Abstract
The production by monocytes of interleukin-1 alpha (IL-1 alpha), interleukin-1 beta (IL-1 beta), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF alpha) in intensive care unit (ICU) patients with sepsis syndrome (n = 23) or noninfectious shock (n = 6) is reported. Plasma cytokines, cell-associated cytokines within freshly isolated monocytes and LPS-induced in vitro cytokine production were assessed at admission and at regular intervals during ICU stay. TNF alpha and IL-6 were the most frequently detected circulating cytokines. Despite the fact that IL-1 alpha is the main cytokine found within monocytes upon in vitro activation of cells from healthy individuals, it was very rarely detected within freshly isolated monocytes from septic patients, and levels of cell-associated IL-1 beta were lower than those of TNF alpha. Cell-associated IL-1 beta and TNF alpha were not correlated with corresponding levels in plasma. Upon LPS stimulation, we observed a profound decrease of in vitro IL-1 alpha production by monocytes in all patients, and of IL-1 beta, IL-6, and TNF alpha in septic patients. This reduced LPS-induced production of cytokines was most pronounced in patients with gram-negative infections. Finally, monocytes from survival patients, but not from nonsurvival ones recovered their capacity to produce normal amounts of cytokines upon LPS stimulation. In conclusion, our data indicate an in vivo activation of circulating monocytes during sepsis as well as in noninfectious shock and suggest that complex regulatory mechanisms can downregulate the production of cytokines by monocytes during severe infections.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. L., Semrad S. D., Czuprynski C. J. Administration of bacterial lipopolysaccharide elicits circulating tumor necrosis factor-alpha in neonatal calves. J Clin Microbiol. 1990 May;28(5):998–1001. doi: 10.1128/jcm.28.5.998-1001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andus T., Geiger T., Hirano T., Kishimoto T., Heinrich P. C. Action of recombinant human interleukin 6, interleukin 1 beta and tumor necrosis factor alpha on the mRNA induction of acute-phase proteins. Eur J Immunol. 1988 May;18(5):739–746. doi: 10.1002/eji.1830180513. [DOI] [PubMed] [Google Scholar]
- Bailly S., Mahe Y., Ferrua B., Fay M., Tursz T., Wakasugi H., Gougerot-Pocidalo M. A. Quinolone-induced differential modification of IL-1 alpha and IL-1 beta production by LPS-stimulated human monocytes. Cell Immunol. 1990 Jun;128(1):277–288. doi: 10.1016/0008-8749(90)90025-m. [DOI] [PubMed] [Google Scholar]
