0.2 ml of 50 mM Tris-HCl, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, pH 7.4. Twenty µl from each well was taken for total protein measurements. Protein concentrations were determined by BCA method (Pierce). The rest of the well content was added to scintillation fluid and used for radioactivity counting. Non-specific binding was determined to be approximately 29% of total binding, and “specific binding” was obtained by normalization of [3H]SCH 23390 binding to non-specific binding and relative protein levels.
Statistical Analysis
Statistical evaluations were made using planned comparisons and Analysis of Variance with the assumptions of normal distribution and homogeneity of variance. Bonferroni post hoc tests were used to determine specific treatment effects. An α level of 0.05 was considered significant for all statistical tests employed.
RESULTS
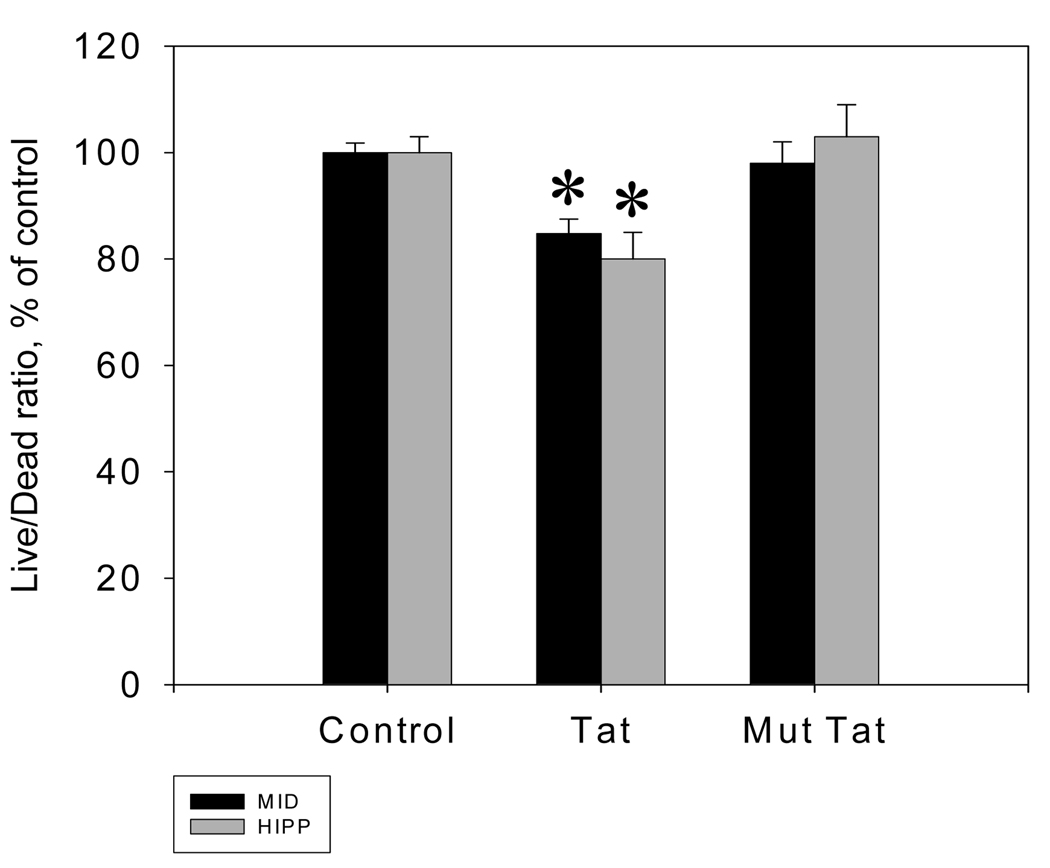
Tat 1–72 produced similar results on cell viability in midbrain and hippocampal neuronal cell cultures. Analysis of Variance revealed a main effect of treatment condition [F(2,90) = 17.31 ; p ≤ 0.01]. Bonferroni post hoc analysis revealed a decrease in Live/Dead ratio in both midbrain (t (15) = 3.02, p ≤ 0.01) and hippocampal (t (15) = 3.92, p ≤ 0.01) neuronal cell cultures Following 48 hours of incubation, the Live/Dead ratio in midbrain cell cultures treated with Tat 1–72 was 84.8 ± 2.7% of control. In Tat-treated hippocampal cell cultures the Live/Dead ratio was 80 ± 5% of control (Figure 1). Exposure to the biologically inactive Tat analog, Tat Δ31–61 did not result in increased cell death in either culture.
