Abstract
Pregnancy is characterized by plasma volume expansion and renal sodium retention with loss of natriuretic response to atrial natriuretic peptide due to increased medullary phosphodiesterase-5 (PDE5). Here, we determined whether natriuretic responses to nitric oxide (NO) are also blunted in pregnancy due to increased PDE5. Anesthetized 16-day pregnant and virgin rats were studied at baseline and during intrarenal infusion of the NO donor spermine NONOate (2.5 nmol/min), the PDE5 inhibitor sildenafil (SILD; 0.5 μg/min), or a combination. The right (noninfused) kidney served as a control. Intrarenal NONOate had no effect on mean arterial pressure (MAP); however, SILD reduced MAP in virgin rats, and the combination of NONOate+SILD reduced MAP in both virgin and pregnant rats. Neither NONOate nor SILD altered glomerular filtration rate. NONOate and SILD each stimulated sodium excretion (UNaV) and fractional excretion of sodium (FENa) in virgin rats, but the combination did not result in an additional natriuretic response. However, NONOate infusion did not increase UNaV or FENa in pregnant rats, but the natriuretic response to NONOate was restored with SILD, and SILD alone produced a natriuresis during pregnancy. Sodium nitroprusside (10−4 mol/l)-stimulated cGMP accumulation from inner medullary collecting duct cells was blunted in cells from pregnant vs. virgin or postpartum rats and was restored by treatment with the PDE5 inhibitor DMPPO (10−7 mol/l). Therefore, increased intrarenal PDE5 mediates the blunted natriuretic response to NO, and loss of responsiveness to the cGMP-dependent, natriuretic agents may contribute to volume expansion during pregnancy.
Keywords: spermine NONOate, sodium nitroprusside, cGMP, inner medulla, sodium retention, sildenafil
during normal pregnancy, women undergo a progressive plasma volume expansion. This increase in circulating volume begins in the first trimester, peaks near gestational week 32, and remains elevated until term to meet the demands of fetal development (12, 24). Failure to expand plasma volume is correlated with complications of pregnancy and intrauterine growth restriction (4, 15). Experimental animal models also exhibit plasma volume expansion during pregnancy, and this is due to net continual renal sodium and fluid retention (1).
Previous findings in our laboratory and from others have indicated that in normal pregnancy the natriuretic response to atrial natriuretic peptide (ANP) and the pressure-natriuresis response are blunted, therefore promoting sodium retention and plasma volume expansion (6, 17, 18, 22, 23). Pressure-induced natriuresis is dependent on the renal tubular actions of nitric oxide (NO, 16). While it is likely that the tubular responsiveness to NO is blunted in pregnancy based on these findings, the direct effect of NO on sodium balance during pregnancy has not been investigated. Therefore, the first goal of this study was to determine whether the natriuretic response to a NO donor is in fact blunted in the pregnant rat.
Both NO and ANP signal through the second messenger cGMP. This signaling pathway is terminated via cGMP specific phosphodiesterases, such as phosphodiesterase-5 (PDE5). We have reported that PDE5 activity and protein abundance in the inner medullary collecting duct (IMCD) are selectively increased in normal pregnancy (21). We hypothesize that an increase in renal PDE5 will degrade cGMP and inhibit the natriuretic response, contributing to the volume expansion of normal pregnancy. In support of this theory, we have demonstrated that selective, intrarenal PDE5 inhibition with sildenafil reverses the blunted response to infused ANP seen in normal pregnancy (14). Furthermore, this appears to be specific to cGMP-mediated natriuresis as cAMP-mediated (i.e., dopamine induced) natriuresis remains intact in pregnant rats (25).
If our hypothesis that the sodium retention of pregnancy is dependent upon increased renal PDE5 activity is correct, then we would also predict that increased PDE5 activity also mediates a loss of tubular responsiveness to NO. The goal of this study was to test the hypothesis that natriuretic responses to NO in pregnancy are blunted due to increased renal PDE5. We proposed that selective inhibition of PDE5 in the kidney of the pregnant rat would promote natriuresis and restore the blunted natriuretic response to a NO donor. We tested this by comparing the natriuretic response to an intrarenal infusion of a specific PDE5 inhibitor (sildenafil) into the left kidney of virgin and pregnant rats in the presence or absence of spermine NONOate, with the right kidney serving as control. We also conducted in vitro studies to measure cGMP accumulation in IMCD cells isolated from virgin, pregnant, and postpartum rats in the presence or absence of the PDE5 inhibitor DMPPO.