- Baumann H., Richards C., Gauldie J. Interaction among hepatocyte-stimulating factors, interleukin 1, and glucocorticoids for regulation of acute phase plasma proteins in human hepatoma (HepG2) cells. J Immunol. 1987 Dec 15;139(12):4122–4128. [PubMed] [Google Scholar]
- Beutler B. A., Milsark I. W., Cerami A. Cachectin/tumor necrosis factor: production, distribution, and metabolic fate in vivo. J Immunol. 1985 Dec;135(6):3972–3977. [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Majeau G. R., Fiers W., Cotran R. S., Gimbrone M. A., Jr Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with the actions of interleukin 1. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4533–4537. doi: 10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
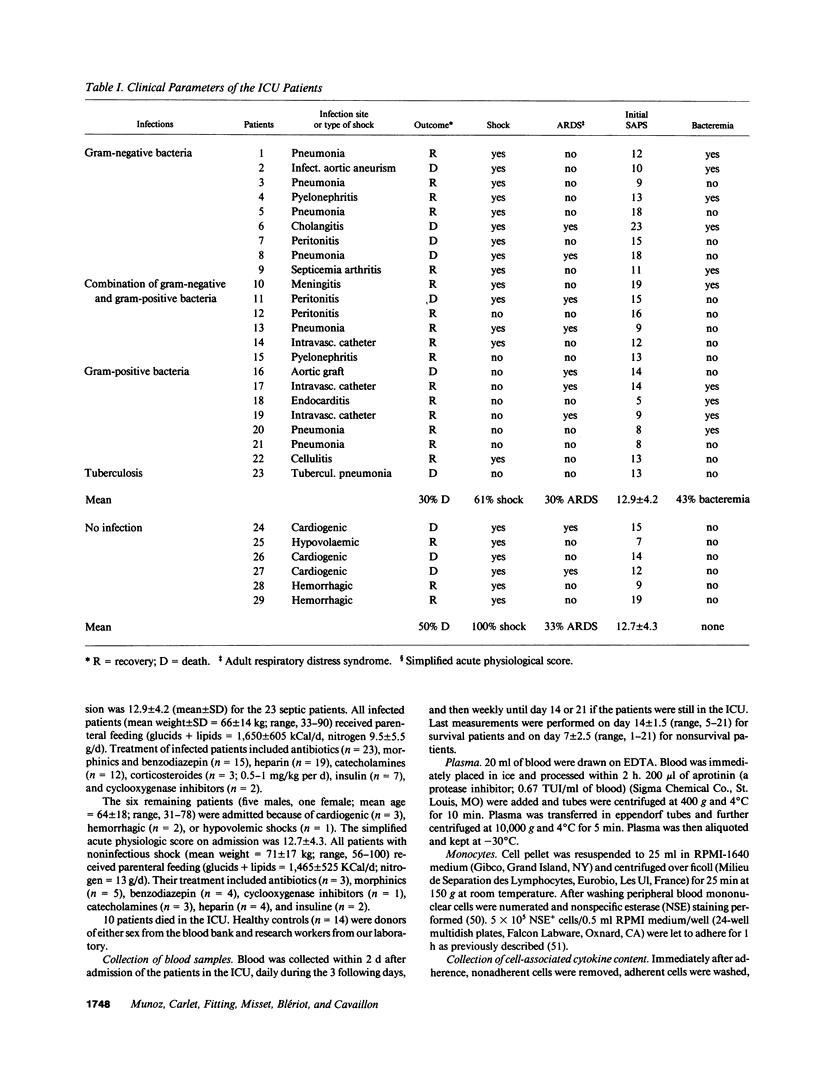
- Bone R. C., Fisher C. J., Jr, Clemmer T. P., Slotman G. J., Metz C. A., Balk R. A. Sepsis syndrome: a valid clinical entity. Methylprednisolone Severe Sepsis Study Group. Crit Care Med. 1989 May;17(5):389–393. [PubMed] [Google Scholar]
- Brouckaert P., Spriggs D. R., Demetri G., Kufe D. W., Fiers W. Circulating interleukin 6 during a continuous infusion of tumor necrosis factor and interferon gamma. J Exp Med. 1989 Jun 1;169(6):2257–2262. doi: 10.1084/jem.169.6.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calandra T., Baumgartner J. D., Grau G. E., Wu M. M., Lambert P. H., Schellekens J., Verhoef J., Glauser M. P. Prognostic values of tumor necrosis factor/cachectin, interleukin-1, interferon-alpha, and interferon-gamma in the serum of patients with septic shock. Swiss-Dutch J5 Immunoglobulin Study Group. J Infect Dis. 1990 May;161(5):982–987. doi: 10.1093/infdis/161.5.982. [DOI] [PubMed] [Google Scholar]
- Cannon J. G., Tompkins R. G., Gelfand J. A., Michie H. R., Stanford G. G., van der Meer J. W., Endres S., Lonnemann G., Corsetti J., Chernow B. Circulating interleukin-1 and tumor necrosis factor in septic shock and experimental endotoxin fever. J Infect Dis. 1990 Jan;161(1):79–84. doi: 10.1093/infdis/161.1.79. [DOI] [PubMed] [Google Scholar]
- Cannon J. G., van der Meer J. W., Kwiatkowski D., Endres S., Lonnemann G., Burke J. F., Dinarello C. A. Interleukin-1 beta in human plasma: optimization of blood collection, plasma extraction, and radioimmunoassay methods. Lymphokine Res. 1988 Winter;7(4):457–467. [PubMed] [Google Scholar]
- Castell J. V., Geiger T., Gross V., Andus T., Walter E., Hirano T., Kishimoto T., Heinrich P. C. Plasma clearance, organ distribution and target cells of interleukin-6/hepatocyte-stimulating factor in the rat. Eur J Biochem. 1988 Nov 1;177(2):357–361. doi: 10.1111/j.1432-1033.1988.tb14384.x. [DOI] [PubMed] [Google Scholar]