Figure 1.
Tat-mediated changes of neuronal viability in rat midbrain and hippocampal cell cultures. Changes in Calcein/Ethidium bromide fluorescence ratio (Live/Dead ratio) following 48 hours of exposure to 50 nM Tat 1–72 presented as mean % vs. vehicle control ± SEM; n of sister cultures analyzed = 16 per treatment. *- marks significant (P<0.05) difference between Tat-treated and non-treated control cell cultures. 50 nM Tat Δ31–61did not cause significant changes in cell viability following 48 hours of exposure.
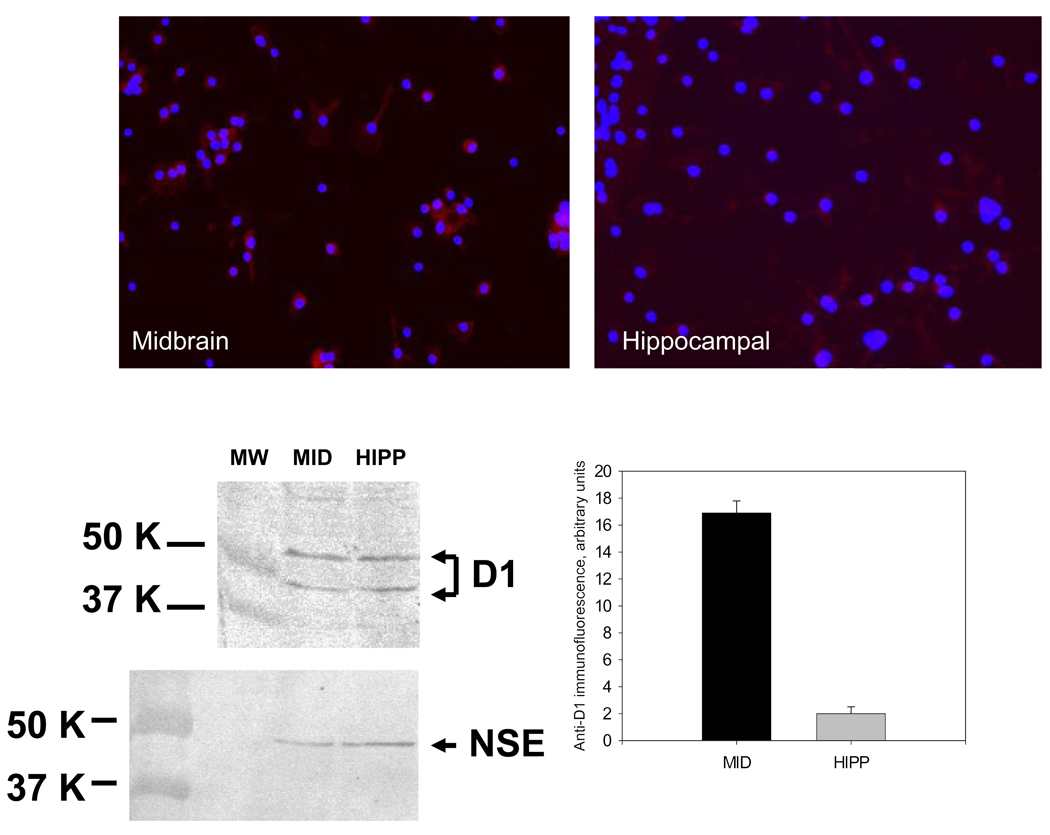
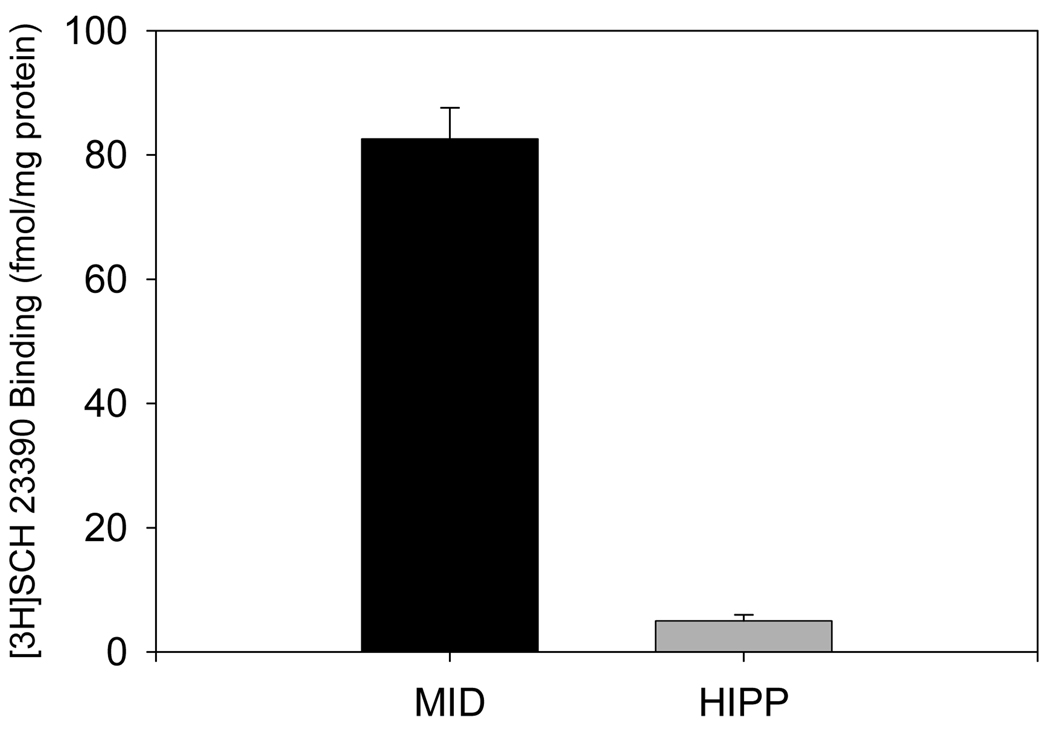
D1 immunoreactivity was present in midbrain and hippocampal cell cultures as identified by anti-D1 immunofluorescent microscopy, immunofluorescent plate readings and immunoblotting. Neurons positively stained with anti-D1 antibodies could be observed in midbrain and hippocampal cell cultures. Levels of anti-D1 immunoreactivity in hippocampal cell cultures were significantly lower than in midbrain cell cultures as it was determined by immunofluorescent plate reading. Anti-D1 immunopositive bands of appropriate molecular weight were could be detected in midbrain and hippocampal cell culture lysates (Figure 2). The presence of functional D1 receptors was also determined in midbrain and hippocampal cell cultures by ligand binding. Specific binding of [3H] SCH 23390 was significantly