METHODS
All experiments were performed using female Sprague-Dawley rats (3–5 mo old; Harlan Laboratories) in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved and monitored by the University of Florida and University of California San Francisco Institutional Animal Care and Use Committees. Rats were housed at a constant temperature and humidity with 12:12-h light-dark cycles and given free access to tap water and standard laboratory chow. Females chosen to become pregnant were housed with males, daily vaginal smears were taken, and the presence of sperm was taken as day 1 of pregnancy and confirmed by the presence of fetuses in utero at the time of acute study.
In vivo renal function measurements in anesthetized rats.
Experiments were conducted in rats at day 16 of pregnancy (n = 24) and in age-matched virgins (n = 28). Rats were anesthetized with 100 μl/100 g body wt of 120 mg/ml inactin (ip, Sigma, St. Louis, MO), placed on a heated table to maintain body temperature at 37 ± 1°C, a tracheotomy was performed, and oxygen was blown across the tracheal tube throughout the experiment. The left femoral vein was cannulated with PE-50 tubing, and an infusion was started at 1 ml/100 g body wt/min of 0.9% NaCl and 2 mg/ml FITC-inulin (Sigma). The left femoral artery was cannulated with PE-50 tubing to monitor mean arterial pressure (MAP) and to collect blood samples. A midline abdominal incision was made, and the left kidney was exposed. In pregnant animals, the uterus was covered with parafilm (Pechiney, WI) and kept warm and moist. Both the left and right ureters were cannulated for the collection of urine with PE-10 tubing. For intrarenal drug infusion, PE-10 tubing was inserted into the left ileolumbar artery, passed up the abdominal aorta, and positioned at the junction of the left renal artery without obstructing renal blood flow. Saline vehicle was infused at a rate of 5.1 μl/min. After a 60-min stabilization period, 2 × 20-min baseline urine collections and midpoint blood samples (250 μl) were taken. Then, the intrarenal infusion was switched to deliver spermine NONOate (Cayman Chemical) into the left kidney at 2.5 nmol/min, sildenafil (Pfizer) at 0.5 μg/min, or a combination of the two, with the right kidney acting as a control. Two further 20-min urine collections and blood samples were taken. The uterus and pups were checked to confirm viability at the end of the experiment, and the intrarenal cannula was flushed with lissamine green dye to confirm the correct positioning of the intrarenal catheter. The rat was killed with an overdose of anesthetic, and the left kidney was removed and weighed. The blood was centrifuged, and plasma was used to measure inulin and sodium concentrations.
To calculate glomerular filtration rate (GFR), the inulin concentration in urine and plasma was measured in a black, clear bottom 96-well plate on a Tecan Safire optical system, with fluorescence read at 485-nm excitation/530-nm emission. Sodium concentrations were measured on a flame photometer using cesium as the internal standard [1:100 dilution of sample in 1.5 mmol/l CsCl solution (Instrumentation Laboratory)].
Nitroprusside-dependent cGMP accumulation in IMCD cells.