- Cavaillon J. M., Fitting C., Haeffner-Cavaillon N., Kirsch S. J., Warren H. S. Cytokine response by monocytes and macrophages to free and lipoprotein-bound lipopolysaccharide. Infect Immun. 1990 Jul;58(7):2375–2382. doi: 10.1128/iai.58.7.2375-2382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillon J. M., Fitting C., Haeffner-Cavaillon N. Recombinant C5a enhances interleukin 1 and tumor necrosis factor release by lipopolysaccharide-stimulated monocytes and macrophages. Eur J Immunol. 1990 Feb;20(2):253–257. doi: 10.1002/eji.1830200204. [DOI] [PubMed] [Google Scholar]
- Cavaillon J. M., Haeffner-Cavaillon N. Signals involved in interleukin 1 synthesis and release by lipopolysaccharide-stimulated monocytes/macrophages. Cytokine. 1990 Sep;2(5):313–329. doi: 10.1016/1043-4666(90)90061-w. [DOI] [PubMed] [Google Scholar]
- Cybulsky M. I., Movat H. Z., Dinarello C. A. Role of interleukin-1 and tumour necrosis factor-alpha in acute inflammation. Ann Inst Pasteur Immunol. 1987 May-Jun;138(3):505–512. doi: 10.1016/s0769-2625(87)80068-5. [DOI] [PubMed] [Google Scholar]
- De Simoni M. G., Sironi M., De Luigi A., Manfridi A., Mantovani A., Ghezzi P. Intracerebroventricular injection of interleukin 1 induces high circulating levels of interleukin 6. J Exp Med. 1990 May 1;171(5):1773–1778. doi: 10.1084/jem.171.5.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn C. J., Schaub R. G., Fleming W. E., Gibbons A. J. Interleukin-1 induced vascular pathology "in vivo": a scanning electron-microscopy study. Agents Actions. 1989 Jun;27(3-4):287–289. doi: 10.1007/BF01972800. [DOI] [PubMed] [Google Scholar]
- Endres S., Cannon J. G., Ghorbani R., Dempsey R. A., Sisson S. D., Lonnemann G., Van der Meer J. W., Wolff S. M., Dinarello C. A. In vitro production of IL 1 beta, IL 1 alpha, TNF and IL2 in healthy subjects: distribution, effect of cyclooxygenase inhibition and evidence of independent gene regulation. Eur J Immunol. 1989 Dec;19(12):2327–2333. doi: 10.1002/eji.1830191222. [DOI] [PubMed] [Google Scholar]
- Evans G. F., Snyder Y. M., Butler L. D., Zuckerman S. H. Differential expression of interleukin-1 and tumor necrosis factor in murine septic shock models. Circ Shock. 1989 Dec;29(4):279–290. [PubMed] [Google Scholar]
- Ferrari-Baliviera E., Mealy K., Smith R. J., Wilmore D. W. Tumor necrosis factor induces adult respiratory distress syndrome in rats. Arch Surg. 1989 Dec;124(12):1400–1405. doi: 10.1001/archsurg.1989.01410120046010. [DOI] [PubMed] [Google Scholar]
