Abstract
Sulfonylurea receptor-containing ATP-sensitive potassium (KATP) channels have been implicated in cardioprotection, but the cell type and constitution of channels responsible for this protection have not been clear. Mice deleted for the first nucleotide binding region of sulfonylurea receptor 2 (SUR2) are referred to as SUR2 null since they lack full-length SUR2 and glibenclamide-responsive KATP channels in cardiac, skeletal, and smooth muscle. As previously reported, SUR2 null mice develop electrocardiographic changes of ST segment elevation that were shown to correlate with coronary artery vasospasm. Here we restored expression of the cardiomyocyte SUR2-KATP channel in SUR2 null mice by generating transgenic mice with ventricular cardiomyocyte-restricted expression of SUR2A. Introduction of the cardiomyocyte SUR2A transgene into the SUR2 null background restored functional cardiac KATP channels. Hearts isolated from rescued mice, referred to as MLC2A, had significantly reduced infarct size (27 ± 3% of area at risk) compared with SUR2 null mice (36 ± 3% of area at risk). Compared with SUR2 null hearts, MLC2A hearts exhibited significantly improved cardiac function during the postischemia reperfusion period primarily because of preservation of low diastolic pressures. Additionally, restoration of cardiac SUR2-KATP channels significantly reduced the degree and frequency of ST segment elevation episodes in MLC2A mice. Therefore, cardioprotective mechanisms both dependent and independent of SUR2-KATP channels contribute to cardiac function.
Keywords: ATP-sensitive potassium channel, SUR2, SUR2A, ischemia, vasospasm
atp-sensitive potassium (KATP) channels respond to changes in the intracellular ADP-to-ATP ratio and link the energy state of the cell to the membrane voltage potential (40). The protein architecture of sulfonylurea receptor (SUR)-KATP channels consists of a relatively mild inward K+ rectifier pore-forming subunit (Kir6.1 or Kir6.2) coupled to a regulatory SUR subunit (SUR1 or SUR2) (21, 25, 40, 54). Full-length SUR is a multitransmembrane protein with two nucleotide binding folds. Alternative splicing within the regions encoding the nucleotide binding folds and at the carboxy terminus of SUR2 potentially produces a diversity of channels (4, 16, 22, 44, 45, 63). The Abcc9 gene encodes SUR2, and alternative splicing of the 3′ terminal exons of Abcc9 results in SUR2A and SUR2B (22). SUR2A and SUR2B are found at the plasma membrane.
Within the heart, SUR-KATP channels are also expressed in multiple cell types. In cardiomyocytes, SUR-KATP channels play a critical role in several vital cellular processes important for stress response such as ischemic preconditioning, where a brief period of ischemia is cardioprotective for subsequent periods of prolonged ischemia (38, 58). Broad pharmacological blockade of KATP channels has been shown to attenuate preconditioning. However, the targets of these KATP agents likely include the sarcolemma-associated channels and mitochondria-associated channels, and there are potentially off-target effects (17, 30, 46, 55). Several hypotheses have been proposed to explain the role of both sarcolemmal and mitochondrial KATP channels in cardioprotection. During stress, sarcolemmal KATP channel activation shortens the action potential duration, which conserves ATP by decreasing myofilament contraction (66). The opening of mitochondrial KATP channels during ischemia is thought to prevent mitochondrially mediated necrosis including the release of apoptotic signals and further decreases in ATP levels (17, 55).
In addition to cardiomyocytes, SUR-KATP channels are also expressed in multiple cell types such as endothelial and vascular smooth muscle cells (6, 23, 37, 65). SUR2B is mainly present in endothelial and vascular smooth muscle cells and results in KATP channels relatively insensitive to ATP and less sensitive to the sulfonylurea agents than SUR2A-KATP channels (53, 64, 65). In the coronary vasculature, the vasodilation effect of SUR-KATP channel activation during both hypoxia and hyperemia is thought to be mediated by both vascular smooth muscle and endothelial cells (7, 8, 10–12). Activation of smooth muscle SUR-KATP channels results in a decreased membrane potential (49) and activation of endothelial SUR-KATP channels increases NO release (60), both of which lead to vasculature relaxation.
To gain insight into how cardiomyocyte SUR2-KATP channels affect cardiac function, we previously characterized mice with a deletion of exons 14 to 18 in the Abcc9 gene encoding SUR2 (SUR2 null) (5, 6). These mice lack full-length sarcolemmal SUR2-KATP channels, including SUR2A and SUR2B, and have no sulfonylurea-sensitive KATP channels in cardiomyocytes, vascular smooth muscle, and skeletal myofibers (5, 6, 23). SUR2 null mice develop repetitive and episodic ST segment elevations on conscious telemetric electrocardiographic monitoring (5). ST segment elevation, a marker of myocardial injury, was also evident in mice lacking the partner protein of SUR2, Kir6.1 (34). Indeed, these investigators found that stimulation with ergonovine elicited ST segment elevation. Together, the findings from the SUR2- and Kir6.1-null mice supported a role for the SUR2-KATP channel in the regulation of vascular tone and served as models of Prinzmetal variant angina. Electrocardiographic findings of ST elevation were coincident with angiographic evidence of coronary artery vasospasm and abnormal coronary artery perfusion pressure (5, 23). Surprisingly, restoration of vascular smooth muscle SUR2 expression with the use of a transgenic approach to express SUR2 under the control of a vascular smooth muscle promoter did not rescue coronary artery vasospasm, suggesting that vasospasm resulted from dysfunction in multiple cell types (23).
Cardioprotection relies on many different pathways (18, 20). SUR2 null mice have increased cardioprotection at baseline against both ischemia and adrenergic stress (56, 63). In the cardiomyocyte, SUR2 partners with Kir6.2 to form the major sarcolemmal SUR2-KATP channel in the ventricle. Increased cardioprotection in SUR2 null hearts was an unexpected finding given that mice lacking Kir6.2 have an impaired adaptation to stress (67). It was recently shown that a smaller protein, SUR2–55kDa, derives from the Abcc9 locus, and that this smaller protein associates with the mitochondria in cardiomyocytes (63). The 55-kDa SUR2 protein is generated from an intraexonic splicing event linking exon 6 to exon 31 and missing the intervening sequences. The SUR2 null mouse was generated by deleting exons 14–18. Therefore, the splicing event that produces SUR2–55kDa remains intact, where it is poised to contribute to cardioprotection.
In the present study, we tested the effect of restoring full-length, plasma membrane-associated SUR2 only in the cardiomyocyte in SUR2 null mice. Cardiomyocyte SUR2A was restored by transgenically expressing SUR2A, as a complete, single-exon cDNA, under the control of the myosin light chain 2v (MLC-2v) promoter. Mice bearing this transgene were then bred into SUR2 null mice to generate SUR2 null mice carrying the MLC-2v SUR2A transgene (MLC2A mice). Hearts isolated from MLC2A mice exhibited significantly increased resistance to ischemia compared with SUR2 null mice, as well as significantly improved cardiac function during reperfusion. In addition, restoration of cardiomyocyte KATP channels significantly reduced ST segment elevation events on monitoring. Together, these data demonstrate that cardiomyocyte SUR2-KATP channels contribute to coronary function and suggest that full-length, plasma membrane-associated SUR2-KATP channels contribute to cardioprotection.