IMCD cells from rat kidneys were isolated by collagenase digestion according to the method of Zeidel et al. (31) as performed previously (21, 30). Aliquots of freshly prepared IMCD cells (n = 5) were suspended in 350 μl of buffer containing the following: 124 mmol/l NaCl, 5 mmol/l KCl, 1 mmol/l CaCl2; 0.4 mmol/l MgSO4; 1 mmol/l Na2HPO4, 50 mmol/l HEPES, and 7.5 mmol/l glucose, pH 7.4. Cells were preincubated for 10 min at 37°C in a shaking water bath. In some preparations, the specific PDE5 inhibitor DMPPO (10−7 mol/l) (7) was included. Incubation was started by adding sodium nitroprusside (NaNP; Sigma) at a concentration of 10−8 to 10−4 mol/l and terminated after 10 min by adding 750 μl of ice-cold TCA. The precipitated protein was sedimented by centrifugation, and the pellet was dissolved in 1 N NaOH and assayed for protein concentration by the Bradford dye-binding method (Bio-Rad Laboratories, Hercules, CA). The supernatant fluid was extracted four times with five volumes of water-saturated ethyl ether to remove the TCA before being evaporated to dryness under a stream of air and stored at −80°C until assayed for cGMP content. For the cGMP assay, samples were dissolved in 50 mmol/l sodium acetate buffer, pH 6.2, and 100-μl aliquots were assayed with a commercial kit (Amersham, Piscataway, NJ) after acetylation according to the manufacturer's instructions. Results of duplicate determinations were averaged and expressed as femtomoles accumulated per 10-min incubation per milligram of protein.
Statistical analysis.
Results are presented as means ± SE. Paired and unpaired t-tests with a two-tailed P value were used to assess differences within and between groups, respectively, and one-way and repeated measures ANOVA with the Bonferroni post hoc test were used for multiple comparisons among and within groups. A P value <0.05 was taken to indicate a significant difference.
RESULTS
As shown in Table 1, baseline MAP was similar in virgin and pregnant rats, and infusion of NONOate had no effect on MAP in any group. However, NONOate did lower MAP in the virgin and pregnant rats that also received sildenafil (P < 0.05), and sildenafil alone reduced MAP in the virgin rats. Baseline hematocrit was lower in pregnant than in virgin rats, and neither NONOate nor sildenafil infusion altered hematocrit levels in any group. Baseline values of urine flow and GFR were similar among all groups. NONOate infusion decreased urine flow in the contralateral kidney of pregnant rats, but sildenafil infusion alone increased urine flow in both kidneys of virgin rats. None of the drugs alone or in combination affected GFR in any group.
Table 1.
Measurements of rat groups at baseline and after intrarenal infusion of spermine NONOate, sildenafil, or a combination of spermine NONOate and sildenafil
| UV, μl/min |
GFR, ml/min |
|||||
|---|---|---|---|---|---|---|
| MAP, mmHg | Hct, ml/100 ml | Right | Left | Right | Left | |
| Virgin | ||||||
| Baseline | 98 ± 3 | 47 ± 0.4 | 3.9 ± 0.4 | 4.8 ± 0.9 | 0.9 ± 0.1 | 0.8 ± 0.1 |
| NONOate | 96 ± 3 | 46 ± 0.5 | 3.5 ± 0.5 | 5.1 ± 1.0 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| Baseline | 96 ± 5 | 44 ± 1.1 | 3.9 ± 0.4 | 4.3 ± 0.6 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| SILD | 87 ± 5* | 44 ± 1.2 | 5.5 ± 0.9* | 7.0 ± 1.5* | 0.9 ± 0.1 | 1.1 ± 0.2 |
| Baseline | 96 ± 2 | 46 ± 0.6 | 4.0 ± 1.3 | 5.2 ± 1.5 | 1.2 ± 0.1 | 1.1 ± 0.1 |
| SILD/NONOate | 87 ± 2* | 45 ± 0.6 | 3.4 ± 0.9 | 4.4 ± 0.8 | 1.1 ± 0.2 | 1.0 ± 0.1 |
| Pregnant | ||||||
| Baseline | 93 ± 4 | 40 ± 0.7† | 4.7 ± 0.3 | 5.2 ± 0.4 | 0.8 ± 0.04 | 0.8 ± 0.04 |
| NONOate | 92 ± 5 | 39 ± 0.6† | 3.6 ± 0.5* | 4.1 ± 0.6 | 0.6 ± 0.1 | 0.6 ± 0.1 |
| Baseline | 92 ± 3 | 40 ± 0.5† | 3.4 ± 0.2 | 3.6 ± 0.2 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| SILD | 88 ± 4 | 39 ± 0.5† | 3.3 ± 0.4 | 3.8 ± 0.3 | 0.7 ± 0.1 | 0.8 ± 0.04 |
| Baseline | 92 ± 4 | 41 ± 0.6† | 4.4 ± 0.6 | 3.9 ± 0.7 | 0.9 ± 0.1 | 0.8 ± 0.1 |
| SILD/NONOate | 86 ± 2* | 40 ± 0.4† | 3.7 ± 0.5* | 3.3 ± 0.6 | 0.8 ± 0.1 | 0.8 ± 0.1 |
Values are means ± SE; n = 7–11. MAP, mean arterial pressure; Hct, hemotocrit; V, urine flow rate; GFR, glomerular filtration rate; SILD, sildenafil.