- Fieren M. W., Van den Bemd G. J., Bonta I. L. Endotoxin-stimulated peritoneal macrophages obtained from continuous ambulatory peritoneal dialysis patients show an increased capacity to release interleukin-1 beta in vitro during infectious peritonitis. Eur J Clin Invest. 1990 Aug;20(4):453–457. doi: 10.1111/j.1365-2362.1990.tb01883.x. [DOI] [PubMed] [Google Scholar]
- Fong Y., Lowry S. F. Tumor necrosis factor in the pathophysiology of infection and sepsis. Clin Immunol Immunopathol. 1990 May;55(2):157–170. doi: 10.1016/0090-1229(90)90094-7. [DOI] [PubMed] [Google Scholar]
- Fong Y., Moldawer L. L., Marano M., Wei H., Tatter S. B., Clarick R. H., Santhanam U., Sherris D., May L. T., Sehgal P. B. Endotoxemia elicits increased circulating beta 2-IFN/IL-6 in man. J Immunol. 1989 Apr 1;142(7):2321–2324. [PubMed] [Google Scholar]
- Gauldie J., Northemann W., Fey G. H. IL-6 functions as an exocrine hormone in inflammation. Hepatocytes undergoing acute phase responses require exogenous IL-6. J Immunol. 1990 May 15;144(10):3804–3808. [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardin E., Grau G. E., Dayer J. M., Roux-Lombard P., Lambert P. H. Tumor necrosis factor and interleukin-1 in the serum of children with severe infectious purpura. N Engl J Med. 1988 Aug 18;319(7):397–400. doi: 10.1056/NEJM198808183190703. [DOI] [PubMed] [Google Scholar]
- Goldblum S. E., Yoneda K., Cohen D. A., McClain C. J. Provocation of pulmonary vascular endothelial injury in rabbits by human recombinant interleukin-1 beta. Infect Immun. 1988 Sep;56(9):2255–2263. doi: 10.1128/iai.56.9.2255-2263.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas J. G., Thiel C., Blömer K., Weiss E. H., Riethmüller G., Ziegler-Heitbrock H. W. Downregulation of tumor necrosis factor expression in the human Mono-Mac-6 cell line by lipopolysaccharide. J Leukoc Biol. 1989 Jul;46(1):11–14. doi: 10.1002/jlb.46.1.11. [DOI] [PubMed] [Google Scholar]
- Hack C. E., De Groot E. R., Felt-Bersma R. J., Nuijens J. H., Strack Van Schijndel R. J., Eerenberg-Belmer A. J., Thijs L. G., Aarden L. A. Increased plasma levels of interleukin-6 in sepsis. Blood. 1989 Oct;74(5):1704–1710. [PubMed] [Google Scholar]
- Haeffner-Cavaillon N., Cavaillon J. M., Ciancioni C., Bacle F., Delons S., Kazatchkine M. D. In vivo induction of interleukin-1 during hemodialysis. Kidney Int. 1989 May;35(5):1212–1218. doi: 10.1038/ki.1989.112. [DOI] [PubMed] [Google Scholar]
- Haeffner-Cavaillon N., Roussellier N., Ponzio O., Carreno M. P., Laude M., Carpentier A., Kazatchkine M. D. Induction of interleukin-1 production in patients undergoing cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1989 Dec;98(6):1100–1106. [PubMed] [Google Scholar]