METHODS
Generation of transgene construct and transgenic mice.
To generate the MLC-2v SUR2A transgene construct, SUR2A (Abcc9), full-length SUR2B was amplified from mouse cDNA and inserted into pCR2.1 Topo vector (Invitrogen, Carlsbad, CA). The full-length SUR2A was then placed under the control of the MLC-2v promoter (gift of Ju Chen, University of California San Diego). The 5.2-kb transgene construct was linearized and purified with a XhoI restriction digest. Fertilized oocytes from C57/C3H × B6 breeding pairs were isolated and injected with transgene DNA. Resulting pups were screened by PCR, and two founders were identified. Transgenic founders were bred with SUR2 null mice for two consecutive generations to generate SUR2 null mice expressing the MLC-2v SUR2A transgene. SUR2 null mice were previously generated by targeted disruption of exons 14–18 encoding nucleotide binding fold 1 as described previously (6). SUR2 null mice with restoration of cardiomyocyte SUR2A (MLC2A) are on a mixed background (75% FVB, 12.5% C57/C3H, 12.5% B6). Interbreeding of heterozygous littermates resulted in the male and female mice (10–20 wk of age) used for all experiments, with nontransgenic littermates used for control. Protocols were reviewed and approved by the University of Chicago's Institutional Animal Care and Use Committee (IACUC), and animals were housed, treated, and handled in accordance with guidelines set forth by the IACUC, Animal Welfare Act regulations, and the NIH Guide for the Care and Use of Laboratory Animals.
Immunofluorescence microscopy.
An anti-SUR2 antibody [transmembrane domain 1 (TMD1)] that only binds to full-length SUR2 was raised in rabbits against the peptide sequence YEEQKKKAADHPNRTPSIWL localized in the first transmembrane domain and purified as described previously (23). Cryosections were made from hearts frozen in liquid nitrogen-cooled isopentane. The TMD1 antibody was used at 1:2,000 in blocking buffer. Goat anti-rabbit secondary antibody conjugated to Cy3 (Jackson ImmunoResearch Laboratories, West Grove, PA) was used at 1:3,000 in blocking buffer. VectaShield with DAPI (Vector Laboratories, Burlingame, CA) was used to view nuclei and to mount slides. Fluorescent images were collected with the Axioskop, AxioCam, and AxioVision microscope, camera, and software systems (Carl Zeiss, Oberkochen, Germany). Heart sections from all cohorts were stained and processed at the same time and imaged with identical exposure times and gains. Immunoblotting was performed with anti-Kir6.1 and anti-Kir6.2 antibodies (Santa Cruz Biotechnology, catalog nos. 11225 and 11227, respectively).
Isolation of mouse ventricular myocytes.
Adult mice (>3 mo of age) were killed via cervical dislocation in accordance with our IACUC. The heart was quickly excised, and the aorta was cannulated and perfused at 37°C with oxygenated (5% CO2-95% O2) Krebs-Henseleit bicarbonate (KHB) buffer containing (in mM) 118 NaCl, 4.7 KCl, 1.25 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 10 HEPES, and 11.1 glucose on a Langendorff apparatus. Hearts were then perfused with Ca2+-free KHB buffer containing Liberase Blendzyme 4 (0.2 mg/ml; Hoffmann-La Roche) for 5–7 min. The ventricles were removed in Ca2+-free KHB buffer. Extracellular Ca2+ was added incrementally back to 1.25 mM. Cell viability ranged from 60% to 80% after the isolation protocol; viability was assessed by appearance, including cell lucency and cell shape.
Single-channel KATP recordings.
Inside-out patch-clamp recording was performed at room temperature (22–24°C) as previously described (44) with an Axopath 200A amplifier and pCLAMP10 software (Axon Instruments Molecular Devices, Union City, CA). The electrodes were pulled (P-97; Sutter Instrument) from borosilicate glass with a resistance of 2–3 MΩ when filled with recording solutions. The bath solution contained (in mM) 140 KCl, 5 HEPES, 2 EGTA, 0.2 MgCl2, and 5.5 glucose (pH 7.3 with KOH). The pipette solution contained (in mM) 10 KCl, 130 NaCl, 5 HEPES, 1 CaCl2, 0.2 MgCl2, and 5.5 glucose (pH 7.4 set with NaOH). To acquire the inside-out patch clamp configuration, a seal of >2 GΩ was attained on isolated cardiomyocytes and the membrane patch was withdrawn from the cell. Single channels were recorded at a holding potential of 0 mV under constant perfusion, and channel activity was probed in the presence of 1 mM ATP.
ECG telemetry and data collection.
Continuous ambulatory ECG recordings were obtained from MLC-2v-SUR2A transgenic SUR2 null mice (MLC2A) and SUR2 null mice with the use of PhysioTel Implants (model TA10EA-F20, Data Sciences International, St. Paul, MN) as described previously (23). ECG data were analyzed with customized software coded in C++. ECG segments with a low signal-to-noise ratio were uninterpretable and excluded by only analyzing QRS complexes with an amplitude >0.3 μV and imposing a minimal interbeat interval or “eye closing period” of 60 ms. Less than 5% of each ECG recording was disregarded. Regions of ST elevation were defined as segments where the T-wave amplitude exceeded 2 standard deviations of the mean T-wave amplitude. Each detected ST elevation segment was manually verified, and false positives were removed. ST elevation is used as an indicator of vasospasm clinically in patients and in animal models (5, 34, 59). Since our previous ECG characterization of the SUR2 null mice demonstrated that ST elevation correlated with vasospasm, ST elevations in MLC2A mice were evaluated as a indirect reflection of vascular spasm (5, 23).
Langendorff experiments.
Mice were heparinized (0.5 U/g body mass ip) 30 min before surgical explant and then anesthetized with inhaled 3% isoflurane. Hearts were rapidly excised and dissected. The left atrial appendage and part of the left atrium were removed to allow placement of a fluid-filled balloon into the left ventricle. The aorta was then cannulated with a 22-gauge cannula (Fine Science Tools, Foster City, CA) placed immediately distal to the intact aortic valve and secured with 5-0 silk surgical suture. Hearts were perfused at constant flow (∼3–3.5 ml/min) with Krebs-Henseleit solution (in mM: 120 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 15 glucose, 25 NaHCO3, 1.75 CaCl2, 0.05 EDTA) equilibrated with 95% O2 and 5% CO2 at 37°C with a standard Langendorff setup (Radnoti Glass Technology, Monrovia, CA) as described previously (56). Hearts were paced at 400 beats/min via epicardial pacing leads. Left ventricular pressures were measured with a pressure-sensing catheter (model SPR-839, Millar Instruments, Houston, TX) connected to the inflated fluid-filled balloon. Diastolic pressure was set at ∼5 mmHg during baseline stabilization. Hearts were maintained at 37°C with a water-jacketed tissue-organ bath for the duration of the experiment. Baseline cardiac function was recorded for 30 min, followed by 40 min of no-flow global ischemia and 60-min reperfusion. Cardiac function data were collected throughout the duration of the experiment and analyzed with Chart5 software (ADInstruments, Colorado Springs, CO). On completion of reperfusion, hearts were snap-frozen in liquid nitrogen and stored at −80°C for later infarct size analysis.