P < 0.05 vs. respective baseline.
P < 0.05 vs. virgin.
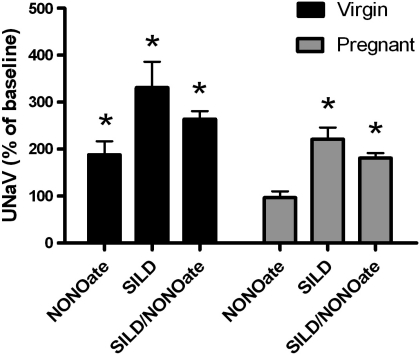
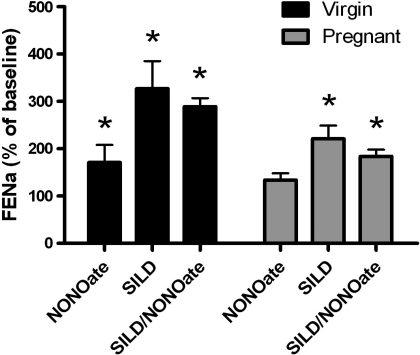
NONOate infusion produced a significant rise in sodium excretion in kidneys from virgin rats (from 0.20 ± 0.05 μeq/min at baseline to 0.38 ± 0.08 μeq/min during NONOate infusion, P = 0.033), and sildenafil infusion had no further effect on the natriuretic response when combined with NONOate (from 0.16 ± 0.06 to 0.30 ± 0.05 μeq/min, P = 0.026, shown as percent increase in Fig. 1). PDE5 inhibition alone via intrarenal sildenafil infusion also increased sodium excretion in virgin rats (from 0.20 ± 0.05 μeq/min at baseline to 0.66 ± 0.18 μeq/min, P = 0.011). At baseline, sodium excretion was lower in pregnant rats (0.044 ± 0.010 μeq/min, P = 0.038), and the natriuretic effect of NONOate was absent in kidneys of the vehicle-infused pregnant rats (0.040 ± 0.006 μeq/min during NONOate infusion). This blunting was reversed in the left kidney of the pregnant rats receiving intrarenal sildenafil in addition to NONOate, and sodium excretion increased from 0.030 ± 0.010 μeq/min at baseline to 0.073 ± 0.023 μeq/min during sildenafil+NONOate infusion (P = 0.026). Sildenafil infusion alone also increased sodium excretion from 0.042 ± 0.007 μeq/min at baseline to 0.088 ± 0.015 μeq/min (P = 0.007) during sildenafil infusion. No significant changes in sodium excretion were observed in the right (noninfused) kidney of any group. Figure 2 presents the increase in fractional sodium excretion in response to NONOate infusion. Fractional sodium excretion was increased in response to either NONOate or sildenafil alone in kidneys from virgin rats, and there was no greater natriuretic effect by intrarenal infusion of the combination of NONOate and sildenafil. NONOate infusion had no effect on fractional sodium excretion in kidneys from pregnant rats, while intrarenal sildenafil infusion increased fractional sodium excretion in pregnant rats when infused alone or in combination with NONOate.
Fig. 1.
Increase in urinary sodium excretion (UNaV) from the left kidney in virgin and pregnant rats after spermine NONOate and/or sildenafil (SILD) infusion. NONOate infusion increased UNaV in virgin but not pregnant rats. The natriuretic response was restored in the pregnant kidney by sildenafil. Sildenafil alone increased UNaV in both groups. *P < 0.05 vs. respective control kidney; n = 7–11.
Fig. 2.