- Hansen M. B., Nielsen S. E., Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989 May 12;119(2):203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- Helfgott D. C., Tatter S. B., Santhanam U., Clarick R. H., Bhardwaj N., May L. T., Sehgal P. B. Multiple forms of IFN-beta 2/IL-6 in serum and body fluids during acute bacterial infection. J Immunol. 1989 Feb 1;142(3):948–953. [PubMed] [Google Scholar]
- Helminen M., Vesikari T. Interleukin-1 production in bacterial meningitis. Scand J Infect Dis. 1990;22(1):105–108. doi: 10.3109/00365549009023128. [DOI] [PubMed] [Google Scholar]
- Ikejima T., Okusawa S., Ghezzi P., van der Meer J. W., Dinarello C. A. Interleukin-1 induces tumor necrosis factor (TNF) in human peripheral blood mononuclear cells in vitro and a circulating TNF-like activity in rabbits. J Infect Dis. 1990 Jul;162(1):215–223. doi: 10.1093/infdis/162.1.215. [DOI] [PubMed] [Google Scholar]
- Jablons D. M., Mulé J. J., McIntosh J. K., Sehgal P. B., May L. T., Huang C. M., Rosenberg S. A., Lotze M. T. IL-6/IFN-beta-2 as a circulating hormone. Induction by cytokine administration in humans. J Immunol. 1989 Mar 1;142(5):1542–1547. [PubMed] [Google Scholar]
- Jacobs R. F., Tabor D. R., Burks A. W., Campbell G. D. Elevated interleukin-1 release by human alveolar macrophages during the adult respiratory distress syndrome. Am Rev Respir Dis. 1989 Dec;140(6):1686–1692. doi: 10.1164/ajrccm/140.6.1686. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Klapproth J., Castell J., Geiger T., Andus T., Heinrich P. C. Fate and biological action of human recombinant interleukin 1 beta in the rat in vivo. Eur J Immunol. 1989 Aug;19(8):1485–1490. doi: 10.1002/eji.1830190821. [DOI] [PubMed] [Google Scholar]
- Last-Barney K., Homon C. A., Faanes R. B., Merluzzi V. J. Synergistic and overlapping activities of tumor necrosis factor-alpha and IL-1. J Immunol. 1988 Jul 15;141(2):527–530. [PubMed] [Google Scholar]
- Le Gall J. R., Loirat P., Alperovitch A., Glaser P., Granthil C., Mathieu D., Mercier P., Thomas R., Villers D. A simplified acute physiology score for ICU patients. Crit Care Med. 1984 Nov;12(11):975–977. doi: 10.1097/00003246-198411000-00012. [DOI] [PubMed] [Google Scholar]
- LeMay D. R., LeMay L. G., Kluger M. J., D'Alecy L. G. Plasma profiles of IL-6 and TNF with fever-inducing doses of lipopolysaccharide in dogs. Am J Physiol. 1990 Jul;259(1 Pt 2):R126–R132. doi: 10.1152/ajpregu.1990.259.1.R126. [DOI] [PubMed] [Google Scholar]