Infarct size was determined by sectioning each heart into six to eight equivalent sections from apex to base. Each section was then stained with 1% 2,3,5-triphenyltetrazolium chloride (TTC, Sigma-Aldrich, catalog no. T8877) in PBS for 20 min at 37°C. Sections were fixed in 10% buffered formalin overnight at 4°C and then weighed and photographed for infarct size calculations (3, 36, 47, 51). TTC stains the viable tissue red due to dehydrogenases, and nonviable while infarcted tissue remains a pale white. Photographs were blinded, and the areas of viable tissue (red) and nonviable infarcted tissue (pale white) were determined with ImageJ. The percent infarct for each slice was defined as the area of the nonviable infarcted area divided by the total area.Total infarct size was then calculated as (P1 × W1)+(P2 × W2)+...+(Pn × Wn) where P is the percent infarct and W is the weight of each respective section. Atrial tissue was excluded from infarct size analysis.
Statistical analysis.
Data are reported as means ± SE, and statistical analysis was conducted with GraphPad Prism version 4.0 for Macintosh (GraphPad Software, San Diego CA; www.graphpad.com). Student t-tests (2 experimental groups), one-way ANOVA in conjunction with Tukey post hoc test (x > 2 experimental groups, 1 variable), or two-way ANOVA in conjunction with Tukey post hoc test (x > 2 experimental groups, 2 variables) were used where appropriate. Significance was set at a level of P = 0.05.
RESULTS
Restoration of cardiomyocyte SUR2A expression in SUR2 null mouse.
SUR2 null mice lack the full-length SUR2 protein, develop repetitive ST segment elevation and anatomic evidence of vascular spasm (5), and display protection against myocardial infarction (56). We refer to these mice as null for expression of the SUR2 protein, referring specifically to the full-length protein that conveys glibenclamide-sensitive KATP activity. At least two smaller mRNAs are produced from the Abcc9 locus, and these smaller forms are still expressed in the SUR2 null mouse (44, 63). These smaller forms encode shorter proteins that couple with Kir subunits but do not glibenclamide-sensitive KATP channels (44, 63), and SUR2–55kDa has been found in mitochondrial fractions. To evaluate the contribution of cardiomyocyte SUR2-KATP channels to coronary vasospasm and cardioprotection, we restored cardiomyocyte full-length SUR2A cDNA in SUR2 null mice, using a transgene under the control of the cardiac-specific MLC-2v promoter (19, 28, 29). This approach does not alter the smaller forms since they are produced from splicing events that cannot occur from the full-length single-exon cDNA used in transgene construction. MLC-2v SUR2A transgenic mice were bred to SUR2 null mice, and the resulting mice were termed MLC2A, indicating that the MLC-2v SUR2A transgene was present in the SUR2 null background. Transgenic mice are viable and generate viable offspring. No adverse effect on weight or mortality due to the MLC-2v SUR2A transgene was noted.
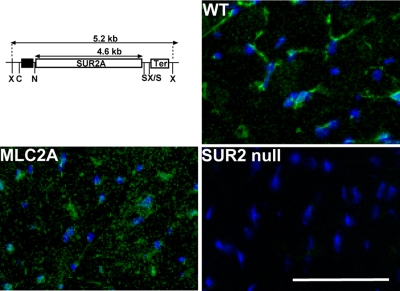
Immunofluorescence microscopy with a full-length SUR2 specific antibody raised against TMD1 was used to evaluate SUR2 expression in cardiomyocytes. Hearts from wild-type (WT), SUR2 null, and MLC2A transgenic mice were immunostained with the TMD1 antibody directed against SUR2 protein. Cardiac tissue sections from WT mice exhibited SUR2 expression primarily along the sarcolemma, with light, diffuse staining of the cardiomyocyte cytoplasm (Fig. 1). MLC2A hearts exhibited SUR2 immunoreactivity of the sarcolemma as well as diffuse staining of the cardiomyocytes. No SUR2 immunoreactivity was detected by immunostaining in SUR2 null hearts. Kir6.X subunits were not altered in their expression (Supplemental Fig. S1).1
Fig. 1.
Restoration of cardiomyocyte sulfonylurea receptor 2 (SUR2)A protein in the SUR2 null heart. Top left: schematic map of MLC-2v SUR2A transgene construct. The 270-bp MLC-2v promoter (black box) is immediately proximal to the MLC2 gene and drives cardiomyocyte-specific expression. The SUR2A variant of SUR2 contains exon 39 and is the primary SUR2 splice variant expressed in heart and skeletal muscle. “Ter” represents the bovine growth hormone terminator. Restriction enzyme sites are indicated: X, XhoI; N, NotI; C, SacII; S, SalI. MLC2A transgene-bearing mice were bred to SUR2 null mice to restore cardiomyocyte SUR2-containing ATP-sensitive potassium (KATP) channels. Immunofluorescence microscopy of heart sections from wild-type (WT) control (top right), MLC2A (bottom left), and SUR2 null (bottom right) mice is also shown. All sections were stained with anti-SUR2 antibodies to visualize SUR2 (green) and DAPI (blue) to visualize nuclei. SUR2 staining was evident in control and MLC2A hearts but not in hearts from SUR2 null mice. Scale bars = 50 μm in all micrographs.
Restoration of cardiomyocyte KATP channel activity.
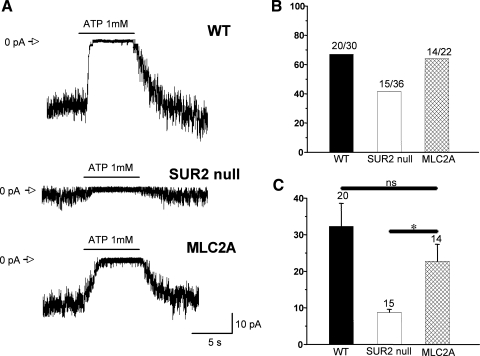
To verify that the restored full-length SUR2-KATP channels observed in immunofluorescence staining were functional, cardiomyocytes were isolated from hearts and single-channel KATP current (IKATP) traces from WT, SUR2 null, and MLC2A ventricular cardiomyocytes were recorded by inside-out patches. The presence of IKATP was determined by application of ATP on the cytoplasmic side, which is a known inhibitor for cardiomyocyte IKATP (Fig. 2A) (39, 44). IKATP was demonstrated in a comparable percentage of WT, SUR2 null, and MLC2A cardiomyocyte patches (Fig. 2B). The IKATP amplitudes recorded from the WT and MLC2A cardiomyocytes were similar and significantly higher than those from the SUR2 null cardiomyocytes (Fig. 2C). These data demonstrate the restoration of functional SUR2-KATP channels in the SUR2 null cardiomyocytes.