higher in midbrain cell cultures than hippocampal cell cultures [t-test, t (13) = 12.63, p ≤ 0.01). Specific binding of [3H] SCH 23390 was approximately 82.6 ± 5 fmol/mg protein in midbrain cultures of rat fetal neurons. In hippocampal cell cultures the level of [3H] SCH 23390 specific binding was very low (5 ± 1 fmol/mg protein, Figure 3).
Figure 2.
Anti-D1 immunoreactivity in primary cultures of rat fetal midbrain and hippocampal neurons. Microscopic images of midbrain and hippocampal cell cultures show the representative result of anti-D1/Hoechst staining. Images were captured under 20X magnification. The graph shows comparative levels of anti-D1 immunofluorescence in midbrain and hippocampal cell cultures. Immunofluorescent signal was measured in a microplate reader using 590 excitation/645 emission filter set. Results presented as mean arbitrary units of anti-D1 immunofluorescence per culture (well) ± SEM. Number of midbrain or hippocampal sister cultures analyzed = 8. Images of Western blots show the results of the detection of D1 and NSE immunoreactive protein in cell lysates prepared from in 14-day old midbrain and hippocampal cell cultures.
Figure 3.
Comparative levels of [3H]SCH 23390 binding in midbrain and hippocampal cell cultures. Results presented as mean fmols of [3H]SCH 23390 per mg of total protein ± SEM; n of sister cultures analyzed = 8.