- LeMay L. G., Otterness I. G., Vander A. J., Kluger M. J. In vivo evidence that the rise in plasma IL 6 following injection of a fever-inducing dose of LPS is mediated by IL 1 beta. Cytokine. 1990 May;2(3):199–204. doi: 10.1016/1043-4666(90)90016-m. [DOI] [PubMed] [Google Scholar]
- Marano M. A., Fong Y., Moldawer L. L., Wei H., Calvano S. E., Tracey K. J., Barie P. S., Manogue K., Cerami A., Shires G. T. Serum cachectin/tumor necrosis factor in critically ill patients with burns correlates with infection and mortality. Surg Gynecol Obstet. 1990 Jan;170(1):32–38. [PubMed] [Google Scholar]
- Marks J. D., Marks C. B., Luce J. M., Montgomery A. B., Turner J., Metz C. A., Murray J. F. Plasma tumor necrosis factor in patients with septic shock. Mortality rate, incidence of adult respiratory distress syndrome, and effects of methylprednisolone administration. Am Rev Respir Dis. 1990 Jan;141(1):94–97. doi: 10.1164/ajrccm/141.1.94. [DOI] [PubMed] [Google Scholar]
- Mathison J. C., Virca G. D., Wolfson E., Tobias P. S., Glaser K., Ulevitch R. J. Adaptation to bacterial lipopolysaccharide controls lipopolysaccharide-induced tumor necrosis factor production in rabbit macrophages. J Clin Invest. 1990 Apr;85(4):1108–1118. doi: 10.1172/JCI114542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie H. R., Manogue K. R., Spriggs D. R., Revhaug A., O'Dwyer S., Dinarello C. A., Cerami A., Wolff S. M., Wilmore D. W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988 Jun 9;318(23):1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Movat H. Z., Burrowes C. E., Cybulsky M. I., Dinarello C. A. Acute inflammation and a Shwartzman-like reaction induced by interleukin-1 and tumor necrosis factor. Synergistic action of the cytokines in the induction of inflammation and microvascular injury. Am J Pathol. 1987 Dec;129(3):463–476. [PMC free article] [PubMed] [Google Scholar]
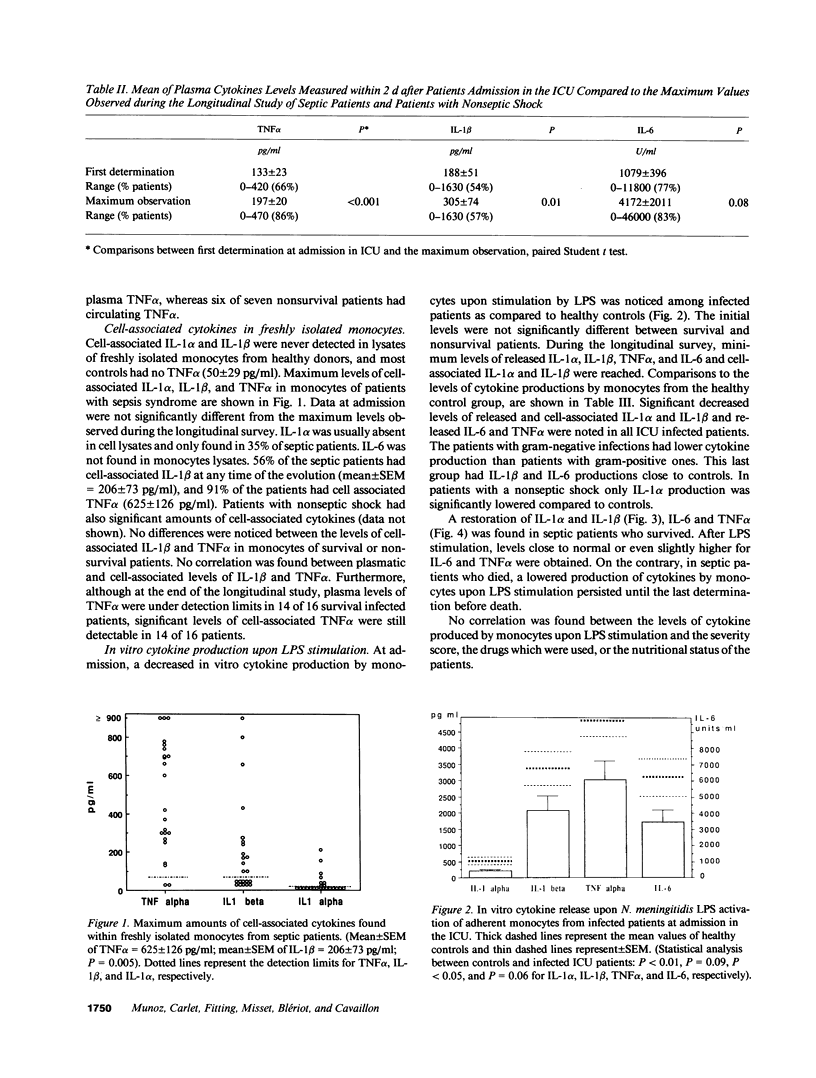
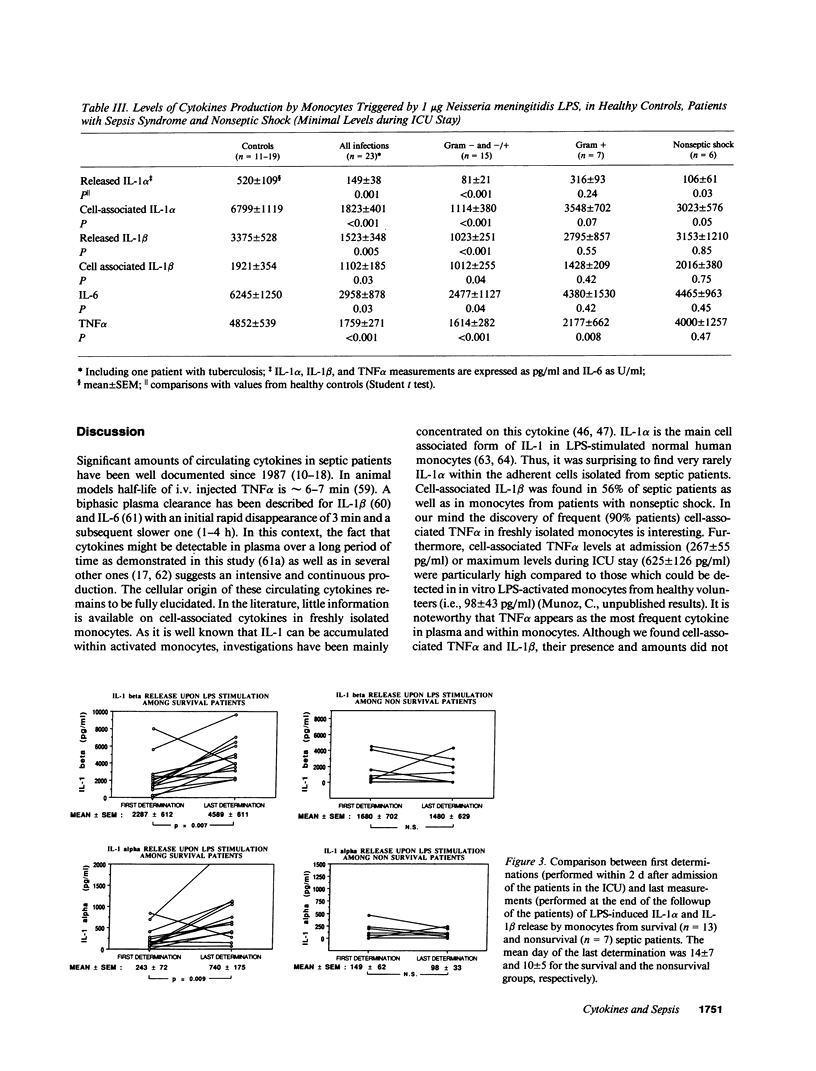
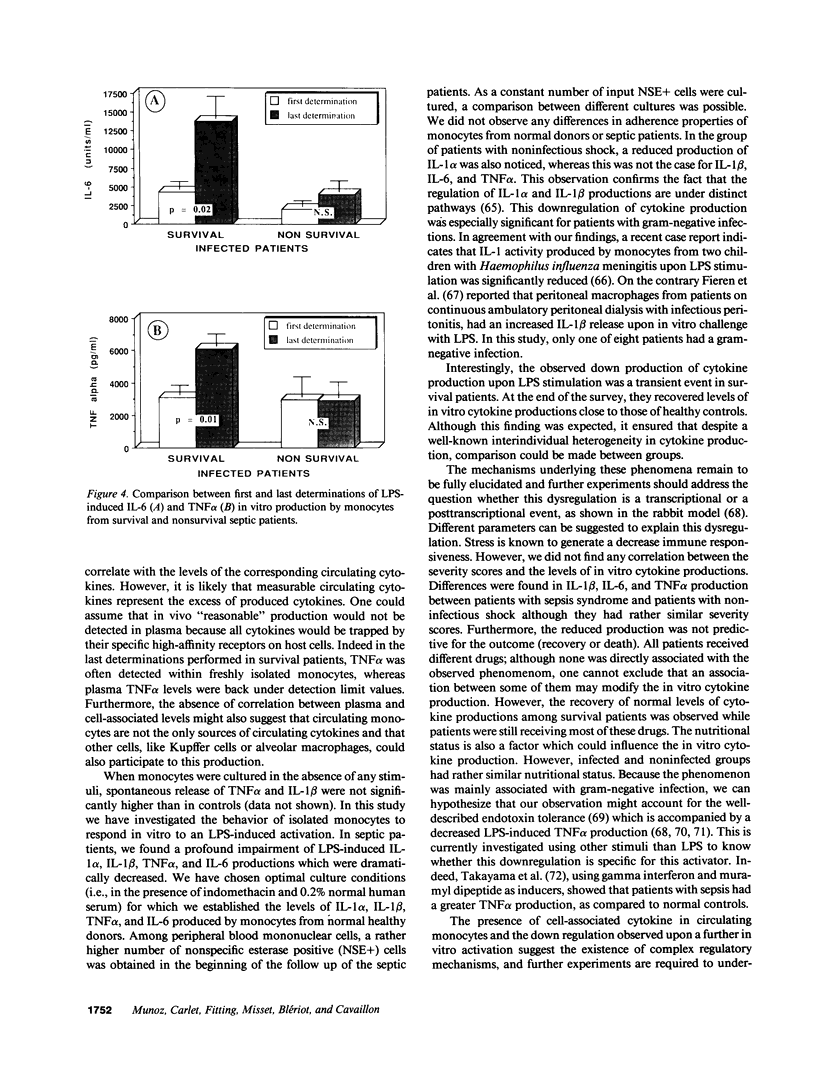
- Munoz C., Misset B., Fitting C., Blériot J. P., Carlet J., Cavaillon J. M. Dissociation between plasma and monocyte-associated cytokines during sepsis. Eur J Immunol. 1991 Sep;21(9):2177–2184. doi: 10.1002/eji.1830210928. [DOI] [PubMed] [Google Scholar]
- Natanson C., Eichenholz P. W., Danner R. L., Eichacker P. Q., Hoffman W. D., Kuo G. C., Banks S. M., MacVittie T. J., Parrillo J. E. Endotoxin and tumor necrosis factor challenges in dogs simulate the cardiovascular profile of human septic shock. J Exp Med. 1989 Mar 1;169(3):823–832. doi: 10.1084/jem.169.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner F., Philippé J., Vogelaers D., Colardyn F., Baele G., Baudrihaye M., Vermeulen A., Leroux-Roels G. Serum tumor necrosis factor levels in patients with infectious disease and septic shock. J Lab Clin Med. 1990 Jul;116(1):100–105. [PubMed] [Google Scholar]