Fig. 2.
Restoration of cardiomyocyte KATP channel activity in MLC2A mice. Inside-out patches of KATP currents were determined from cardiomyocytes isolated from hearts of each genotype. A: representative examples of multichannel excised patch recordings of KATP currents at a holding potential of 0 mV. Open channel currents are represented as downward deflections. The closed level is marked as 0 pA. KATP currents were measured in ventricular myocytes isolated from litter-matched control, SUR2 null, and MLC2A mice, with records taken before, during, and after 1 mM ATP administration as shown by the bars. B: % of patches that contained at least some KATP currents in ventricular myocytes isolated from 4 control, 3 SUR2 null, and 3 MLC2A mice. The actual number of patches with current/total number of patches is shown above each bar. C: summary of mean amplitude of KATP currents for those patches containing currents in the control, SUR2 null, and MLC2A cells. *P < 0.001 vs. SUR2 null. ns, Not significant.
MLC2A hearts exhibit enhanced cardioprotection over SUR2 null hearts.
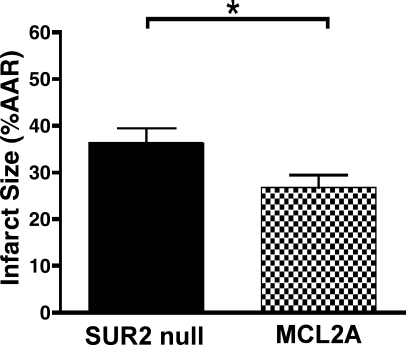
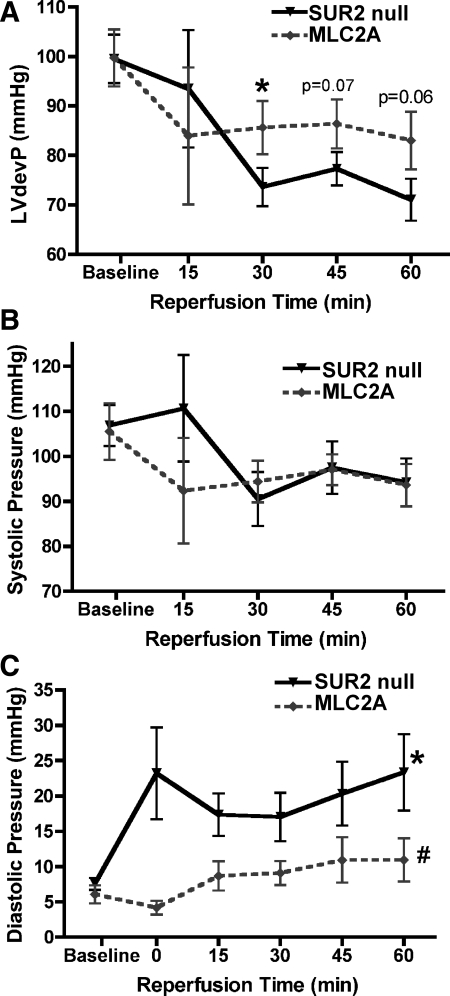
To examine how restoration of cardiac specific full-length SUR2 influenced cardioprotection, isolated hearts from MLC2A and SUR2 null mice were subjected to 40 min of “no-flow” ischemia followed by 60 min of reperfusion. Hearts were sectioned and stained with TTC dye for measurement of infarct size. Hearts from MLC2A mice (n = 7) exhibited significantly reduced infarct size [27 ± 3% of area at risk (AAR)] compared with SUR2 null mice (n = 13, 36 ± 3% AAR, P < 0.05) (Fig. 3). We measured left ventricular pressure with a pressure-sensing catheter connected to a fluid-filled balloon during the ischemia-reperfusion protocol. At baseline, left ventricular developed pressure (LVdevP) was similar between MLC2A (n = 5, 100 ± 6 mmHg) and SUR2 null isolated hearts (n = 10, 100 ± 5 mmHg) (Fig. 4A). After ischemia, isolated hearts from MLC2A mice exhibited consistently higher and more stable LVdevP than those from SUR2 null mice. After 30 min of reperfusion, LVdevP from MLC2A hearts (n = 5, 86 ± 5 mmHg) was significantly greater than that from SUR2 null hearts (n = 10, 74 ± 4 mmHg, P < 0.05) (Fig. 4A).
Fig. 3.
Cardiomyocyte expression of SUR2A in MLC2A mice results in smaller infarct sizes. Isolated hearts from SUR2 null (n = 13) and MLC2A (n = 7) mice were exposed to 40 min of “no-flow” ischemia via a standard Langendorff setup. After 60 min of reperfusion, hearts were sectioned and stained with 1% 2,3,5-triphenyltetrazolium chloride (TTC) to visualize infarct size. Restoration of cardiomyocyte SUR2A expression resulted in increased resistance to ischemia [area at risk (AAR)]. *P < 0.05.
Fig. 4.
MLC2A mice exhibit improved cardiac function after ischemia compared with SUR2 null mice. Left ventricular developed pressure (LVdevP) was measured with a pressure-sensing catheter attached to a fluid-filled balloon placed inside the left ventricle. Baseline parameters were similar for all cohorts. A: isolated hearts from MLC2A mice (n = 5) exhibited increased LVdevP after ischemia compared with hearts from SUR2 null mice (n = 10). *P < 0.05. B: systolic pressure was similar between cohorts. C: diastolic pressure was significantly reduced in isolated hearts from MLC2A mice compared with SUR2 null and MLC2A Line 8 isolated hearts. *P < 0.01 vs. SUR2 null baseline LVdevP; #P < 0.001 vs. SUR2 null.
The observed difference in LVdevP between MLC2A and SUR2 null mice is due to differences in diastolic pressure (Fig. 4, B and C). Left ventricular systolic pressure from isolated hearts was similar throughout the baseline and reperfusion periods (Fig. 4B). However, left ventricular diastolic pressure was significantly higher in isolated hearts from SUR2 null mice compared with hearts from MLC2A mice (P < 0.001 by 2-way ANOVA, Fig. 4C). In MLC2A hearts, diastolic pressure remained more stable and exhibited a slight, insignificant increase throughout the reperfusion period (6 ± 1 and 11 ± 1 mmHg at baseline and 60 min of reperfusion, respectively; P = 0.09). This improvement may result from reduced infarction or attenuated stunning. In contrast, isolated hearts from SUR2 null mice exhibited a significant increase in diastolic pressure from 8 ± 1 mmHg at baseline to 23 ± 5 mmHg after 60 min (P < 0.01).