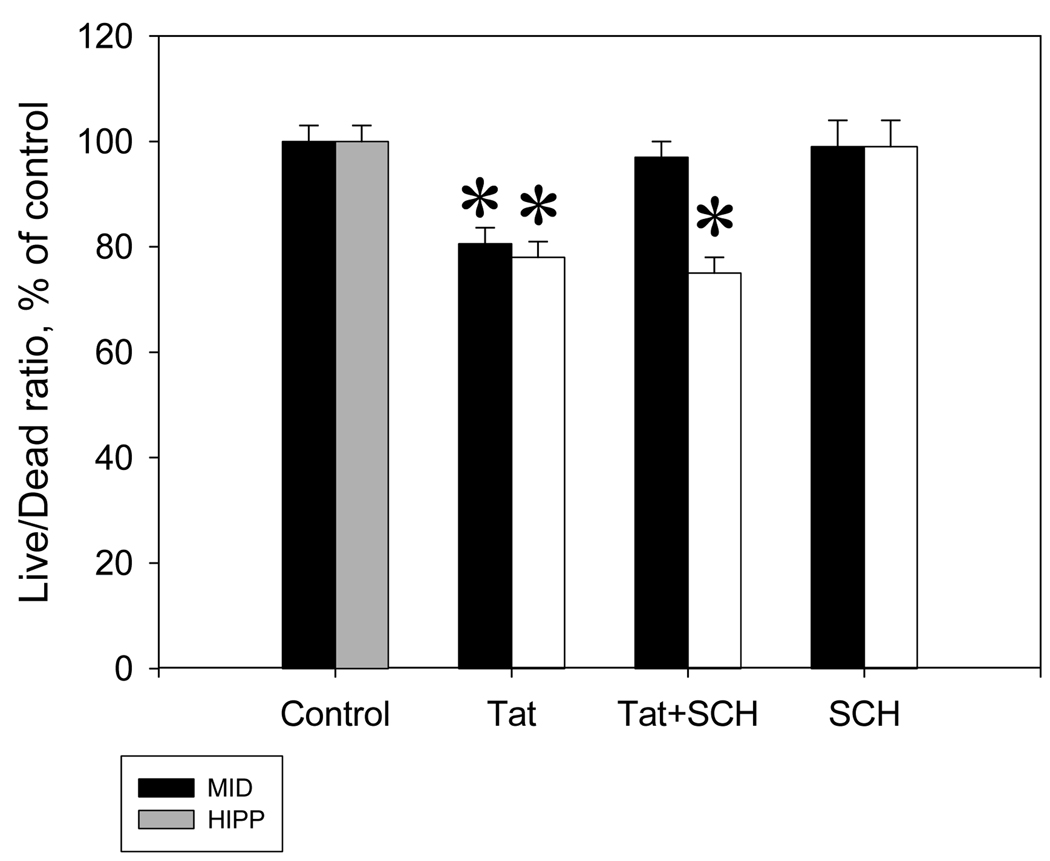
In midbrain cultures only, the addition of 10 µM SCH 23390 prior to Tat prevented Tat-induced decreases in cell viability (Figure 4). When Live/Dead ratio was compared across regions for all treatment groups, Analysis of Variance demonstrated a significant interaction between region and treatment (F(3,72) = 4.92 ; p ≤ 0.01). Post hoc analysis revealed that SCH 23390 alone caused no change in cell viability. The addition of Tat was again shown to cause toxicity in both midbrain (t = 4.40, p ≤ 0.01) and hippocampal (t = 4.44, p ≤ 0.01) cultures. Midbrain cultures which received SCH 23390 + Tat did not differ from control cultures and displayed significantly higher cell viability than the cultures receiving Tat alone (t = 3.529, p ≤ 0.01). Hippocampal cell cultures which received SCH 23390 + Tat displayed significantly lower cell viability than the control cultures (t = 5.07, p ≤ 0.01) and did not differ significantly from cultures which received Tat alone, indicating that the 10 µM dose of SCH 23390 did not decrease the toxicity of Tat in hippocampal cells.
Figure 4.
The specific D1 dopamine receptor antagonist, SCH 23390, eliminated the neurotoxicity of Tat 1–72 in rat midbrain fetal cultures, but not in rat hippocampal fetal cell cultures. Changes in Calcein/Ethidium bromide fluorescence ratio (Live/Dead ratio) following 48 hours of exposure to 50 nM Tat 1–72 or 50 nM Tat 1–72 + 10 µM SCH 23390 presented as mean % vs. non-treated control ± SEM; n of sister cultures analyzed = 16 per treatment. *- marks significant (p ≤ 0.05) difference between Tat-treated and vehicle control cell cultures. 10 µM SCH 23390 did not affect the viability of rat fetal brain cultures.
