Abstract
Apoptosis of podocytes is considered critical in the pathogenesis of diabetic nephropathy (DN). Free fatty acids (FFAs) are critically involved in the pathogenesis of diabetes mellitus type 2, in particular the regulation of pancreatic β cell survival. The objectives of this study were to elucidate the role of palmitic acid, palmitoleic, and oleic acid in the regulation of podocyte cell death and endoplasmic reticulum (ER) stress. We show that palmitic acid increases podocyte cell death, both apoptosis and necrosis of podocytes, in a dose and time-dependent fashion. Palmitic acid induces podocyte ER stress, leading to an unfolded protein response as reflected by the induction of the ER chaperone immunoglobulin heavy chain binding protein (BiP) and proapoptotic C/EBP homologous protein (CHOP) transcription factor. Of note, the monounsaturated palmitoleic and oleic acid can attenuate the palmitic acid-induced upregulation of CHOP, thereby preventing cell death. Similarly, gene silencing of CHOP protects against palmitic acid-induced podocyte apoptosis. Our results offer a rationale for interventional studies aimed at testing whether dietary shifting of the FFA balance toward unsaturated FFAs can delay the progression of DN.
Keywords: diabetic nephropathy, apoptosis, endoplasmic reticulum, stress, palmitic acid
diabetic nephropathy (DN) is the major cause of end-stage renal disease (ESRD) in many industrialized countries, and as the prevalence of type 2 diabetes is much greater, most diabetic patients starting renal replacement therapy today have type 2 diabetes (1, 25). Podocyte injury and loss are critical events in the course of DN (58) and precede albuminuria and renal dysfunction (9, 35, 42, 50, 53). Type 2 diabetes mellitus is characterized by hyperglycemia and dyslipidemia with increased plasma levels of long-chain free fatty acids (FFAs) (43, 55). Saturated FFAs such as palmitic acid are proapoptotic factors in other cell types, including pancreatic β-cells (28, 33, 48) and hepatocytes (29, 57). Monounsaturated FFAs such as palmitoleic or oleic acid are able to prevent/attenuate palmitic acid-induced impaired insulin secretion and increased apoptosis of pancreatic β-cells (27), and diets rich in unsaturated FFAs were shown to lower glucose levels in type 2 diabetic mice (54). It is now widely accepted that a disbalance between saturated and unsaturated FFAs critically contributes to the pathogenesis of type 2 diabetes (37).
Previous studies with cultured human mesangial cells demonstrated that palmitic acid stimulates apoptosis that can be prevented by unsaturated FFAs (36). However, as it stands, the role of FFAs in the pathogenesis of DN and their role in podocyte viability are largely unknown. In cultured podocytes, palmitic acid leads to insulin resistance and blockade of insulin-dependent glucose uptake (21), reflecting profound alterations in podocyte function. In proteinuric kidney disease, FFAs bound to albumin are filtered and reabsorbed by the proximal tubule, which may contribute to tubulointerstitial inflammation and fibrosis (51, 52).
The endoplasmic reticulum (ER) is the organelle where secretory and membrane proteins are folded. Correctly folded proteins exit the ER and are transported to the Golgi and other destinations within the cell, but mis- or unfolded proteins are retained in the ER (44). The accumulation of unfolded proteins constitutes a form of cellular stress that has been termed ER stress. Importantly, the aforementioned toxicity of palmitic acid involves ER stress (15, 18, 33, 56, 57). As a result of ER stress, several signaling pathways, collectively known as the unfolded protein response (UPR), are being activated, thereby maintaining proper ER function. This involves attenuation of translation and transcriptional induction of ER chaperones, whose functions are to increase the folding capacity of the ER and to prevent protein aggregation (17, 26). Under conditions with severe ER stress, however, the protective mechanisms activated by the UPR are not sufficient and a particular branch of the UPR evolves leading to induction of the proapoptotic transcription factor C/EBP homologous protein (CHOP; also known as DDIT3), which can trigger apoptosis (61). The expression of CHOP is mainly regulated at the transcriptional level by several transcription factors, including X-box binding protein-1 (XBP-1) (41). Of note, only the spliced form of XBP-1 has transcriptional activity (60).
The induction of ER stress markers has been described in human kidney biopsies of different glomerulopathies, including membranous nephropathy, focal segmental glomerulosclerosis, and minimal change disease (3, 32). Gene expression analysis of the tubulointerstitial compartment of renal biopsies obtained from patients with DN found a significant upregulation of major genes involved in the UPR, including the ER chaperones BiP (also known as HSPA5 or GRP78) and hypoxia-upregulated 1, but no changes in the expression of proapoptotic CHOP (22).
The objectives of the present study were to investigate the effects of palmitic acid, a saturated FFA, and monounsaturated FFAs such as palmitoleic or oleic acid on podocyte cell death and the involvement of ER stress in this process. In addition, we explored the mechanistic role of the proapoptotic transcription factor CHOP in palmitic acid-induced podocyte cell death by gene silencing of CHOP using a specific short-hairpin (sh) RNA.
MATERIALS AND METHODS
Materials.
Palmitic acid, palmitoleic acid, oleic acid, and essential fatty acid-free BSA were purchased from Sigma, anti-BiP and anti-activated caspase 3 antibodies from Cell Signaling, anti-CHOP from Santa-Cruz Biotechnology, and anti-β-actin from Sigma. The horseradish peroxidase-conjugated secondary antibodies were obtained from Dako and Jackson. The primary antibodies were applied at dilutions of 1:1,000 in blocking solution (5% milk in TBS-Tween) except anti-CHOP (1:200) and anti- β-actin (1:100,000). Alexa 647 annexin V and propidium iodide (PI) were purchased from Invitrogen.