MLC2A mice exhibit coronary vasospasm.
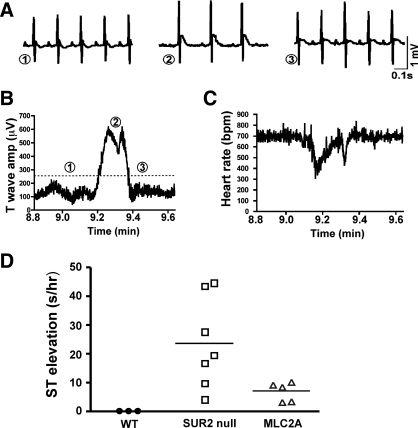
SUR2 null mice exhibit coronary vasospasm at baseline (5). The effect of restoration of cardiomyocyte SUR2A on coronary vasospasm in SUR2 null mice was studied by ambulatory ECG recording. We recorded 48 h of telemetric data and screened this for incidence of ST segment elevation, an indicator of acute myocardial injury. SUR2 null (n = 9) mice exhibited 23.0 ± 5.7 s of ST segment elevation per hour of ECG data (Fig. 5). MLC2A mice (n = 5) exhibited significantly less coronary vasospasm than SUR2 null mice (7.0 ± 1.5 s of ST segment elevation per hour of ECG data; P < 0.05). The average episode durations of each episode of coronary vasospasm in SUR2 null (n = 9) and MLC2A (n = 5) mice were similar (12 ± 1.5 vs. 9 ± 2.6 s per episode, respectively; data not shown). However, MLC2A mice (n = 5) had significantly fewer episodes of vasospasm in the recording period [19 ± 1 vs. 28 ± 3 episodes in SUR2 null mice (n = 9), P < 0.05; data not shown]. These data indicated that vasospasm in SUR2 null mice arises, in part, from defects in cardiomyocyte sulfonylurea-KATP channel, underscoring a paracrine mechanism between the cardiomyocytes and their surrounding vasculature.
Fig. 5.
ST segment elevation episodes are reduced in frequency in MLC2A compared with SUR2 null mice. Conscious telemetric ECG recording was conducted on SUR2 null (n = 9) and MLC2A (n = 5) cohorts for 48-h intervals. A: examples of ST segment elevation are shown from an episode lasting ∼1 min. A baseline tracing just before an ST segment episode is shown in 1. This was followed by an ST segment episode shown in 2. Recovery following the ST segment elevation is shown in 3. B: T-wave amplitude was plotted as an indicator of ST segment elevation over the time frame that includes the data shown in A, where 1, 2, and 3 correlate with those same time points in A. The T-wave amplitude is increased at point 2, reflecting ST segment elevation. C: heart rate [in beats/min (bpm)] was plotted for the same time window as in A and B, showing that heart rate declines during ST segment elevation, as we previously noted (5). D: we used an algorithm to measure T-wave amplitude and found that that MLC2A rescued mice had significantly less ST segment elevation than SUR2 null mice, reflecting a reduction in frequency and duration of episodes. The observed reduction of coronary vasospasm in MLC2A mice was significant (P < 0.05).
DISCUSSION
Role of full-length SUR2-KATP channels in mouse heart.
Pharmacological studies using potassium channel openers such as pinacidil and nicorandil have suggested that SUR2-KATP channels are important targets for cardioprotection and response to stress. The absence of full-length SUR2-KATP channels leads to hypertension and coronary artery vascular spasm (5). However, despite hypertension and ST segment elevation, these SUR2 null mice are protected from infarct and develop smaller infarcts compared with control mice (56). This result indicates that some component of cardioprotection is independent of the full-length SUR2-KATP channel since these channels are absent in this model, and the recent finding that smaller proteins produced from this gene remain intact could explain this finding (63). Here we reintroduced SUR2A in the SUR2 null background and found that this cardiac-specific rescue of SUR2A-KATP channels improved ischemic protection beyond the basal cardioprotection of the SUR2 null mice. Comparing our present results with our previous study, SUR2 null and MLC2A mice were both significantly protected against ischemia compared with control. In our prior study (56), control FVB mice studied with an identical protocol had an infarct size of 54 ± 4%. In comparison, SUR2 null mice had an infarct size of 36 ± 3% and MLC2A mice had a further reduction in infarct size of 27 ± 3% (Fig. 3). These data demonstrate that full-length SUR2-KATP channels provide an additional mode of cardioprotection over and above what occurs in the absence of full-length SUR2-KATP channels. A limitation of our present study is that it addresses infarct size after only 60 min of reperfusion. Longer periods of reperfusion may allow for additional recovery or may uncover molecular deficits that the full-length SUR2 cannot correct. Damage to the myocardium was not completely abrogated after global ischemia in the MLC2A mice. Restoration with the shorter forms encoded by the Abbc9 locus may lead to further improvement in function and reduction in infarct size.
The SUR2 null model used in each of these studies eliminates only the full-length glibenclamide-sensitive channel. The shorter mRNAs that encode KATP channels insensitive to sulfonylurea remain present in the SUR2 null model (44, 63). The smaller Abcc9-encoded proteins generate sulfonylurea-insensitive KATP channels, and we hypothesize that it is these channels that contribute to the baseline cardioprotection in the SUR2 null mouse. The SUR2–55kDa form is found in mitochondria and can couple with Kir6.X subunits (63). The role of mitochondrial KATP channels is well established in cardioprotection, where a number of agonists that target these channels convey cardioprotection (for reviews see Refs. 1, 25). During reperfusion, there is an increase in reactive oxygen species including hydroxyl radicals that induce cardiac dysfunction. Activation of mitochondrial KATP channels is thought to protect against reperfusion-induced injury in multiple states including heart failure (31). Blocking mitochondrial complex II with atpenin, which also activates the mitochondrial KATP channel, conveys cardioprotection (61). Full-length SUR2 expressed in the MLC2A myocardium may exert its effect via a direct or indirect effect on the mitochondrial KATP channel. There are subsarcolemmal mitochondria that can share protein components with the plasma membrane. Recently it was shown that connexin 43 directly modulates mitochondrial KATP function (48). Interestingly, ischemic preconditioning does not further protect SUR2 null hearts from ischemia, suggesting that they may be maximally protected and further supporting the concept that sulfonylurea-sensitive and -insensitive KATP channels have distinct roles (56, 63).