- Okusawa S., Gelfand J. A., Ikejima T., Connolly R. J., Dinarello C. A. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest. 1988 Apr;81(4):1162–1172. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opp M., Obal F., Jr, Cady A. B., Johannsen L., Krueger J. M. Interleukin-6 is pyrogenic but not somnogenic. Physiol Behav. 1989 May;45(5):1069–1072. doi: 10.1016/0031-9384(89)90239-4. [DOI] [PubMed] [Google Scholar]
- Ramadori G., Van Damme J., Rieder H., Meyer zum Büschenfelde K. H. Interleukin 6, the third mediator of acute-phase reaction, modulates hepatic protein synthesis in human and mouse. Comparison with interleukin 1 beta and tumor necrosis factor-alpha. Eur J Immunol. 1988 Aug;18(8):1259–1264. doi: 10.1002/eji.1830180817. [DOI] [PubMed] [Google Scholar]
- Rothstein J. L., Schreiber H. Synergy between tumor necrosis factor and bacterial products causes hemorrhagic necrosis and lethal shock in normal mice. Proc Natl Acad Sci U S A. 1988 Jan;85(2):607–611. doi: 10.1073/pnas.85.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Cantu L., Rode H. N., Christou N. V. Endotoxin tolerance is associated with reduced secretion of tumor necrosis factor. Arch Surg. 1989 Dec;124(12):1432–1436. doi: 10.1001/archsurg.1989.01410120082016. [DOI] [PubMed] [Google Scholar]
- Saukkonen K., Sande S., Cioffe C., Wolpe S., Sherry B., Cerami A., Tuomanen E. The role of cytokines in the generation of inflammation and tissue damage in experimental gram-positive meningitis. J Exp Med. 1990 Feb 1;171(2):439–448. doi: 10.1084/jem.171.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby M. R., Waage A., Aarden L., Espevik T. Endotoxin, tumor necrosis factor-alpha and interleukin 1 induce interleukin 6 production in vivo. Clin Immunol Immunopathol. 1989 Dec;53(3):488–498. doi: 10.1016/0090-1229(89)90010-x. [DOI] [PubMed] [Google Scholar]
- Smith D. M., Lackides G. A., Epstein L. B. Coordinated induction of autocrine tumor necrosis factor and interleukin 1 in normal human monocytes and the implications for monocyte-mediated cytotoxicity. Cancer Res. 1990 Jun 1;50(11):3146–3153. [PubMed] [Google Scholar]
- Stephens K. E., Ishizaka A., Wu Z. H., Larrick J. W., Raffin T. A. Granulocyte depletion prevents tumor necrosis factor-mediated acute lung injury in guinea pigs. Am Rev Respir Dis. 1988 Nov;138(5):1300–1307. doi: 10.1164/ajrccm/138.5.1300. [DOI] [PubMed] [Google Scholar]