Cell culture.
Podocytes were cultured as described before (39). Briefly, conditionally immortalized mouse podocytes were cultured under permissive conditions (33°C) in RPMI-1640 supplemented with 10% FBS, penicillin/streptomycin (all from Invitrogen), and interferon-γ (Cell Sciences) on type I collagen (BD Biosciences). Differentiation was induced by thermoshift to 37°C without interferon-γ in six-well plates (apoptosis assays) and 10-cm dishes (protein isolation). All experiments were performed with cells that had been allowed to differentiate for at least 11 days.
Fatty acid preparation.
Fatty acids were prepared as described previously (23). In brief, 20 mM solutions of palmitic, palmitoleic, or oleic acids in 0.01 M NaOH (vehicle) were incubated at 70°C for 30 min and complexed to 10% BSA in a molar ratio of 6.6:1, shaken overnight at 37°C under N2-atmosphere, sonicated for 10 min, sterile filtrated, and stored at −20°C. Before use, the complexes were heated at 60°C for 15 min followed by dilution in medium. The fatty acid concentrations were determined with a commercial kit according to the manufacturer's instructions (Wako). The endotoxin concentration was measured using a commercial kit (GenScript), and the final concentration in all experiments was equal or below 0.5 ng/ml. BSA used for control experiments was prepared and handled exactly the same as BSA complexed to FFAs.
Apoptosis assay.
Podocyte apoptosis and necrosis were determined by flow cytometry exactly as recently reported (2). Briefly, podocytes were trypsinized and resuspended in annexin V binding buffer (10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2, pH 7.4). Annexin V staining was applied for 15 min at room temperature (see producer protocol) and before analysis 0.5 μg PI was added. For the assay, 20,000–25,000 cells were analyzed by flow cytometry (Dako).
RT-PCR analysis of XBP-1 mRNA splicing.
Total RNA was extracted with a Nucleospin kit (Macherey-Nagel), and first-strand cDNA was synthesized using oligo(dT) primers (Fermentas). Amplification (initial denaturation at 94°C for 1 min, 30 cycles of PCR at 94°C for 30 s, 58°C for 30 s, and 72°C for 1 min, and a final extension at 72°C for 10 min) was performed with a pair of primers corresponding to nucleotides 392–415 (TGAGAACCAGGAGTTAAGAACACGC) and 720–696 (TTCTGGGTAGACCTCTGGGAGTTCC) of mouse XBP-1 cDNA (AF027963) (40). PCR products were separated by electrophoresis on 2.5% agarose gels and visualized by ethidium bromide staining.
Western blotting.
Podocytes were washed with ice-cold PBS and scraped into 200 μl RIPA lysis buffer (50 mM Tris·HCl, pH 7.5, 200 mM NaCl, 1% Triton, 0.25% deoxycholic acid, 1 mM EDTA, 1 mM EGTA) containing EDTA-free protease inhibitors (Roche) and phosphatase inhibitors (Pierce). To include the floating and detached cells, the culture medium and PBS (the cells were washed with) were collected, centrifuged (530 g), and resuspended in 20 μl lysis buffer. The pooled cells were lysed mechanically and rotated for 1 h at 4°C. To remove nuclei, the samples were spun down (10,000 rpm, 10 min) and the protein concentration of the supernatant was determined by DC protein assay (Bio-Rad). Then, 30–80 μg of protein/well were loaded on 12 or 15% gels and separated by SDS-PAGE. Transfer to nitrocellulose membranes was applied at 100 V in the cold room for 1 h, and the blots were blocked for 2 h with 5% milk powder in TBS-Tween. Primary antibodies were applied overnight, secondary antibodies for 1 h. The immunoblots were detected by enhanced chemiluminescence (Pierce) on Kodak BioMax light films (Sigma).
Lentivirus production and CHOP knockdown.
A CHOP-specific oligonucleotide (GGAAACGAAGAGGAAGAATCA) (11) was cloned into a FUGW vector coexpressing green fluorescent protein under a ubiquitin promotor. A 21-nt scrambled sequence (GACCGCGACTCGCCGTCTGCG) with no murine homology served as a nonsilencing control (2). Lentivirus production was performed as previously reported (13). In brief, HEK293 cells were transfected using Fugene (Roche) with the FUGW vector and two helper plasmids, vesicular stomatitis virus (VSV) G protein and Δ8.9. After 72 h, the supernatant was spun down at 780 g and filtered using a filter with 0.45-μm pore size. Podocytes were transduced by adding virus-containing medium after 5-min pretreatment with 10 μg/ml polybrene (Sigma). All experiments were performed 4 days after viral transduction (2).
Statistical analysis.
Data are expressed as means ± SD unless otherwise indicated. The significance of differences was calculated with a two-sided, unpaired t-test unless otherwise indicated.
RESULTS
Palmitic acid induces podocyte cell death.