Our present study reinforces a role for the full-length SUR2 protein and the sulfonylurea-sensitive KATP channel in cardioprotection. Kir6.2-null mice lack cardiomyocyte sulfonylurea-sensitive and -insensitive KATP channels and are impaired in their ability to respond to ischemic preconditioning or cannot adequately respond to cardiac stress due to volume overload, increased afterload, or sympathetic stimulation (24, 25, 34, 57, 62, 67). One potential KATP channel-dependent mechanism for protection may be improved calcium handling by cardiomyocytes. During ischemia, energy stores become depleted and intracellular calcium levels become elevated because of impaired calcium handling (35). As a result, cardiomyocytes remain partially contracted even during diastole. SUR2 null hearts exhibited significantly increased diastolic pressure after ischemia, whereas hearts from MLC2A mice maintained near-baseline diastolic pressures after ischemia. Consistent with a role for KATP channels in calcium handling, hearts from Kir6.2-null mice accumulated intracellular calcium and developed contracture more quickly than control hearts (15). Contracture was evident by increasing diastolic pressure during the reperfusion period. In addition, cardiomyocytes from Kir6.2-null mice displayed elevated intracellular calcium levels primarily during diastole following cardiac stress induced by isoproterenol, a β-adrenergic agonist (67). The defects in diastole and calcium handling in the KATP mutants suggests that the cardioprotective effects of KATP channels may be related to maintenance of calcium homeostasis, most likely due to the KATP channel's role in the regulation of the cell's energy state during stress. These features may work to reduce infarct size or attenuate stunning. These data must all be taken in the context of the mouse heart and its high heart rate and concomitant energy demands and sensors. The relative important of sarcolemmal versus mitochondrial channels may differ between larger and smaller mammalian hearts. In a porcine model glibenclamide was not shown to change infarct size, but it did abolish the effects of preconditioning (52).
Two different transgenic mouse models with overexpression of SUR2A have previously been generated that suggested a contribution to cardiac function (9, 14). Du and colleagues (9) generated transgenic mice with overexpressed SUR2A under the control of a nonspecific cytomegalovirus (CMV) promoter and found increased KATP channel density and cardioprotection. In contrast, transgenic mice with cardiomyocyte-specific overexpression of SUR2A in WT background had reduced channel density (14). The experimental data from our MLC2A transgenic mice align with the protective effects observed in the CMV-SUR2A mice, but with an important caveat. The MLC2A mice exhibit cardiomyocyte SUR2A overexpression in a SUR2 null background, a background that is associated with increased cardioprotection at baseline.
ST segment elevation and cardiomyocyte SUR2-based KATP channels.
SUR2 null mice have a curious phenotype of ST segment elevation associated with coronary vasospasm (5). ST elevation occurs in the clinical setting of myocardial infarction, where it is a sign of myocardial injury. In Prinzmetal variant angina, ST segment elevation occurs repetitively in the setting of coronary artery vascular spasm, rather than acute infarction from a flow-limiting atherosclerotic lesion. Recurrent ST segment elevation in the SUR2 null model correlates with coronary vascular spasm (5) and is identical to what is seen in mice lacking Kir6.1, the partner protein of SUR2 in vascular smooth muscle (34). Since the etiology of ST segment elevation with spontaneous vasospasm is not well understood, determining the role of KATP channels in this process is challenging and most likely involves cross talk between multiple cell types (27). Dysfunction in both vascular smooth muscle and endothelial cells has been discussed as a prominent cause of vasospasm. A candidate molecule for triggering vascular spasm is acetylcholine. Clinically, the presence of a paradoxical acetylcholine response is used to diagnose Prinzmetal angina (41). In normal vasculature, acetylcholine results in vascular smooth muscle relaxation. However, endothelial dysfunction can result in a failure of acetylcholine to induce nitric oxide release. Acetylcholine can also activate KATP channels in cardiomyocytes and endothelial cells (42, 60). In addition to acetylcholine, an increase in extracellular K+ concentration is known to regulate vascular function in multiple tissues (10, 13, 26, 50) and may also be important for coronary artery vasculature. We previously (23) rescued transgenic expression of vascular smooth muscle SUR2B using the SM22α promoter and found that despite production of functional SUR2-KATP channels in vascular smooth muscle, the coronary artery vascular spasm was not attenuated. Therefore, our result that restoration of cardiomyocyte SUR2-KATP channels attenuates vasospasm in the SUR2 null mouse was not expected and suggests that cardiomyocytes play a previously unidentified pivotal role in vasospasm function. This finding necessitates a cross talk between the cardiomyocytes and the embedded, neighboring vasculature.
Although the mechanism by which cardiomyocyte SUR2-KATP channels regulate vascular function has not been identified, the interdependence of vasculature and cardiomyocyte has been well documented (2). Interaction between tissue-specific subsets of KATP channels has been previously demonstrated in glucose homeostasis (32, 33, 43). In addition to acetylcholine, several factors are known to signal between cardiomyocytes and the vasculature that regulate or are regulated by KATP channels and ischemia, including endothelin-1, angiotensin II, and adenosine (12). We hypothesize that the increased cardioprotection in the MLC2A cardiomyocytes may alter the signaling milieu and affect the nearby vessels. Together these data support a paracrine mechanism between the cardiomyocyte and surrounding vasculature.
Episodes of ST segment elevation in the SUR2 null mice are short-lived, lasting seconds, and tend to occur clustered in time. When multiple episodes of ST segment elevation occur in sequence, this is associated with bradycardia and death (5). However, many episodes of ST segment elevation are of insufficient magnitude and frequency to produce bradycardia and death. This raises the likely possibility that ST segment elevation episodes may produce short bouts of ischemia that induce cardioprotection. However, if this were the only mechanism of cardioprotection, then the reduction of ST segment elevation in the MLC2A model would be expected to reverse cardioprotection. Thus, while there may be SUR2-KATP-independent cardioprotective mechanisms, the mechanism mediated by KATP channel expression is also sufficient to protect against ischemia.
GRANTS
Supported by National Heart, Lung, and Blood Institute Grants R01-HL-078926 (E. M. McNally), R01-HL-57414 (J. C. Makielski) and F32-HL-097587 (J. P. Fahrenbach). N.-Q. Shi is supported by The American Heart Association National Center (0630268N).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
Footnotes
Supplemental Material for this article is available online at the Journal website.