DISCUSSION
HIV-1 Tat is neurotoxic and nanomolar concentrations of this viral protein can cause degeneration of neurons in primary cortical (Bonavia et al., 2001), hippocampal (Aksenov et al., 2006), and midbrain cell cultures (Aksenova et al., 2006). Interactions of Tat with neuronal membrane-bound receptors play an essential role in the mechanism of Tat neurotoxicity (Strijbos et al., 1995; Wallace et al., 2006(a,b); Chandra et al., 2005). Previous studies in different cell culture models have focused on the role of NMDA receptor-controlled pathways in the mechanism of Tat neurotoxicity (King et al., 2006; Perez et al., 2001; Wang et al., 1999; Magnuson et al., 1995). There is considerable evidence of functional interactions and cross-talk between NMDA subtype glutamate receptors and dopamine D1-like receptors (Pei et al., 2004; Lee and Liu, 2004; Wirkner et al., 2004). Physical protein-protein interactions also can occur between the NMDA-receptor subunit-1 (NR1) and C-terminal peptides of D1 receptors; suggesting intracellular associations of direct relevance to dopaminergic modulation of NMDA currents (Pei et al., 2004). Several mechanisms are involved in this interaction: 1) D1R-dependent, second messenger-mediated phosphorylation of NMDAR subunits; 2) coordinated regulation of receptor trafficking at synaptic sites; 3) formation of a heteromeric D1/NMDA receptor complex (Missale et al., 2006). The number and outcomes of reciprocal interactions between D1 receptors and NMDA receptors have been intensively investigated (For review, Cepeda and Levine, 1998; 2006).
Rat fetal mesencephalic neurons exhibit NMDA and D1 dopamine receptor activities (Chneiweiss et al., 1984). Primary neuronal cell cultures prepared from rat fetal midbrain are known to contain sizeable populations of dopaminergic neurons (Prasad and Amara, 2001). Rat fetal hippocampal neurons in culture are known to express functional NMDA receptors (Harris and Miller, 1989). Moderate levels of D1/D5 dopamine receptor protein expression were recently documented in rat hippocampal cell cultures (Smialowski and Bijak, 1987; Lezcano and Bergson, 2002; Smith et al., 2005). Our results of the determination of D1 immunoreactivity and SCH 23390 specific binding in midbrain and hippocampal cell cultures are consistent with the literature.
In this study we demonstrated that the D1 receptor antagonist SCH 23390 was able to prevent Tat-induced decrease of cell viability in midbrain cell cultures but did not change toxic effects of Tat in hippocampal cell cultures. Levels of D1 dopamine receptor expression were significantly different in midbrain and hippocampal cell cultures. According to previous reports (Hoyt and Reynolds, 1996), 15–35% of cultured striatal neurons express D1 receptors. In hippocampal culture, as it follows from our results, low levels of D1 receptors are expressed in rat hippocampal neurons. This observation is consistent with a recently published report (Smith et al, 2005). Thus, it was not surprising that effects of SCH 23390 on Tat neurotoxicity were different in these two types of rat fetal brain cell cultures. The fact that the protection of neurons by this D1 receptor antagonist correlates with D1 immunoreactivity and [H3] SCH 23390 binding potential in Tat-treated rat fetal brain cell cultures confirms that the action of 10 µM SCH 23390 on Tat 1–72 toxicity is linked to the expression of functional D1 dopamine receptors.
For the first time the results presented in this study demonstrate that D1-mediated pathways are involved in the mechanism of Tat neurotoxicity in midbrain cell cultures. HIV-1 Tat was recently shown to inhibit DA uptake and DAT-specific ligand binding in different cell culture models (Aksenova et al., 2006; Wallace et al., 2006a). Thus, Tat-mediated inhibition of DA re-uptake in “presynaptic” DA neurons may influence the activity of D1 dopamine receptors in “postsynaptic” neurons and trigger NMDA receptor-controlled apoptotic cascades through the D1/NMDAR interactions. Alternatively, activation of NMDA receptors in D1-expressing neurons exposed to Tat may increase pro-apoptotic D1–controlled signaling. Blockade of D1 receptors with SCH 23390 has been demonstrated to alleviate c-fos and cleaved caspase-3 expression in rat striatum after perinatal asphyxia and cocaine binge (Mitchell and Snyder-Keller, 2003). Future studies are needed to further elucidate the role of reciprocal NMDAR/D1 regulation in the mechanism of Tat neurotoxicity.