Increase in fractional excretion of sodium (FENa) from the left kidney in virgin and pregnant rats after spermine NONOate and/or SILD infusion. NONOate infusion increased FENa in virgin but not pregnant rats. The natriuretic response was restored in the pregnant kidney by SILD. SILD alone increased FENa in both groups. *P < 0.05 vs. respective control kidney; n = 7–11.
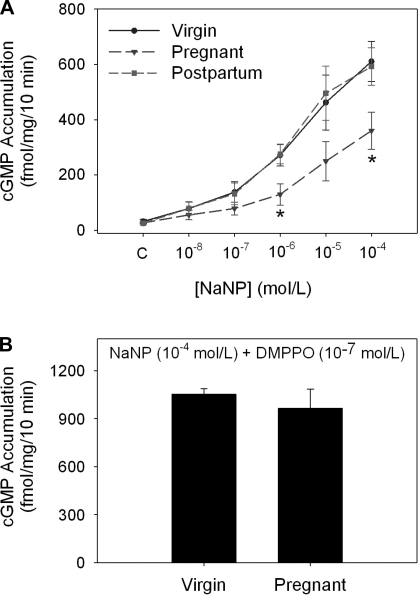
As shown in Fig. 3A, accumulation of cGMP increased in a dose-dependent manner in response to added NaNP in isolated IMCD cells from kidneys from virgin, pregnant, and postpartum rats; however, IMCD cells from kidneys of pregnant rats exhibited blunted cGMP accumulation compared with cells from virgin and postpartum rats. This difference in response was abolished in the presence of the selective and potent PDE5 inhibitor DMPPO (10−7 mol/l, Fig. 3B).
Fig. 3.
Sodium nitroprusside (NaNP)-dependent cGMP accumulation by isolated inner medullary collecting duct (IMCD) cells from kidneys of virgin, pregnant, and postpartum rats. A: NaNP led to a dose-dependent increase in cGMP accumulation in all groups; however, the IMCD cells from pregnant rat kidneys displayed a markedly blunted response at NaNP concentrations of 10−6 and 10−4 mol/l. *P < 0.05 vs. virgin; n = 5. B: phosphodiesterase-5 (PDE5) inhibitor DMPPO (10−7 mol/l) increased NaNP (10−4 mol/l)-stimulated cGMP accumulation in cells from both virgin and pregnant rats and abolished the difference between the groups.
DISCUSSION
The main novel findings of this study are that the natriuretic response to a NO donor is blunted in the pregnant rat and that inhibition of renal PDE5 restores the natriuresis in pregnant rats. Previous work from our laboratory showed that pressure-natriuresis is blunted during pregnancy, suggesting that the renal tubular response to NO is altered (18), and the present study is the first to demonstrate a tubular refractoriness to a NO donor in vivo during pregnancy. In addition, we have previously shown that PDE5 abundance and activity are increased in normal pregnancy and that increased PDE5 activity in pregnant rats inhibits both ANP-induced cGMP accumulation in isolated IMCD cells and the natriuretic response to ANP in vivo. The present study extends that work by demonstrating blunted cGMP accumulation in response to the NO donor NaNP in IMCD cells isolated from pregnant rats. In addition, we report that intrarenal infusion of a PDE5 selective inhibitor promotes sodium excretion in both virgin and pregnant rats even in the absence of an exogenous NO donor. These findings support our overall hypothesis that an increase in renal PDE5 abundance and activity mediates the sodium retention of pregnancy by blunting the natriuretic actions of both NO and ANP and also provide evidence that renal PDE5 regulates sodium excretion in the nonpregnant rat.
Volume homeostasis is maintained in the normal, nonpregnant state via a balance between antinatriuretic (the renin-angiotensin-aldosterone and sympathetic nervous systems) and natriuretic (NO and ANP) systems. However, in normal pregnancy, this balance is disrupted so that antinatriuretic signals predominate in the kidney, and sodium and water retention occurs to allow a physiological volume expansion (1). This antinatriuresis occurs even though there is a significant increase in NO production during pregnancy, at least in the rat (8). We and others have previously shown that pressure-natriuresis, which is dependent on the renal NO system (16), is blunted during late pregnancy in the rat (13, 18); however, the direct effect of NO on sodium excretion during pregnancy has not been determined. The present study demonstrates a direct tubular refractoriness to NO using the NO donors spermine NONOate and NaNP both in vivo and in IMCD cells isolated from pregnant rats, respectively.