REFERENCES
- 1.Ardehali H, O'Rourke B. Mitochondrial KATP channels in cell survival and death. J Mol Cell Cardiol 39: 7–16, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brutsaert DL. Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev 83: 59–115, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Budas GR, Disatnik MH, Chen CH, Mochly-Rosen D. Activation of aldehyde dehydrogenase 2 (ALDH2) confers cardioprotection in protein kinase C epsilon (PKCepsilon) knockout mice. J Mol Cell Cardiol 48: 757–764, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chutkow WA, Makielski JC, Nelson DJ, Burant CF, Fan Z. Alternative splicing of sur2 exon 17 regulates nucleotide sensitivity of the ATP-sensitive potassium channel. J Biol Chem 274: 13656–13665, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Chutkow WA, Pu J, Wheeler MT, Wada T, Makielski JC, Burant CF, McNally EM. Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 KATP channels. J Clin Invest 110: 203–208, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chutkow WA, Samuel V, Hansen PA, Pu J, Valdivia CR, Makielski JC, Burant CF. Disruption of Sur2-containing KATP channels enhances insulin-stimulated glucose uptake in skeletal muscle. Proc Natl Acad Sci USA 98: 11760–11764, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daut J, Maier-Rudolph W, von Beckerath N, Mehrke G, Gunther K, Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science 247: 1341–1344, 1990 [DOI] [PubMed] [Google Scholar]
- 8.Dick GM, Bratz IN, Borbouse L, Payne GA, Dincer UD, Knudson JD, Rogers PA, Tune JD. Voltage-dependent K+ channels regulate the duration of reactive hyperemia in the canine coronary circulation. Am J Physiol Heart Circ Physiol 294: H2371–H2381, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Du Q, Jovanovic S, Clelland A, Sukhodub A, Budas G, Phelan K, Murray-Tait V, Malone L, Jovanovic A. Overexpression of SUR2A generates a cardiac phenotype resistant to ischemia. FASEB J 20: 1131–1141, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev 88: 1009–1086, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Duncker DJ, Van Zon NS, Altman JD, Pavek TJ, Bache RJ. Role of K+ATP channels in coronary vasodilation during exercise. Circulation 88: 1245–1253, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Duncker DJ, van Zon NS, Pavek TJ, Herrlinger SK, Bache RJ. Endogenous adenosine mediates coronary vasodilation during exercise after KATP+ channel blockade. J Clin Invest 95: 285–295, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci 9: 1397–1403, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Flagg TP, Remedi MS, Masia R, Gomes J, McLerie M, Lopatin AN, Nichols CG. Transgenic overexpression of SUR1 in the heart suppresses sarcolemmal KATP. J Mol Cell Cardiol 39: 647–656, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Gumina RJ, O'Cochlain DF, Kurtz CE, Bast P, Pucar D, Mishra P, Miki T, Seino S, Macura S, Terzic A. KATP channel knockout worsens myocardial calcium stress load in vivo and impairs recovery in stunned heart. Am J Physiol Heart Circ Physiol 292: H1706–H1713, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Hambrock A, Preisig-Muller R, Russ U, Piehl A, Hanley PJ, Ray J, Daut J, Quast U, Derst C. Four novel splice variants of sulfonylurea receptor 1. Am J Physiol Cell Physiol 283: C587–C598, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Hanley PJ, Daut J. KATP channels and preconditioning: a re-examination of the role of mitochondrial KATP channels and an overview of alternative mechanisms. J Mol Cell Cardiol 39: 17–50, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Heinzel FR, Luo Y, Li X, Boengler K, Buechert A, Garcia-Dorado D, Di Lisa F, Schulz R, Heusch G. Impairment of diazoxide-induced formation of reactive oxygen species and loss of cardioprotection in connexin 43 deficient mice. Circ Res 97: 583–586, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Henderson SA, Spencer M, Sen A, Kumar C, Siddiqui MA, Chien KR. Structure, organization, and expression of the rat cardiac myosin light chain-2 gene. Identification of a 250-base pair fragment which confers cardiac-specific expression. J Biol Chem 264: 18142–18148, 1989 [PubMed] [Google Scholar]
- 20.Heusch G, Boengler K, Schulz R. Cardioprotection: nitric oxide, protein kinases, mitochondria. Circulation 118: 1915–1919, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Inagaki N, Gonoi T, Seino S. Subunit stoichiometry of the pancreatic beta-cell ATP-sensitive K+ channel. FEBS Lett 409: 232–236, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Isomoto S, Kondo C, Yamada M, Matsumoto S, Higashiguchi O, Horio Y, Matsuzawa Y, Kurachi Y. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. J Biol Chem 271: 24321–24324, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Kakkar R, Ye B, Stoller DA, Smelley M, Shi NQ, Galles K, Hadhazy M, Makielski JC, McNally EM. Spontaneous coronary vasospasm in KATP mutant mice arises from a smooth muscle-extrinsic process. Circ Res 98: 682–689, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Kane GC, Behfar A, Dyer RB, O'Cochlain DF, Liu XK, Hodgson DM, Reyes S, Miki T, Seino S, Terzic A. KCNJ11 gene knockout of the Kir6.2 KATP channel causes maladaptive remodeling and heart failure in hypertension. Hum Mol Genet 15: 2285–2297, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Kane GC, Liu XK, Yamada S, Olson TM, Terzic A. Cardiac KATP channels in health and disease. J Mol Cell Cardiol 38: 937–943, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko EA, Han J, Jung ID, Park WS. Physiological roles of K+ channels in vascular smooth muscle cells. J Smooth Muscle Res 44: 65–81, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Konidala S, Gutterman DD. Coronary vasospasm and the regulation of coronary blood flow. Prog Cardiovasc Dis 46: 349–373, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Lee KJ, Hickey R, Zhu H, Chien KR. Positive regulatory elements (HF-1a and HF-1b) and a novel negative regulatory element (HF-3) mediate ventricular muscle-specific expression of myosin light-chain 2-luciferase fusion genes in transgenic mice. Mol Cell Biol 14: 1220–1229, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee KJ, Ross RS, Rockman HA, Harris AN, O'Brien TX, van Bilsen M, Shubeita HE, Kandolf R, Brem G, Price J. Myosin light chain-2 luciferase transgenic mice reveal distinct regulatory programs for cardiac and skeletal muscle-specific expression of a single contractile protein gene. J Biol Chem 267: 15875–15885, 1992 [PubMed] [Google Scholar]