Acknowledgments
Support provided by grants from the NIH: DA013137, DA014401 (RMB), and HD043680 (CFM)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aksenov MY, Hasselrot U, Bansal AK, Wu G, Nath A, Anderson C, Mactutus CF, Booze RM. Oxidative damage induced by the injection of HIV-1 Tat protein in the rat striatum. Neurosci Lett. 2001;305:5–8. doi: 10.1016/s0304-3940(01)01786-4. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Hasselrot U, Wu G, Nath A, Anderson C, Mactutus CF, Booze RM. Temporal relationships between HIV-1 Tat-induced neuronal degeneration, OX-42 immunoreactivity, reactive astrocytosis, and protein oxidation in the rat striatum. Brain Res. 2003;987:1–9. doi: 10.1016/s0006-8993(03)03194-9. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Aksenova MV, Nath A, Ray PD, Mactutus CF, Booze RM. Cocaine-mediated enhancement of Tat toxicity in rat hippocampal cell culture: The role of oxidative stress and D1 dopamine receptor. Neurotoxicol. 2006;27:217–228. doi: 10.1016/j.neuro.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Aksenova MV, Silvers JM, Aksenov MY, Nath A, Ray PD, Mactutus CF, Booze RM. HIV-1 neurotoxicity in primary cultures of rat midbrain fetal neurons: Changes in dopamine transporter binding and immunoreactivity. Neurosci Lett. 2006;395:235–239. doi: 10.1016/j.neulet.2005.10.095. [DOI] [PubMed] [Google Scholar]
- Basarsky TA, Parpura V, Haydon PG. Hippocampal synaptogenesis in cell culture: developmental time course of synapse formation, calcium influx, and synaptic protein distribution. J. Neurosci. 1994;14:6402–6411. doi: 10.1523/JNEUROSCI.14-11-06402.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger JR, Arendt G. HIV dementia: the role of the basal ganglia and dopaminergic systems. J Psychopharmacol. 2000;14:214–221. doi: 10.1177/026988110001400304. [DOI] [PubMed] [Google Scholar]
- Bonavia R, Bajetto A, Barbero S, Albini A, Noonan DM, Schettini G. HIV-1 Tat causes apoptotic death and calcium homeostasis alterations in rat neurons. Biochem Biophys Res Commun. 2001;288:301–308. doi: 10.1006/bbrc.2001.5743. [DOI] [PubMed] [Google Scholar]
- Chandra T, Maier W, Konig HG, Hirzel K, Kogel D, Schuler T, Chandra A, Demirhan I, Laube B. Molecular interactions of the type 1 human immunodeficiency virus transregulatory protein Tat with N-methyl-d-aspartate receptor subunits. Neuroscience. 2005;134:145–153. doi: 10.1016/j.neuroscience.2005.02.049. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Levine MS. Dopamine and N-methyl-D-aspartate receptor interactions in the neostriatum. Dev Neurosci. 1998;20:1–18. doi: 10.1159/000017294. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Levine MS. Where do you think you are going? The NMDA-D1 receptor trap. Sci STKE. 2006;333:20. doi: 10.1126/stke.3332006pe20. [DOI] [PubMed] [Google Scholar]
- Chneiweiss H, Prochiantz A, Glowinski J, Premont J. Biogenic amine-sensitive adenylate cyclases in primary culture of neuronal or glial cells from mesencephalon. Brain Res. 1984;302:363–370. doi: 10.1016/0006-8993(84)90251-8. [DOI] [PubMed] [Google Scholar]
- Farnie G, Clark RB, Spence K, Pinnock N, Brennan K, Anderson NG, bunred NJ. Novel cell culture technique for primary ductal carcinoma in situ: role of notch and epidermal growth factor receptor signaling pathways. JNCI J. Natl. Cancer Institute. 2007;99:616–627. doi: 10.1093/jnci/djk133. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Hasselrot U, Mactutus CF. Intrahippocampal injections of Tat: Effects on prepulse inhibition of the auditory startle response in adult male rats. Pharmacol Biochem Behav. 2006(a);84:189–196. doi: 10.1016/j.pbb.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal hippocampal Tat injections: Developmental effects on prepulse inhibition (PPI) of the auditory startle response. Int J Dev Neurosci. 2006(b);24:275–283. doi: 10.1016/j.ijdevneu.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Miller RJ. Excitatory amino acid-evoked release of [3H]GABA from hippocampal neurons in primary culture. Brain Res. 1989;482:23–33. doi: 10.1016/0006-8993(89)90538-6. [DOI] [PubMed] [Google Scholar]