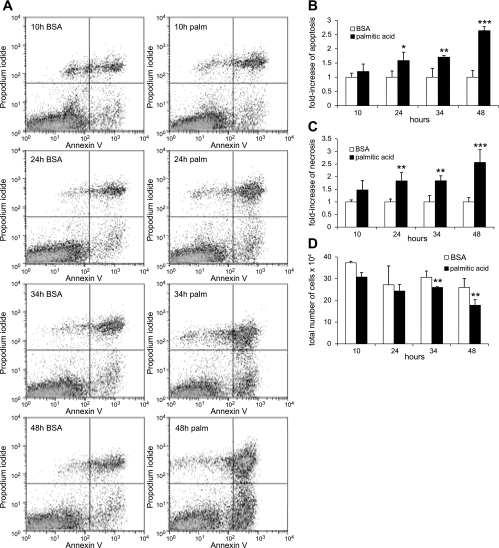
First, we investigated whether palmitic acid may also induce podocyte cell death as reported for other cell types, e.g., pancreatic β-cells (28, 33, 48). Palmitic acid complexed to BSA in concentrations from 125 to 500 μM enhanced cell death of podocytes in a concentration-dependent manner (Fig. 1). Figure 1A shows representative flow cytometry data with the abscissa and ordinate representing the fluorescence intensity of annexin V and PI, respectively. In BSA not complexed to palmitic acid (BSA containing control medium), 5.2 ± 0.1% of podocytes were annexin V-positive/PI-negative cells, representing early apoptotic podocytes, and 5.9 ± 0.9% were annexin V positive/PI positive, representing late apoptotic/necrotic cells (Fig. 1B). Palmitic acid increased apoptosis significantly at 250 (9.1 ± 0.9%, P < 0.01; Fig. 1B) and at 500 μM (14.0 ± 0.2%, P < 0.01; Fig. 1B). We also found a dose-dependent increase in necrotic cells (at 500 μM, 17.3 ± 1.2%, P < 0.01; Fig. 1B). To be able to detect an effect of low palmitic acid concentrations (125 and 250 μM), a relatively long incubation time (38 h) was chosen for the experiments displayed in Fig. 1. Next, we tested the time dependency of palmitic acid-induced podocyte apoptosis by analyzing cell death over time after exposure to 500 μM palmitic acid (Fig. 2A). Palmitic acid induced cell death, both apoptosis and necrosis, in a time-dependent manner, and significant differences became apparent at 24 h (Fig. 2, B and C). After 48 h, a 2.5- to 3-fold increase in apoptotic and necrotic podocytes was observed (P < 0.01; Fig. 2, B and C). Of note, the increase in annexin V/PI double-positive late apoptotic/necrotic cells was underestimated by flow cytometry, as more floating cellular debris were formed in the supernatants of cell pellets used for flow experiments over time. This was reflected in a decreased total number of cells recovered in cell pellets after 34 and 48 h (P < 0.05; Fig. 2D).
Fig. 1.
Palmitic acid induces apoptosis and necrosis of podocytes in a dose-dependent manner. A: representative flow cytometry results for podocytes exposed to increasing concentrations of palmitic acid (125–500 μM) or BSA (at a concentration equivalent to cells treated with 500 μM palmitic acid complexed to BSA) for 38 h. The abscissa and ordinate represent the fluorescence intensity of annexin V Alexa 647 and propidium iodide (PI), respectively. B: quantitative analysis of palmitic acid-induced podocyte cell death. Bar graph represents the mean percentages ± SD of annexin V-positive/PI-negative (early apoptotic) and annexin V-positive/PI-positive (late apoptotic/necrotic) podocytes (n = 3). *P < 0.05, **P < 0.01.
Fig. 2.
Palmitic acid induces time-dependent podocyte apoptosis. A: palmitic acid-induced podocyte cell death was determined by annexin V/PI staining followed by flow cytometry. B: quantitative analysis. Bar graph represents the mean fold-increase ± SD in annexin V-positive/PI-negative (apoptotic) cells incubated with palmitic acid or BSA for indicated time points (n = 3). *P = 0.05, **P < 0.05, ***P < 0.01. C: bar graph representing the mean fold-increase ± SD in annexin V-positive/PI-positive (necrotic) cells incubated with palmitic acid or BSA for indicated time points (n = 3). **P < 0.05, ***P < 0.01. D: bar graphs representing intact cells recovered in cell pellets (= total cells in culture dishes minus floating cellular debris in supernatant after centrifugation at 550 g), which were used for flow experiments shown in A–C. Palmitic acid significantly decreased the number of recovered cells after 34 and 48 h (**P < 0.05), reflecting the increase in necrotic cellular debris that could not be recovered in cell pellets.
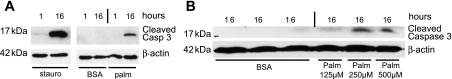
Palmitic acid activates effector caspase 3.
To confirm the effect of palmitic acid on podocyte apoptosis with a second, independent approach, we examined the activation of effector caspase 3 by Western immunoblotting using an antibody specific for cleaved and therefore activated caspase 3 (49) (Fig. 3). As activation of caspase 3 typically occurs before externalization of phosphatidylserine (30) as assessed by annexin V staining in the flow cytometry assay, we incubated podocytes with 500 μM palmitic acid for 1 or 16 h (Fig. 3A). For staurosporine (positive control), a faint band for cleaved caspase 3 was seen as early as after 1 h, whereas for palmitic acid a band for cleaved caspase 3 was seen after 16 h (Fig. 3A). Consistent with the flow cytometry data (Fig. 1), cleaved caspase 3 could be observed at lower concentrations of palmitic acid with a faint band at a concentration of 125 μM and a strong band at 250 and 500 μM (Fig. 3B).
Fig. 3.
Palmitic acid activates caspase 3 in podocytes. Western blot analysis of active, cleaved caspase 3 protein steady-state levels in podocytes exposed to staurosporine (stauro), fatty acid-free BSA (BSA), or palmitic acid (palm) is shown. A: 0.25 μM staurosporine (serving as a positive control) and palmitic acid caused a strong activation of caspase 3 after 16 h. No signal was seen after 1-h exposure to palmitic acid or after treatment with BSA (negative control). β-Actin served as a loading control. Representative results of 3 independent experiments are shown. B: dose-dependent activation of caspase 3 by palmitic acid. For control condition (BSA), the BSA concentration equivalent to cells exposed to 500 μM palmitic acid complexed to BSA was used. β-Actin served as a loading control. Representative results of 2 independent experiments are shown.