Our previous work has demonstrated a tubular refractoriness to ANP in pregnancy (14, 21, 17), cirrhosis (19, 20), and nephrotic syndrome (29, 30). ANP and NO both activate guanylyl cyclase (particulate and soluble isoforms, respectively) to produce the second messenger cGMP, and interference in this pathway would explain the similar blunted response to both agents. PDE5 degrades cGMP specifically, and our previous studies have shown that increased activity of PDE5 blunts ANP natriuresis in pregnancy (14, 21), cirrhosis (19, 20), and nephrotic syndrome (29, 30). In the current study, we demonstrated that the blunted response to NaNP in IMCD cells isolated from pregnant rats was abolished by treatment with the PDE5 inhibitor DMPPO. While DMPPO was initially reported to be a highly selective PDE5 inhibitor (7), it is possible that DMPPO could also inhibit the activity of the more recently discovered PDE isoforms PDE9 or PDE11. However, our in vitro data show marked increases in medullary PDE5 in pregnancy, and we show here that intrarenal infusion of the specific PDE5 inhibitor sildenafil restores the natriuretic response to spermine NONOate in the pregnant rat in vivo. These findings lend support to our overall hypothesis that increased renal PDE5 activity in pregnant rats enables sodium retention in the face of increased natriuretic stimuli. We propose that this increase in PDE5 activity is specific to the renal tubular epithelium since the NO/cGMP pathway is an important mediator of the renal and systemic vasodilation of pregnancy. A generalized increase in PDE5 would prevent this component of the vasodilatory response of pregnancy that is essential to accommodate the plasma volume expansion without leading to a rise in blood pressure. Indeed, we have previously shown that PDE5 protein abundance is increased in the renal inner medulla during pregnancy, with no change in expression observed in the renal cortex (21).
While future studies are needed to investigate the pathways involved in this specific renal tubular upregulation of PDE5, we have shown that this increase in PDE5 is secondary to systemic vasodilation (26). We suggest that a primary vasodilation during pregnancy, due to increased NO production (2–3, 5, 8–9), drives increased inner medullary PDE5 expression by a currently unknown mechanism and facilitates continual renal sodium retention causing “refilling” of the vasculature and a net volume expansion.
In this study, we employed an intrarenal infusion of spermine NONOate and sildenafil to minimize the marked antihypertensive effects of systemic NO and PDE5 inhibition. In pilot studies, the spermine NONOate concentration was tested in virgin female rats to determine a concentration that would elicit a natriuretic response with a minimal effect on MAP. There was minimal spillover into the general circulation, as indicated by the stability of MAP in the NONOate-infused groups. There was, however, a greater decline in MAP seen in both virgin and pregnant rats during the combined intrarenal infusion of NONOate and sildenafil, suggesting a small cumulative effect of systemic NONOate and sildenafil. Of importance, this fall in MAP did not interfere with the natriuretic response to spermine NONOate in the virgin rat, and the natriuretic response to NONOate was restored by sildenafil in the pregnant rat despite the lower blood pressure. Furthermore, the natriuretic effect of intrarenal NONOate infusion is due to its tubular action, as indicated by the rise in the fractional excretion of sodium in the virgin rats and in the sildenafil-treated pregnant rats.
PDE5 inhibition alone increased sodium excretion by the infused kidney in both virgin and pregnant rats, indicating that PDE5 actively regulates the natriuretic effects of endogenous ANP and NO in the kidney. However, PDE5 inhibition had no additive effect on natriuresis in the nonpregnant rat in the presence of exogenous NO. This is consistent with our previous work which showed no additive effect of renal PDE5 inhibition in the presence of exogenous ANP. Other cGMP-specific phosphodiesterases, PDE1 and PDE9, are expressed in the kidney (10, 27), and PDE10 and PDE11, both dual substrate PDEs that act on both cAMP and cGMP, have been found in the kidney (11, 28). Therefore, PDE5 along with other PDEs may play an important role in the regulation of natriuresis and volume homeostasis in the virgin female or male kidney.