- Hoyt KR, Reynolds IJ. Localization of D1 dopamine receptors on live cultured striatal neurons by quantitative fluorescence microscopy. Brain Res. 1996;731:21–30. doi: 10.1016/0006-8993(96)00436-2. [DOI] [PubMed] [Google Scholar]
- Hudson L, Liu J, Nath A, Jones M, Raghavan R, Narayan O, Male D, Everall I. Detection of the human immunodeficiency virus regulatory protein tat in CNS tissues. J Neurovirol. 2000;6:145–155. doi: 10.3109/13550280009013158. [DOI] [PubMed] [Google Scholar]
- Iacovitti L, Stull ND, Mishizen A. Neurotransmitters, KCl and antioxidants rescue striatal neurons from apoptotic cell death in culture. Brain Res. 1999;816:276–285. doi: 10.1016/s0006-8993(98)00955-x. [DOI] [PubMed] [Google Scholar]
- Kendall SL, Anderson CF, Nath A, Turchan-Cholewo J, Land CL, Mactutus CF, Booze RM. Gonadal steroids differentially modulate neurotoxicity of HIV and cocaine: Testosterone and ICI 182,780 sensitive mechanism. BMC Neurosci. 2005;6:40. doi: 10.1186/1471-2202-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JE, Eugenin EA, Buckner CM, Berman JW. HIV Tat and neurotoxicity. Microbes and Infection. 2006;8:1347–1357. doi: 10.1016/j.micinf.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Koutsilieri E, Scheller C, Sopper S, ter Meulen V, Riederer P. Psychiatric complications in human immunodefieciency virus infection. J Neurovirol. 2002;8:129–133. doi: 10.1080/13550280290167948. [DOI] [PubMed] [Google Scholar]
- Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload and oxidative stress. Exp Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Liu F. Direct interactions between NMDA and D1 receptors: A tale of tails. Biochem Soc Trans. 2004;32:1032–1036. doi: 10.1042/BST0321032. [DOI] [PubMed] [Google Scholar]
- Lezcano N, Bergson C. D1/D5 Dopamine receptors stimulate intracellular calcium release in primary cultures of neocortical and hippocampal neurons. J Neurophysiol. 2002;87:2167–2175. doi: 10.1152/jn.00541.2001. [DOI] [PubMed] [Google Scholar]
- Magnuson DS, Knudsen BE, Geiger JD, Brownstone RM, Nath A. Human immunodeficiency virus type 1 tat activates non-N-methyl-D-aspartate excitatory amino acid receptors and causes neurotoxicity. Ann Neurol. 1995;37:373–380. doi: 10.1002/ana.410370314. [DOI] [PubMed] [Google Scholar]
- Missale C, Fiorentini C, Busi C, Collo G, Spano PF. The NMDA/D1 receptor complex as a new target in drug development. Curr Top Med Chem. 2006;6:801–808. doi: 10.2174/156802606777057562. [DOI] [PubMed] [Google Scholar]
- Mitchell ES, Snyder-Keller A. Blockade of D1 dopaminergic transmission alleviates c-fos induction and cleaved caspase-3 expression in the brains of rat pups exposed to prenatal cocaine or perinatal asphyxia. Exp Neurol. 2003;182:64–74. doi: 10.1016/s0014-4886(03)00026-8. [DOI] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186 Suppl 2:S193–S198. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- Nath A, Anderson C, Jones M, Maragos W, Booze R, Mactutus C, Bell J, Hauser KF, Mattson M. Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. J Psychopharmacol. 2000;14:222–227. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- Pei L, Lee FJ, Moszynska A, Vukusic B, Liu F. Regulation of dopamine D1 receptor function by physical interaction with the NMDA receptors. J Neurosci. 2004;24:1149–1158. doi: 10.1523/JNEUROSCI.3922-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez A, Probert AW, Wang KK, Sharmeen L. Evaluation of HIV-1 Tat induced neurotoxicity in rat cortical cell culture. J Neurovirol. 2001;7:1–10. doi: 10.1080/135502801300069575. [DOI] [PubMed] [Google Scholar]