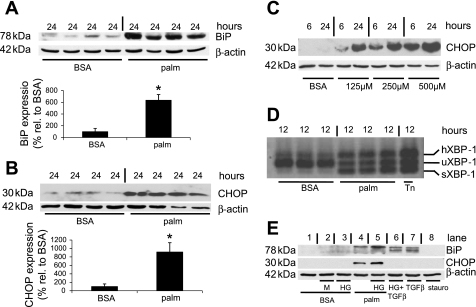
Palmitic acid induces ER stress in podocytes.
The toxicity of palmitic acid has been attributed to the induction of ER stress (15, 18, 33, 56, 57). Therefore, we studied the effect of palmitic acid on protein levels of BiP, an ER chaperone that is upregulated during ER stress (19). We observed a strong upregulation of BiP protein expression after incubation with 500 μM palmitic acid for 16 and 24 h (P < 0.01, Fig. 4A, and Supplemental Fig. S1A; supplemental material for this article is available online at the Journal website). In addition, the proapoptotic transcription factor CHOP, which is typically upregulated during severe ER stress (61), was increased ninefold after 24 h (P < 0.01, Fig. 4B).
Fig. 4.
Palmitic acid induced the endoplasmic reticulum (ER) chaperone immunoglobulin heavy chain binding protein (BiP) and C/EBP homologous protein (CHOP) in podocytes. A: palmitic acid-induced upregulation of BiP (top) and quantitative analysis of BiP levels normalized to β-actin (bottom). The expression level of the control condition (BSA) was set to 100%; n = 4. *P < 0.01. B: palmitic acid induced upregulation of CHOP (top) and quantitative analysis of CHOP levels normalized to β-actin (bottom). The expression level of the control condition (BSA) was set to 100%; n = 4. *P < 0.01. C: time- and dose-dependent upregulation of CHOP protein levels by palmitic acid. For the control condition (BSA), the BSA concentration was equivalent to cells exposed to 500 μM palmitic acid complexed to BSA. β-Actin served as a loading control. Representative results of 3 independent experiments are shown. D: RT-PCR of X-box binding protein-1 (XBP-1) mRNA in podocytes treated with 500 μM palmitic acid for 12 h. Tunicamycin (Tn; 5 ng/ml) was used as a positive control. In the control condition (BSA), a strong band for unspliced (u) XBP-1 is visible, whereas sXBP-1 and hXBP-1 are strongly increased after treatment with palmitic acid or tunicamycin. Representative results of 3 independent experiments. E. BiP and CHOP expression after exposure for 24 h as follows. Lane 1, control medium containing 5 mM glucose and fatty acid free BSA (BSA); lane 2, control medium (BSA) supplemented with additional 17 mM mannitol (M); lane 3, control medium (BSA) supplemented with additional 17 mM glucose [high glucose (HG)]; lane 4, BSA complexed with 500 μM palmitic acid (palm); lane 5, palmitic acid (palm) and HG; lane 6, 5 ng/ml TGF-β with HG; lane 7, 5 ng/ml TGF-β; lane 8, 0.25 μM staurosporine (stauro). Upregulation of CHOP is only seen with palmitic acid, whereas BiP is induced by palmitic acid and TGF-β (faint band also for HG and M). β-Actin served as a loading control. Representative results of 4 independent experiments are shown.
As the effect of palmitic acid on podocyte cell death started to become visible at a concentration of 125 μM and even more at 250 μM (Fig. 1), we tested whether palmitic acid at 125 and 250 μM could also upregulate BiP and CHOP expression. Indeed, already lower concentrations of palmitic acid upregulated CHOP (Fig. 4C) and BiP expression (Supplemental Fig. S1B). Moreover, as the induction of CHOP expression should precede palmitic acid-induced cell death, we explored changes in CHOP expression at an earlier time point (6 h). Taken together, we found a dose- and time-dependent increase in CHOP protein levels as early as after 6 h (Fig. 4C).
XBP-1 is involved in the transcriptional activation of CHOP in ER stress, but only the spliced form of XBP-1 (sXBP-1) has transcriptional activity (60). Therefore, we used RT-PCR to amplify fragments of XBP-1 representing both the unspliced (uXBP-1) and the spliced (sXBP-1) forms of XBP-1 mRNA. Palmitic acid and tunicamycin (Tn), an established inducer of ER stress, strongly induced sXBP-1 and an additional slowly migrating band (Fig. 4D). The slowly migrating band represents a hybrid form of uXBP-1 and sXBP-1 (hXBP-1), which can form during annealing in the last PCR step (47). XBP-1 splicing was increased as early as after 4 h after exposure to palmitic acid (data not shown), implying a potential role for XBP-1 splicing in transcriptional activation of CHOP.
High glucose concentrations (50) and TGF-β at a concentration of 5 ng/ml (62) can also induce podocyte injury and death. Therefore, we tested whether high glucose or TGF-β can induce BiP or CHOP expression. Neither high glucose (22 mM) nor TGF-β induced CHOP (Fig. 4E), but TGF-β (5 ng/ml) alone or in combination with high glucose induced BiP (Fig. 4E). The weak effect of high glucose on BiP expression was similar to the effect of mannitol, which was used to adjust osmolality to the high-glucose condition, and therefore, the difference in osmolality rather than a high glucose concentration may account for this observation. Staurosporine, a strong inducer of podocyte apoptosis (2), did not increase BiP or CHOP expression (Fig. 4E).