In conclusion, this study reinforces our previous work that the natriuretic response is blunted in normal pregnancy and that renal PDE5 is upregulated during pregnancy and plays an important role in blunting the cGMP-dependent natriuretic response. Therefore, PDE5 may play an essential role in the sodium retention and plasma volume expansion required for optimal pregnancy and fetal development. In addition, the current study reveals a role for PDE5 in the regulation of natriuresis in nonpregnant rats as well, highlighting the need for future studies to determine the role of renal PDE5 activity in the regulation of renal tubular sodium transport.
GRANTS
This work was supported by postdoctoral fellowships from the American Heart Association and the University of Florida Multidisciplinary Training Program in Hypertension (T32 HL083810) to J. M. Sasser and a grant from the National Institutes of Health (HD041571) to C. Baylis.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
Portions of this work have appeared in abstract form (J Am Soc Nephrol 20: 2009).
REFERENCES
- 1.Baylis C. Glomerular filtration and volume regulation in gravid animal models. Baillieres Clin Obstet Gynaecol 8: 235–264, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Baylis C, Engels K. Adverse interactions between pregnancy and a new model of systemic hypertension produced by chronic blockade of EDRF in the rat. Clin Exp Hypertens 11: 117–129, 1992 [Google Scholar]
- 3.Cadnapaphornchai MA, Ohara M, Morris KG, Jr, Knotek M, Rogachev B, Ladtkow T, Carter EP, Schrier RW. Chronic NOS inhibition reverses systemic vasodilation and glomerular hyperfiltration in pregnancy. Am J Physiol Renal Physiol 280: F592–F598, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Chesley LC, Lindheimer MD. Renal hemodynamics and intravascular volume in normal and hypertensive pregnancy. In: Hypertension in Pregnancy, edited by Rubin PC. Amsterdam: Elsevier, 1988, vol. 10, p. 38 [Google Scholar]
- 5.Conrad KP, Joffe GM, Kruszyna H, Kruszyna R, Rochelle LG, Smith RP, Chavez JE, Mosher MD. Identification of increased nitric oxide biosynthesis during pregnancy in rats. FASEB J 7: 566–571, 1993 [PubMed] [Google Scholar]
- 6.Corwin E, Castro L, Brizzee B, Solomon S. Atrial natriuretic factor: effects on blood pressure and renal function during pregnancy. Biogenic Amines 9: 271–280, 1993 [Google Scholar]
- 7.Coste H, Grondin P. Characterization of a novel potent and specific inhibitor of type V phosphodiesterase. Biochem Pharmacol 50: 1577–1585, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Danielson LA, Conrad KP. Acute blockade of nitric oxide synthase inhibits renal vasodilation and hyperfiltration during pregnancy in chronically instrumented conscious rats. J Clin Invest 96: 482–490, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng A, Engels K, Baylis C. Increased nitric oxide production plays a critical role in the maternal blood pressure and glomerular hemodynamic adaptations to pregnancy in the rat. Kidney Int 50: 1132–1138, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Dousa TP. Cyclic-3′,5′-nucleotide phosphodiesterase isozymes in cell biology and pathophysiology of the kidney. Kidney Int 55: 29–62, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Fawcett L, Baxendale R, Stacey P, McGrouther C, Harrow I, Soderling S, Hetman J, Beavo JA, Phillips SC. Molecular cloning and characterization of a distinct human phosphodiesterase gene family: PDE11A. Proc Natl Acad Sci USA 97: 3702–3807, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallery EDM, Hunyor SN, Gyory AZ. Plasma volume concentration: a significant factor in both pregnancy-associated hypertension (pre-eclampsia) and chronic hypertension in pregnancy. Q J Med 48: 593–602, 1979 [PubMed] [Google Scholar]