- Prasad BM, Amara SG. The dopamine transporter in mesencephalic cultures is refractory to physiological changes in membrane voltage. J Neurosci. 2001;21:7561–7567. doi: 10.1523/JNEUROSCI.21-19-07561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JM, Aksenov MY, Aksenova MV, Beckley J, Olton P, Mactutus CF, Booze RM. Dopaminergic marker proteins in the substantia nigra of human immunodeficiency virus type 1-infected brains. J Neurovirol. 2006;12:140–145. doi: 10.1080/13550280600724319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smialowski A, Bijak M. Excitatory and inhibitory action of dopamine on hippocampal neurons in vitro. Involvement of D2 and D1 receptors. Neuroscience. 1987;1:95–101. doi: 10.1016/0306-4522(87)90274-0. [DOI] [PubMed] [Google Scholar]
- Smith WB, Starck SR, Roberts RW, Schuman EM. Dopaminergic stimulation of local protein synthesis enhances surface expression of GluR1 and synaptic transmission in hippocampal neurons. Neuron. 2005;45:765–779. doi: 10.1016/j.neuron.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Strijbos PJ, Zamani MR, Rothwell NJ, Arbuthnott G, Harkiss G. Neurotoxic mechanisms of transactivating protein Tat of Maedi-Visna virus. Neurosci Lett. 1995;197:215–218. doi: 10.1016/0304-3940(95)11940-x. [DOI] [PubMed] [Google Scholar]
- Turchan J, Anderson C, Hauser KF, Sun Q, Zhang J, Liu Y, Wise PM, Kruman II, Maragos W, Mattson MP, Booze R, Nath A. Estrogen protects against the synergistic toxicity by HIV proteins, methamphetamine and cocaine. BMC Neurosci. 2001;2:3. doi: 10.1186/1471-2202-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle LD, Croul S, Morgello S, Amini S, Rappaport J, Khalili K. Detection of HIV-1 Tat and JCV capsid protein, VP1, in AIDS brain with progressive multifocal leukoencephalopathy. J Neurovirol. 2000;6:221–228. doi: 10.3109/13550280009015824. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Stevens MJ, Backus C, McLean LL, Feldman EL. Cell culture modeling to test therapies against hyperclycemia-mediated oxidative stress and injury. Antioxidants & Redox Signaling. 2005;7:1494–1506. doi: 10.1089/ars.2005.7.1494. [DOI] [PubMed] [Google Scholar]
- Yuferov V, Kroslak T, Laforge KS, Zhou Y, Ho A, Kreek MJ. Differential gene expression in the rat caudate putamen after "binge" cocaine administration: advantage of triplicate microarray analysis. Synapse. 2003;48:157–169. doi: 10.1002/syn.10198. [DOI] [PubMed] [Google Scholar]
- Wallace DR, Dodson SL, Nath A, Booze RM. Estrogen attenuates gp120 and Tat (1–72)-induced oxidative stress and prevents loss of dopamine transporter function. Synapse. 2006(a);59:51–60. doi: 10.1002/syn.20214. [DOI] [PubMed] [Google Scholar]
- Wallace DR, Dodson SL, Nath A, Booze RM. Delta opioid agonists attenuate TAT(1–72)-induced oxidative stress in SK-N-SH cells. Neurotoxicol. 2006(b);27:101–107. doi: 10.1016/j.neuro.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, Fowler JS. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004;127:2452–2458. doi: 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- Wang P, Barks JD, Silverstein FS. Tat, a human immunodeficiency virus-1-derived protein, augments excitotoxic hippocampal injury in neonatal rats. Neuroscience. 1999;88:585–597. doi: 10.1016/s0306-4522(98)00242-5. [DOI] [PubMed] [Google Scholar]
- Wirkner K, Krause T, Koles L, Thummler S, Al-Kharasani M, Illes P. D1 but not D2 dopamine receptors or adrenoceptors mediate dopamine-induced potentiation of N-methyl-d-aspartate currents in the rat prefrontal cortex. Neurosci Lett. 2004;372:89–93. doi: 10.1016/j.neulet.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Zauli G, Secchierro P, Rodella L, Gibellini D, Mirandola P, Mazzoni M, Milani D, Dowd D, Capitani S, Vitale M. HIV-1 Tat-mediated inhibition of the tyrosine hydroxylase gene expression in dopaminergic neuronal cells. J Biol Chem. 2000;275:4159–4165. doi: 10.1074/jbc.275.6.4159. [DOI] [PubMed] [Google Scholar]
- Zang L, Lou D, Jiao H, Zhang D, Wang X, Xia Y, Zhang J, Xu M. Cocaine-induced intracellular signaling and gene expression are oppositely regulated by the dopamine D1 and D3 receptors. J Neurosci. 2004;24:3344–3354. doi: 10.1523/JNEUROSCI.0060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]