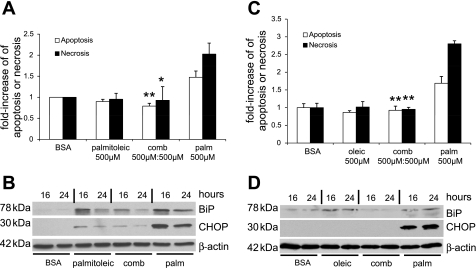
Palmitoleic or oleic acid attenuates palmitic acid-induced UPR and prevents podocyte cell death.
In β cells of the pancreas, certain monounsaturated FFAs can activate antiapoptotic mechanisms (38) and suppress CHOP expression (10), and diets rich in unsaturated FFAs can lower glucose levels in type 2 diabetic mice (54). Therefore, we explored the effect of 500 μM palmitoleic acid (C16:1) or oleic acid (C18:1) on palmitic acid-induced podocyte cell death and CHOP induction. Palmitoleic acid and oleic acid could prevent podocyte apoptosis and necrosis (Fig. 5, A and C). Palmitoleic acid alone or in combination with palmitic acid also induced BiP but strongly attenuated the induction of CHOP (Fig. 5B). Similar results were observed when oleic acid was used instead of palmitoleic acid (Fig. 5D).
Fig. 5.
Palmitoleic or oleic acid prevent palmitic acid-induced podocyte death and attenuate CHOP induction. A: palmitoleic acid blocks palmitic acid-induced podocyte cell death (n = 3, *P < 0.05, **P < 0.01, compared with palmitic acid). B: palmitoleic acid blocks palmitic acid-induced upregulation of BiP and CHOP protein steady-state levels. β-Actin served as a loading control. Representative result of 2 independent experiments is shown. C: oleic acid blocks palmitic acid-induced podocyte cell death (n = 3, *P < 0.01 compared with palmitic acid). D: oleic acid blocks palmitic acid-induced upregulation of BiP and CHOP protein steady-state levels. β-Actin served as a loading control. Representative result of 2 independent experiments is shown.
Gene silencing of CHOP attenuates palmitic acid-induced cell death in podocytes.
CHOP is a proapoptotic transcription factor, and CHOP deletion can protect from ER-stressed induced apoptosis (61). To test whether the marked upregulation of CHOP (Fig. 4, B and C) is mechanistically involved in palmitic acid-induced podocyte cell death, we generated CHOP knockdown podocytes by lentiviral infection using a CHOP-specific shRNA (11). By immunoblotting, we observed a marked suppression of CHOP (Fig. 6B, P < 0.01) and BiP (Fig. 6B, P < 0.05) protein expression in podocytes transduced with CHOP-silencing shRNA after stimulation with tunicamycin, an established inducer of the UPR (Fig. 6, A and B) or palmitic acid (Fig. 6C). The suppression of BiP upregulation (Fig. 6, A–C) was expected as CHOP knockdown leads to lower levels of the UPR as a result of reduced translation, resulting in decreased expression of BiP (31). Functionally, the knockdown of CHOP significantly attenuated the palmitic acid-induced cell death of podocytes (Fig. 6, D and F). These findings are completely consistent with the important role of CHOP in a murine model of DN as well as age-related albuminuria (59). However, the role of CHOP may be more complicated in humans with DN, as CHOP was not upregulated by quantitative RT-PCR in the tubulointerstitial compartment of patients with DN (22). Similarly, we found no upregulation of CHOP mRNA expression in glomerular extracts from eight patients with established DN where CHOP expression was even reduced (Supplemental Fig. S2C).
Fig. 6.
Gene silencing of CHOP protects against palmitic acid-induced podocyte cell death. A: gene-silencing of CHOP suppresses the tunicamycin-induced upregulation of BiP and CHOP protein levels. β-Actin served as a loading control. Representative result of 3 independent experiments are shown. B: quantitative analysis showing a significant reduction of tunicamycin-induced upregulation of CHOP and BiP in cells infected with CHOP short-hairpin (sh) RNA (*P < 0.01, **P < 0.001). C: gene silencing of CHOP suppresses the palmitic acid-induced upregulation of CHOP and BiP protein levels. Representative result of 3 independent experiments is shown. D–F: gene silencing of CHOP protects against palmitic acid-induced apoptosis (D) and overall increase in cell death (F).
DISCUSSION
The present study uncovered that saturated palmitic acid induces podocyte cell death. Our findings are of clinical interest because insulin resistance is associated with increased plasma levels of long-chain FFAs, and DN is characterized by apoptosis and loss of podocytes that precede albuminuria and renal dysfunction in DN, both in type 1 and type 2 diabetes (9, 35, 42, 50, 53). Previous studies found that high glucose (50), TGF-β (62), the renin-angiotensin-aldosterone system (12, 14), and advanced glycation end products (7) can also induce apoptosis of podocytes.
The identification of palmitic acid as another proapoptotic factor for podocytes may be of clinical relevance in the setting of type 2 diabetes because FFAs are elevated in patients with obesity and insulin resistance even before hyperglycemia arises (4). FFAs can also be elevated in type 1 diabetic subjects (34). Moreover, similar to DN, obesity-related glomerulonephropathy is characterized by a decreased density of podocytes (5), raising the intriguing possibility that FFA-induced podocyte apoptosis may also contribute to the development and progression of obesity-related glomerular disease.