- 30.Loubani M, Fowler A, Standen NB, Galinanes M. The effect of gliclazide and glibenclamide on preconditioning of the human myocardium. Eur J Pharmacol 515: 142–149, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Maack C, Dabew ER, Hohl M, Schafers HJ, Bohm M. Endogenous activation of mitochondrial KATP channels protects human failing myocardium from hydroxyl radical-induced stunning. Circ Res 105: 811–817, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y, Horiuchi M, Ashcroft F, Minokoshi Y, Roeper J, Seino S. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci 4: 507–512, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, Tamamoto A, Gonoi T, Iwanaga T, Miyazaki J, Seino S. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci USA 95: 10402–10406, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, Koseki H, Iwanaga T, Nakaya H, Seino S. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med 8: 466–472, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Miklos Z, Ivanics T, Roemen TH, van der Vusse GJ, Dezsi L, Szekeres M, Kemecsei P, Toth A, Kollai M, Ligeti L. Time related changes in calcium handling in the isolated ischemic and reperfused rat heart. Mol Cell Biochem 250: 115–124, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Milano G, Corno AF, Samaja M, Morel S, Vassalli G, von Segesser LK. Daily reoxygenation decreases myocardial injury and improves post-ischaemic recovery after chronic hypoxia. Eur J Cardiothorac Surg 37: 942–949, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Morrissey A, Rosner E, Lanning J, Parachuru L, Dhar Chowdhury P, Han S, Lopez G, Tong X, Yoshida H, Nakamura TY, Artman M, Giblin JP, Tinker A, Coetzee WA. Immunolocalization of KATP channel subunits in mouse and rat cardiac myocytes and the coronary vasculature. BMC Physiol 5: 1, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev 88: 581–609, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nichols CG, Lederer WJ, Cannell MB. ATP dependence of KATP channel kinetics in isolated membrane patches from rat ventricle. Biophys J 60: 1164–1177, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature 305: 147–148, 1983 [DOI] [PubMed] [Google Scholar]
- 41.Okumura K, Yasue H, Horio Y, Takaoka K, Matsuyama K, Kugiyama K, Fujii H, Morikami Y. Multivessel coronary spasm in patients with variant angina: a study with intracoronary injection of acetylcholine. Circulation 77: 535–542, 1988 [DOI] [PubMed] [Google Scholar]
- 42.Oldenburg O, Critz SD, Cohen MV, Downey JM. Acetylcholine-induced production of reactive oxygen species in adult rabbit ventricular myocytes is dependent on phosphatidylinositol 3- and Src-kinase activation and mitochondrial KATP channel opening. J Mol Cell Cardiol 35: 653–660, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic KATP channels control hepatic glucose production. Nature 434: 1026–1031, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Pu JL, Ye B, Kroboth SL, McNally EM, Makielski JC, Shi NQ. Cardiac sulfonylurea receptor short form-based channels confer a glibenclamide-insensitive KATP activity. J Mol Cell Cardiol 44: 188–200, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quesada I, Rovira JM, Martin F, Roche E, Nadal A, Soria B. Nuclear KATP channels trigger nuclear Ca2+ transients that modulate nuclear function. Proc Natl Acad Sci USA 99: 9544–9549, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rainbow RD, Norman RI, Hudman D, Davies NW, Standen NB. Reduced effectiveness of HMR 1098 in blocking cardiac sarcolemmal KATP channels during metabolic stress. J Mol Cell Cardiol 39: 637–646, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Riess ML, Rhodes SS, Stowe DF, Aldakkak M, Camara AK. Comparison of cumulative planimetry versus manual dissection to assess experimental infarct size in isolated hearts. J Pharmacol Toxicol Methods 60: 275–280, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rottlaender D, Boengler K, Wolny M, Michels G, Endres-Becker J, Motloch LJ, Schwaiger A, Buechert A, Schulz R, Heusch G, Hoppe UC. Connexin 43 acts as a cytoprotective mediator of signal transduction by stimulating mitochondrial KATP channels in mouse cardiomyocytes. J Clin Invest 120: 1441–1453, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Russ U, Metzger F, Kickenweiz E, Hambrock A, Krippeit-Drews P, Quast U. Binding and effects of KATP channel openers in the vascular smooth muscle cell line, A10. Br J Pharmacol 122: 1119–1126, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saito T, Sato T, Miki T, Seino S, Nakaya H. Role of ATP-sensitive K+ channels in electrophysiological alterations during myocardial ischemia: a study using Kir6.2-null mice. Am J Physiol Heart Circ Physiol 288: H352–H357, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Salas MA, Valverde CA, Sanchez G, Said M, Rodriguez JS, Portiansky EL, Kaetzel MA, Dedman JR, Donoso P, Kranias EG, Mattiazzi A. The signalling pathway of CaMKII-mediated apoptosis and necrosis in the ischemia/reperfusion injury. J Mol Cell Cardiol 48: 1298–1306, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schulz R, Rose J, Heusch G. Involvement of activation of ATP-dependent potassium channels in ischemic preconditioning in swine. Am J Physiol Heart Circ Physiol 267: H1341–H1352, 1994 [DOI] [PubMed] [Google Scholar]
- 53.Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol 81: 133–176, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Shi NQ, Ye B, Makielski JC. Function and distribution of the SUR isoforms and splice variants. J Mol Cell Cardiol 39: 51–60, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Standen NB. Cardioprotection by preconditioning: KATP channels, metabolism, or both? J Physiol 542: 666, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stoller D, Kakkar R, Smelley M, Chalupsky K, Earley JU, Shi NQ, Makielski JC, McNally EM. Mice lacking sulfonylurea receptor 2 (SUR2) ATP-sensitive potassium channels are resistant to acute cardiovascular stress. J Mol Cell Cardiol 43: 445–454, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Seino S, Marban E, Nakaya H. Role of sarcolemmal KATP channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest 109: 509–516, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Valen G. Cellular signalling mechanisms in adaptation to ischemia-induced myocardial damage. Ann Med 35: 300–307, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Van Spall HG, Overgaard CB, Abramson BL. Coronary vasospasm: a case report and review of the literature. Can J Cardiol 21: 953–957, 2005 [PubMed] [Google Scholar]
- 60.Wang H, Long C, Duan Z, Shi C, Jia G, Zhang Y. A new ATP-sensitive potassium channel opener protects endothelial function in cultured aortic endothelial cells. Cardiovasc Res 73: 497–503, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Wojtovich AP, Brookes PS. The complex II inhibitor atpenin A5 protects against cardiac ischemia-reperfusion injury via activation of mitochondrial KATP channels. Basic Res Cardiol 104: 121–129, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamada S, Kane GC, Behfar A, Liu XK, Dyer RB, Faustino RS, Miki T, Seino S, Terzic A. Protection conferred by myocardial ATP-sensitive K+ channels in pressure overload-induced congestive heart failure revealed in KCNJ11 Kir6.2-null mutant. J Physiol 577: 1053–1065, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ye B, Kroboth SL, Pu JL, Sims JJ, Aggarwal NT, McNally EM, Makielski JC, Shi NQ. Molecular identification and functional characterization of a mitochondrial sulfonylurea receptor 2 splice variant generated by intraexonic splicing. Circ Res 105: 1083–1093, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yokoshiki H, Sunagawa M, Seki T, Sperelakis N. ATP-sensitive K+ channels in pancreatic, cardiac, and vascular smooth muscle cells. Am J Physiol Cell Physiol 274: C25–C37, 1998 [DOI] [PubMed] [Google Scholar]
- 65.Yoshida H, Feig JE, Morrissey A, Ghiu IA, Artman M, Coetzee WA. KATP channels of primary human coronary artery endothelial cells consist of a heteromultimeric complex of Kir6.1, Kir6.2, and SUR2B subunits. J Mol Cell Cardiol 37: 857–869, 2004 [DOI] [PubMed] [Google Scholar]
- 66.Zhang H, Flagg TP, Nichols CG. Cardiac sarcolemmal KATP channels: latest twists in a questing tale! J Mol Cell Cardiol 48: 71–75, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, Pucar D, Bienengraeber M, Dzeja PP, Miki T, Seino S, Alekseev AE, Terzic A. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci USA 99: 13278–13283, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.