- 13.Khraibi AA. Renal interstitial hydrostatic pressure and pressure natriuresis in pregnant rats. Am J Physiol Renal Physiol 279: F353–F357, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Knight S, Snellen H, Humphreys M, Baylis C. Increased renal phosphodiesterase-5 activity mediates the blunted natriuretic response to ANP in the pregnant rat. Am J Physiol Renal Physiol 292: F655–F659, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindheimer MD, Katz AI. Renal Function and Disease in Pregnancy. Philadelphia, PA: Lea and Febiger, 1977 [Google Scholar]
- 16.Majid DS, Williams A, Navar LG. Inhibition of nitric oxide synthesis attenuates pressure-induced natriuretic responses in anesthetized dogs. Am J Physiol Renal Fluid Electrolyte Physiol 264: F79–F87, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Masilamani S, Castro L, Baylis C. Pregnant rats are refractory to the natriuretic actions of atrial natriuretic peptide. Am J Physiol Regul Integr Comp Physiol 267: R1611–R1616, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Masilamani S, Hobbs GR, Baylis C. The acute pressure natriuresis response is blunted and the blood pressure response reset in the normal pregnant rat. Am J Obstet Gynecol 179: 486–491, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Ni XP, Cheng Y, Cao L, Gardner DG, Humphreys MH. Mechanisms contributing to renal resistance to atrial natriuretic peptide in rats with common bile-duct ligation. J Am Soc Nephrol 7: 2110–2118, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Ni XP, Safai M, Gardner DG, Humphreys MH. Increased cGMP phosphodiesterase activity mediates renal resistance to ANP in rats with bile duct ligation. Kidney Int 59: 1264–1273, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Ni XP, Safai M, Rishi R, Baylis C, Humphreys MH. Increased activity of cGMP-specific phosphodiesterase (PDE5) contributes to resistance to atrial natriuretic peptide natriuresis in the pregnant rat. J Am Soc Nephrol 15: 1254–1260, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olsson K, Hossaini-Hilali J, Eriksson L. Atrial natriuretic peptide attenuates pressor but enhances natriuretic responses to angiotensin II in pregnant conscious goats. Acta Physiol (Scand) 145: 385–394, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Olsson K, Karlberg BE, Eriksson L. Atrial natriuretic peptide in pregnant and lactating goats. Acta Endocrinol (Copenh) 120: 519–525, 1989 [DOI] [PubMed] [Google Scholar]
- 24.Pirani BBK, Campbell DM, MacGillivray I. Plasma volume in normal first pregnancy. J Obstet Gynaecol Br Commonw 80: 884–887, 1973 [DOI] [PubMed] [Google Scholar]
- 25.Sasser JM, Baylis C. The natriuretic and diuretic response to dopamine is maintained during rat pregnancy. Am J Physiol Renal Physiol 294: F1342–F1344, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasser JM, Fekete A, Baylis C. Chronic vasodilation mimics plasma volume expansion (pve) seen in pregnancy—support for the underfill theory. J Am Soc Nephrol 20: 827A, 2009. Accessed online at www.asn-online.org/education_and_meetings/renal_week/archives/RW09Abstracts.pdf Accessed on July 30, 2010 [Google Scholar]
- 27.Soderling SH, Bayuga SJ, Beavo JA. Identification and characterization of a novel family of cyclic nucleotide phosphodiesterases. J Biol Chem 273: 15553–15558, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Soderling SH, Bayuga SJ, Beavo JA. Isolation and characterization of a dual-substrate phosphodiesterase gene family: PDE10A. Proc Natl Acad Sci USA 96: 7071–7076, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valentin JP, Qiu C, Muldowney WP, Ying WZ, Gardner DG, Humphreys MH. Cellular basis for blunted volume expansion natriuresis in experimental nephrotic syndrome. J Clin Invest 90: 1302–1312, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valentin JP, Ying WZ, Sechi LA, Ling KT, Qiu C, Couser WG, Humphreys MH. Phosphodiesterase inhibitors correct resistance to natriuretic peptides in rats with Heymann nephritis. J Am Soc Nephrol 7: 582–593, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Zeidel ML, Silva P, Brenner BM, Seifter JL. cGMP mediates effects of atrial peptides on medullary collecting duct cells. Am J Physiol Renal Fluid Electrolyte Physiol 252: F551–F559, 1987 [DOI] [PubMed] [Google Scholar]