The detection of palmitic acid-induced ER stress in podocytes is in line with similar findings in other cell types (15, 18, 33, 56, 57). In pancreatic β cells, the overexpression of the ER-chaperon BiP can reduce palmitic acid-induced apoptosis (20), implying that the upregulation of BiP (Fig. 4A) is part of an adaptive podocyte-protective mechanism. In contrast, the strong upregulation of CHOP (Fig. 4, B and C) makes CHOP a good candidate as a mediator of palmitic acid-induced podocyte apoptosis because CHOP is critically involved in ER stress-induced apoptosis (31, 61). Consistent with the strong induction of CHOP, we also observed palmitic acid-induced XBP-1 splicing (Fig. 4D), which is directly involved in transcriptional regulation of CHOP (41). Together, our data indicate that the palmitic acid-induced upregulation of CHOP in podocytes results, at least in part, from transcriptional activation of CHOP.
Another interesting outcome of the current study was the finding that neither high glucose nor TGF-β at a concentration previously shown to induce podocyte apoptosis (45) caused an upregulation of CHOP (Fig. 4E), suggesting that CHOP is not involved in podocyte apoptosis induced by high glucose or TGF-β.
The antagonistic effects of palmitic acid and monounsaturated palmitoleic acid or oleic acid on ER stress and apoptosis of podocytes (Fig. 5) are in line with studies in other cells (10, 15, 28, 56). The addition of the monounsaturated FFAs to palmitic acid could suppress the induction of CHOP (Fig. 5, B and C), which may explain, at least in part, the prevention of palmitic acid-induced podocyte apoptosis. In addition, we consistently observed an increase in the ER chaperone BiP in podocytes treated with monounsaturated palmitoleic or oleic acid (Fig. 5, B and C). As BiP is known to protect from palmitic acid-induced apoptosis (20), this may explain the protective effect of monounsaturated FFAs.
The gene silencing of CHOP reduces palmitic acid-induced podocyte death (Fig. 6, D–F), thereby establishing a causative role for CHOP in palmitic acid-induced podocyte apoptosis. This outcome is consistent with the known role of CHOP in ER stress-induced apoptosis (31, 61). ER stress has been implicated in podocyte apoptosis caused by advanced glycation end products (6) or excessive protein loading (16), but a causative role of CHOP under these conditions remains to be established.
The notion that CHOP plays a pathogenic role in experimental DN is supported by the observation that CHOP is upregulated in two rodent models of DN (24, 59), and CHOP-deficient mice are protected from DN as well as age-related albuminuria (59). However, in patients with DN, although we found an upregulation of BiP by quantitative RT-PCR analysis in the tubulointerstitial compartment (22) as well as in microdissected glomeruli (Supplemental Fig. S2A), CHOP mRNA expression was unchanged in the tubulointerstitial compartment (22) and downregulated in glomeruli (Supplemental Fig. 2C). Clearly, future studies will be required to address potential differences between our in vitro data, results in murine models, and human DN to determine the precise role of CHOP in patients with DN.
In conclusion, our results unveil the antagonistic effects of palmitic acid vs. monounsaturated FFAs on podocyte survival, ER stress, and the UPR. They support an important role of CHOP in the regulation of podocyte cell death by FFAs. The observed opposing effects of long-chain saturated and unsaturated FFAs on ER stress and podocyte viability provide a rationale for interventional studies that will test whether the progression of DN can be delayed by dietary shifting the FFA balance toward unsaturated FFAs, e.g., by consumption of peanuts and olive oil.
GRANTS
This study was supported by Swiss National Science Foundation Grant 31003A-119974 (A. W. Jehle), a grant from Freie Akademische Gesellschaft Basel (A. W. Jehle), National Institutes of Health Grants DK62472 and DK57683 (P. Mundel), and the Else-Kröner-Fresenius Foundation (C. D. Cohen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Marc Y. Donath, Dr. R. Krapf, and Dr. Alan R. Tall for comments on the manuscript.
We thank all participating centers of the European Renal cDNA Bank-Kroener-Fresenius Biopsy Bank (ERCB-KFB) and patients for cooperation. Active members at the time of the study were as follows: Clemens David Cohen, Holger Schmid, Michael Fischereder, Lutz Weber, Matthias Kretzler, and Detlef Schlöndorff (Munich/Zurich/AnnArbor/New York); Jean Daniel Sraer and Pierre Ronco (Paris); Maria Pia Rastaldi and Giuseppe D'Amico (Milan); Peter Doran and Hugh Brady (Dublin); Detlev Mönks and Christoph Wanner (Würzburg); Andrew Rees (Aberdeen); Frank Strutz and Gerhard Anton Müller (Göttingen); Peter Mertens and Jürgen Floege (Aachen); Norbert Braun and Teut Risler (Tübingen); Loreto Gesualdo and Francesco Paolo Schena (Bari); Jens Gerth and Gunter Wolf (Jena); Rainer Oberbauer and Dontscho Kerjaschki (Vienna); Bernhard Banas and Bernhard Krämer (Regensburg); Moin Saleem (Bristol); Rudolf Wüthrich (Zurich); Walter Samtleben (Munich); Harm Peters and Hans-Hellmut Neumayer (Berlin); Mohamed Daha (Leiden); Katrin Ivens and Bernd Grabensee (Düsseldorf); Francisco Mampaso (Madrid); Jun Oh, Franz Schaefer, Martin Zeier, and Hermann-Joseph Gröne (Heidelberg); Peter Gross (Dresden); Giancarlo Tonolo (Sassari); Vladimir Tesar (Prague); Harald Rupprecht (Bayreuth); Hermann Pavenstädt (Münster); and Hans-Peter Marti (Bern).
