Abstract
Strong evidence that premotor interneurons provide ventral spinocerebellar tract (VSCT) neurons with feedback information on their actions on motoneurons was previously found for Ia inhibitory interneurons and Renshaw cells, while indications for similar actions of other premotor interneurons were weaker and indirect. Therefore the aim of the present study was to reexamine this possibility with respect to interneurons relaying actions of group Ib afferents from tendon organs and group II afferents from muscle spindles. In all, 133 VSCT neurons in the L3–L5 segments (including 41 spinal border neurons) were recorded from intracellularly in deeply anesthetized cats to verify that stimuli applied in motor nuclei evoked monosynaptic inhibitory postsynaptic potentials (IPSPs) attributable to stimulation of axon collaterals of premotor interneurons. IPSPs were found in over two thirds of the investigated neurons. When intraspinal stimuli were preceded by stimuli applied to a muscle nerve at critical intervals, IPSPs evoked from motor nuclei were considerably reduced, indicating a collision of nerve volleys in axons of interneurons activated by group I and group II afferents. In individual VSCT neurons monosynaptic IPSPs were evoked from both biceps–semitendinosus and gastrocnemius–soleus motor nuclei, in parallel with disynaptic IPSPs from group Ib and group II as well as group Ia afferents. These observations indicate that individual VSCT neurons may monitor the degree of inhibition of both flexor and extensor motoneurons by premotor interneurons in inhibitory pathways from group Ib and group II afferents to motoneurons. They may thus be providing the cerebellum with feedback information on actions of these premotor interneurons on motoneurons.
INTRODUCTION
Ventral spinocerebellar tract (VSCT) neurons forward highly diversified information to the cerebellum, as individual VSCT neurons are excited and/or inhibited by different combinations of peripheral afferents and spinal interneurons (Eccles et al. 1961; Lundberg and Weight 1971). However, the most common action of muscle afferents on these neurons is inhibition. In fact in a subpopulation of VSCT neurons the inhibition is not associated with any excitatory input from peripheral afferents and it should therefore play a very special role for motor control. The predominance of inhibitory input to VSCT neurons led to the hypothesis that the most essential information forwarded by them concerns the degree of inhibitory control of spinal activity (Lundberg 1971) rather than peripheral events. Monitoring the degree to which premotor interneurons inhibit alpha-motoneurons would require that these interneurons act in parallel on motoneurons and on VSCT neurons. The strongest evidence to this end was presented for disynaptic inhibitory postsynaptic potentials (IPSPs) evoked from group Ia afferents by showing that they are depressed by Renshaw cells as efficiently in VSCT neurons (Lindström 1973; Lindström and Schomburg 1974) as in motoneurons (Hultborn et al. 1971). Indications that premotor interneurons in pathways from group Ib afferents to motoneurons have collateral actions on VSCT neurons were more indirect. These indications consisted mainly of showing that the pattern of origin of IPSPs evoked in VSCT neurons (Eccles et al. 1961; Lundberg and Weight 1971) is consistent with the pattern of actions of group Ib afferents on motoneurons (Eccles et al. 1957b). Indications for collateral actions of premotor interneurons in pathways from high-threshold muscle, skin and joint afferents, jointly denoted as flexor reflex afferents [FRA; (Eccles and Lundberg 1959)] were similarly indirect, including similar thresholds, time course and origin of IPSPs in VSCT neurons (Lundberg 1971) and in alpha-motoneurons (Eccles and Lundberg 1959). Although it has been demonstrated that some polysynaptic inhibitory FRA actions evoked in both VSCT neurons and motoneurons are mediated via Ia inhibitory interneurons (Lindström 1973; ten Bruggencate and Lundberg 1974) the involvement of other premotor interneurons, in particular those in disynaptic pathways from group II afferents from muscle spindles (Cavallari et al. 1987; Edgley and Jankowska 1987; Lundberg et al. 1987a,b) has not been investigated.
The main aim of the current study was therefore to search for more direct evidence that inhibitory interneurons in reflex pathways from group Ib and group II afferents to motoneurons do indeed exert collateral actions on VSCT neurons via pathways indicated in Fig. 1A. We approached this issue by verifying that stimuli applied in motor nuclei evoke IPSPs attributable to monosynaptic actions of inhibitory interneurons projecting to these nuclei and that these IPSPs are matched by disynaptic IPSPs evoked by group Ib and/or group II afferents stimulated within muscle nerves. To this end we compared effects of stimuli applied in main hindlimb motor nuclei in VSCT neurons with IPSPs evoked from muscle afferents in the same neurons. This was investigated in the two main populations of VSCT neurons, those with peripheral excitatory input mainly from group Ib afferents (to be referred to as Ib-VSCT or VSCT) and those with input mainly from group Ia afferents (to be referred to as the spinal border subdivision of VSCT neurons [SB-VSCT or SB]; for references see Burke et al. 1971; Oscarsson 1973).