- Takayama T. K., Miller C., Szabo G. Elevated tumor necrosis factor alpha production concomitant to elevated prostaglandin E2 production by trauma patients' monocytes. Arch Surg. 1990 Jan;125(1):29–35. doi: 10.1001/archsurg.1990.01410130031004. [DOI] [PubMed] [Google Scholar]
- Tucker S. B., Pierre R. V., Jordon R. E. Rapid identification of monocytes in a mixed mononuclear cell preparation. J Immunol Methods. 1977;14(3-4):267–269. doi: 10.1016/0022-1759(77)90137-5. [DOI] [PubMed] [Google Scholar]
- Van Snick J., Cayphas S., Vink A., Uyttenhove C., Coulie P. G., Rubira M. R., Simpson R. J. Purification and NH2-terminal amino acid sequence of a T-cell-derived lymphokine with growth factor activity for B-cell hybridomas. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9679–9683. doi: 10.1073/pnas.83.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waage A., Brandtzaeg P., Halstensen A., Kierulf P., Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989 Jan 1;169(1):333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waage A., Halstensen A., Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987 Feb 14;1(8529):355–357. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- Waage A., Halstensen A., Shalaby R., Brandtzaeg P., Kierulf P., Espevik T. Local production of tumor necrosis factor alpha, interleukin 1, and interleukin 6 in meningococcal meningitis. Relation to the inflammatory response. J Exp Med. 1989 Dec 1;170(6):1859–1867. doi: 10.1084/jem.170.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groote M. A., Martin M. A., Densen P., Pfaller M. A., Wenzel R. P. Plasma tumor necrosis factor levels in patients with presumed sepsis. Results in those treated with antilipid A antibody vs placebo. JAMA. 1989 Jul 14;262(2):249–251. doi: 10.1001/jama.262.2.249. [DOI] [PubMed] [Google Scholar]
- van der Meer J. W., Endres S., Lonnemann G., Cannon J. G., Ikejima T., Okusawa S., Gelfand J. A., Dinarello C. A. Concentrations of immunoreactive human tumor necrosis factor alpha produced by human mononuclear cells in vitro. J Leukoc Biol. 1988 Mar;43(3):216–223. doi: 10.1002/jlb.43.3.216. [DOI] [PubMed] [Google Scholar]
- van der Poll T., Büller H. R., ten Cate H., Wortel C. H., Bauer K. A., van Deventer S. J., Hack C. E., Sauerwein H. P., Rosenberg R. D., ten Cate J. W. Activation of coagulation after administration of tumor necrosis factor to normal subjects. N Engl J Med. 1990 Jun 7;322(23):1622–1627. doi: 10.1056/NEJM199006073222302. [DOI] [PubMed] [Google Scholar]