REFERENCES
- 1.USRDS The United States Renal Data System. Am J Kidney Dis 42: 1–230, 2003. [PubMed] [Google Scholar]
- 2.Asanuma K, Campbell KN, Kim K, Faul C, Mundel P. Nuclear relocation of the nephrin and CD2AP-binding protein dendrin promotes apoptosis of podocytes. Proc Natl Acad Sci USA 104: 10134–10139, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bek MF, Bayer M, Muller B, Greiber S, Lang D, Schwab A, August C, Springer E, Rohrbach R, Huber TB, Benzing T, Pavenstadt H. Expression and function of C/EBP homology protein (GADD153) in podocytes. Am J Pathol 168: 20–32, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boden G. Fatty acid-induced inflammation and insulin resistance in skeletal muscle and liver. Curr Diab Rep 6: 177–181, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Chen HM, Liu ZH, Zeng CH, Li SJ, Wang QW, Li LS. Podocyte lesions in patients with obesity-related glomerulopathy. Am J Kidney Dis 48: 772–779, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Liu CP, Xu KF, Mao XD, Lu YB, Fang L, Yang JW, Liu C. Effect of taurine-conjugated ursodeoxycholic acid on endoplasmic reticulum stress and apoptosis induced by advanced glycation end products in cultured mouse podocytes. Am J Nephrol 28: 1014–1022, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Chuang PY, Yu Q, Fang W, Uribarri J, He JC. Advanced glycation endproducts induce podocyte apoptosis by activation of the FOXO4 transcription factor. Kidney Int 72: 965–976, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen CD, Frach K, Schlondorff D, Kretzler M. Quantitative gene expression analysis in renal biopsies: a novel protocol for a high-throughput multicenter application. Kidney Int 61: 133–140, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Dalla Vestra M, Masiero A, Roiter AM, Saller A, Crepaldi G, Fioretto P. Is podocyte injury relevant in diabetic nephropathy? Studies in patients with type 2 diabetes. Diabetes 52: 1031–1035, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Diakogiannaki E, Welters HJ, Morgan NG. Differential regulation of the endoplasmic reticulum stress response in pancreatic beta-cells exposed to long-chain saturated and monounsaturated fatty acids. J Endocrinol 197: 553–563, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Di Nardo A, Kramvis I, Cho N, Sadowski A, Meikle L, Kwiatkowski DJ, Sahin M. Tuberous sclerosis complex activity is required to control neuronal stress responses in an mTOR-dependent manner. J Neurosci 29: 5926–5937, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding G, Reddy K, Kapasi AA, Franki N, Gibbons N, Kasinath BS, Singhal PC. Angiotensin II induces apoptosis in rat glomerular epithelial cells. Am J Physiol Renal Physiol 283: F173–F180, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Dittgen T, Nimmerjahn A, Komai S, Licznerski P, Waters J, Margrie TW, Helmchen F, Denk W, Brecht M, Osten P. Lentivirus-based genetic manipulations of cortical neurons and their optical and electrophysiological monitoring in vivo. Proc Natl Acad Sci USA 101: 18206–18211, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durvasula RV, Petermann AT, Hiromura K, Blonski M, Pippin J, Mundel P, Pichler R, Griffin S, Couser WG, Shankland SJ. Activation of a local tissue angiotensin system in podocytes by mechanical strain. Kidney Int 65: 30–39, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Guo W, Wong S, Xie W, Lei T, Luo Z. Palmitate modulates intracellular signaling, induces endoplasmic reticulum stress, and causes apoptosis in mouse 3T3-L1 and rat primary preadipocytes. Am J Physiol Endocrinol Metab 293: E576–E586, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Inagi R, Nangaku M, Onogi H, Ueyama H, Kitao Y, Nakazato K, Ogawa S, Kurokawa K, Couser WG, Miyata T. Involvement of endoplasmic reticulum (ER) stress in podocyte injury induced by excessive protein accumulation. Kidney Int 68: 2639–2650, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest 110: 1389–1398, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kharroubi I, Ladriere L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL. Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology 145: 5087–5096, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature 332: 462–464, 1988 [DOI] [PubMed] [Google Scholar]
- 20.Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 50: 752–763, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Lennon R, Pons D, Sabin MA, Wei C, Shield JP, Coward RJ, Tavare JM, Mathieson PW, Saleem MA, Welsh GI. Saturated fatty acids induce insulin resistance in human podocytes: implications for diabetic nephropathy. Nephrol Dial Transplant 24: 3288–3296, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindenmeyer MT, Rastaldi MP, Ikehata M, Neusser MA, Kretzler M, Cohen CD, Schlondorff D. Proteinuria and hyperglycemia induce endoplasmic reticulum stress. J Am Soc Nephrol 19: 2225–2236, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Listenberger LL, Ory DS, Schaffer JE. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J Biol Chem 276: 14890–14895, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Liu G, Sun Y, Li Z, Song T, Wang H, Zhang Y, Ge Z. Apoptosis induced by endoplasmic reticulum stress involved in diabetic kidney disease. Biochem Biophys Res Commun 370: 651–656, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Locatelli F, Pozzoni P, DelVecchio L. Renal replacement therapy in patients with diabetes and end-stage renal disease. J Am Soc Nephrol 15, Suppl 1: S25–S29, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Ma Y, Hendershot LM. The unfolding tale of the unfolded protein response. Cell 107: 827–830, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes 52: 726–733, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Maedler K, Spinas GA, Dyntar D, Moritz W, Kaiser N, Donath MY. Distinct effects of saturated and monounsaturated fatty acids on beta-cell turnover and function. Diabetes 50: 69–76, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem 281: 12093–12101, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Mandal D, Moitra PK, Saha S, Basu J. Caspase 3 regulates phosphatidylserine externalization and phagocytosis of oxidatively stressed erythrocytes. FEBS Lett 513: 184–188, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 18: 3066–3077, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Markan S, Kohli HS, Joshi K, Minz RW, Sud K, Ahuja M, Anand S, Khullar M. Up regulation of the GRP-78 and GADD-153 and down regulation of Bcl-2 proteins in primary glomerular diseases: a possible involvement of the ER stress pathway in glomerulonephritis. Mol Cell Biochem 324: 131–138, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Martinez SC, Tanabe K, Cras-Meneur C, Abumrad NA, Bernal-Mizrachi E, Permutt MA. Inhibition of Foxo1 protects pancreatic islet beta-cells against fatty acid and endoplasmic reticulum stress-induced apoptosis. Diabetes 57: 846–859, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Mevorach M, Kaplan J, Chang CJ, Rossetti L, Shamoon H. Hormone-independent activation of EGP during hypoglycemia is absent in type 1 diabetes mellitus. Am J Physiol Endocrinol Metab 278: E421–E429, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with type II diabetes and microalbuminuria. Diabetologia 42: 1341–1344, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Mishra R, Simonson MS. Saturated free fatty acids and apoptosis in microvascular mesangial cells: palmitate activates pro-apoptotic signaling involving caspase 9 and mitochondrial release of endonuclease G. Cardiovasc Diabetol 4: 2, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgan NG. Fatty acids and beta-cell toxicity. Curr Opin Clin Nutr Metab Care 12: 117–122, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Morgan NG, Dhayal S, Diakogiannaki E, Welters HJ. The cytoprotective actions of long-chain mono-unsaturated fatty acids in pancreatic beta-cells. Biochem Soc Trans 36: 905–908, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Mundel P, Reiser J, Zuniga Mejia Borja A, Pavenstadt H, Davidson GR, Kriz W, Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Nozaki J, Kubota H, Yoshida H, Naitoh M, Goji J, Yoshinaga T, Mori K, Koizumi A, Nagata K. The endoplasmic reticulum stress response is stimulated through the continuous activation of transcription factors ATF6 and XBP1 in Ins2+/Akita pancreatic beta cells. Genes Cells 9: 261–270, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11: 381–389, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99: 342–348, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1: 785–789, 1963 [DOI] [PubMed] [Google Scholar]
- 44.Rasheva VI, Domingos PM. Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis 14: 996–1007, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Schiffer M, Mundel P, Shaw AS, Bottinger EP. A novel role for the adaptor molecule CD2-associated protein in transforming growth factor-beta-induced apoptosis. J Biol Chem 279: 37004–37012, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Schmid H, Henger A, Cohen CD, Frach K, Grone HJ, Schlondorff D, Kretzler M. Gene expression profiles of podocyte-associated molecules as diagnostic markers in acquired proteinuric diseases. J Am Soc Nephrol 14: 2958–2966, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Shang J, Lehrman MA. Discordance of UPR signaling by ATF6 and Ire1p-XBP1 with levels of target transcripts. Biochem Biophys Res Commun 317: 390–396, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci USA 95: 2498–2502, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slee EA, Adrain C, Martin SJ. Serial killers: ordering caspase activation events in apoptosis. Cell Death Differ 6: 1067–1074, 1999 [DOI] [PubMed] [Google Scholar]
- 50.Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55: 225–233, 2006 [PubMed] [Google Scholar]
- 51.Thomas ME, Harris KP, Walls J, Furness PN, Brunskill NJ. Fatty acids exacerbate tubulointerstitial injury in protein-overload proteinuria. Am J Physiol Renal Physiol 283: F640–F647, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Thomas ME, Schreiner GF. Contribution of proteinuria to progressive renal injury: consequences of tubular uptake of fatty acid bearing albumin. Am J Nephrol 13: 385–398, 1993 [DOI] [PubMed] [Google Scholar]
- 53.Toyoda M, Najafian B, Kim Y, Caramori ML, Mauer M. Podocyte detachment and reduced glomerular capillary endothelial fenestration in human type 1 diabetic nephropathy. Diabetes 56: 2155–2160, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Vassiliou EK, Gonzalez A, Garcia C, Tadros JH, Chakraborty G, Toney JH. Oleic acid and peanut oil high in oleic acid reverse the inhibitory effect of insulin production of the inflammatory cytokine TNF-alpha both in vitro and in vivo systems. Lipids Health Dis 8: 25, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wahba IM, Mak RH. Obesity and obesity-initiated metabolic syndrome: mechanistic links to chronic kidney disease. Clin J Am Soc Nephrol 2: 550–562, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Wei Y, Wang D, Gentile CL, Pagliassotti MJ. Reduced endoplasmic reticulum luminal calcium links saturated fatty acid-mediated endoplasmic reticulum stress and cell death in liver cells. Mol Cell Biochem 331: 31–40, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab 291: E275–E281, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Wolf G, Chen S, Ziyadeh FN. From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes 54: 1626–1634, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Wu J, Zhang R, Torreggiani M, Ting A, Xiong H, Striker GE, Vlassara H, Zheng F. Induction of diabetes in aged C57B6 mice results in severe nephropathy: an association with oxidative stress, endoplasmic reticulum stress, and inflammation. Am J Pathol 176: 2163–2176, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107: 881–891, 2001 [DOI] [PubMed] [Google Scholar]
- 61.Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 12: 982–995, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ziyadeh FN, Hoffman BB, Han DC, Iglesias-De La Cruz MC, Hong SW, Isono M, Chen S, McGowan TA, Sharma K. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci USA 97: 8015–8020, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.