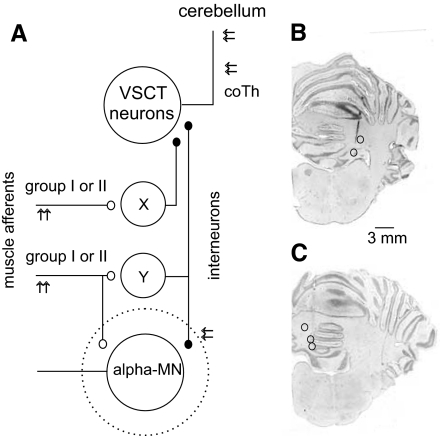
Fig. 1.
Diagram of coupling between group I and group II muscle afferents and ventral spinocerebellar tract (VSCT) neurons via interneurons in reflex pathways from group I and group II muscle afferents investigated in this study. A: diagram of putative connections. X and Y denote interneurons that might mediate inhibitory actions of group I and/or group II afferents on VSCT neurons. X represents interneurons in “private” pathways between these afferents and VSCT neurons and Y represents interneurons acting on both VSCT neurons and hindlimb alpha-motoneurons (MNs). The dotted circle represents motor nuclei within which the intraspinal stimuli were applied and within which axon collaterals of both afferent fibers and interneurons were stimulated. The findings in this study provide evidence for coupling via interneurons Y, albeit without excluding contribution of interneurons X. Stimulation sites in the cerebellum and at the level of the contralateral lateral funiculus (co Th) are indicated by double arrows. Peripheral afferents were stimulated to evoke disynaptic postsynaptic potentials (PSPs) in VSCT neurons, whereas stimulation in motor nuclei aimed at exciting terminals of interneurons synapsing with motoneurons to examine whether monosynaptic PSPs could be evoked in this way in VSCT neurons. B and C: location of stimulation sites within the cerebellum from which the investigated neurons were antidromically activated in 5 experiments.
METHODS
Preparation
The experiments were performed on 10 deeply anesthetized cats weighing 2.6–4.2 kg. Anesthesia was induced with sodium pentobarbital (Apoteksbolaget, Sweden; 40–44 mg/kg, administered intraperitoneally) and maintained with intermittent doses of α-chloralose (Rhône-Poulenc Santé, France; 5 mg/kg, administered intravenously [iv], every 1–2 h ≤50 mg/kg). Additional doses of α-chloralose were given when changes in the continuously monitored blood pressure or heart rate were evoked by peripheral or central stimulation, or if the pupils dilated. During recordings, neuromuscular transmission was blocked by pancuronium bromide (Pavulon, Organon, Sweden; ∼0.2 mg·kg−1·h−1 iv) and the animals were artificially ventilated. The effectiveness of synaptic transmission was increased by iv application of 4-aminopyridine (4-AP; Sigma, St. Louis, MO) in doses 0.1–0.2 mg/kg, iv. Mean blood pressure was kept at 100–130 mmHg and the end-tidal concentration of CO2 at about 4% by adjusting the parameters of artificial ventilation and the rate of continuous infusion of a bicarbonate buffer solution with 5% glucose (1–2 ml·h−1·kg−1). Core body temperature was maintained at about 38°C by servo-controlled infrared lamps. The experiments were terminated by a lethal dose of anesthetic. All procedures were approved by the local Ethics Committee (Göteborgs Djurförsöksetiska nämnd) and followed National Institutes of Health and European Union guidelines for animal care.
The spinal cord was exposed by a laminectomy from the second to the sixth lumbar (L2–L6) segments and at the level of the low thoracic (Th10–Th12) segments. To restrict the antidromic activation following stimuli applied in the cerebellum to neurons belonging to spinocerebellar tract neurons with axons ascending through the contralateral spinal cord (VSCT, SB-VSCT) and to exclude spinocerebellar neurons with axons ascending ipsilaterally, the spinal cord was hemisectioned ipsilaterally at the level of the twelfth thoracic or second lumbar segments in four experiments. In the remaining experiments the axonal projection of neurons was verified by comparing effects of strong stimuli (≤1 mA) applied to the left and right lateral funiculi at the level of the twelfth thoracic segment and only neurons in which stimulation to the ipsilateral thoracic lateral funiculus failed to elicit antidromic activation were included in the sample. The dura mater over the lumbosacral enlargement was left intact except for small holes (∼1 mm2) through which the microelectrodes were advanced. Axon collaterals of premotor interneurons expected to provide input to VSCT neurons were stimulated within the gastrocnemius–soleus and posterior biceps–semitendinosus motor nuclei in the caudal part of the seventh lumbar segment. Because both these motor nuclei extend to the most caudal part of the lumbosacral enlargement (see e.g., Vanderhorst and Holstege 1997) they could be accessed within the same electrode track by advancing or retracting the stimulating electrode, respectively. The cerebellar stimulation sites were at locations from which distinct descending volleys were evoked by stimuli of 20–50 μA, with examples of electrolytic lesions at the site of stimulation in Fig. 1, B and C. They were close to or within either the contralateral (n = 7) or the ipsilateral (n = 3) nucleus interpositus (at Horsley–Clarke coordinates P 6.5–7, L 3–3.5, H about −1) accessed by an electrode introduced at an angle of 35–45° from vertical (with the tip directed rostrally). The electrode was advanced using a manipulator designed by Y. Källström (Gothenburg University).
The following left hindlimb nerves were dissected free, transected, and mounted on stimulating electrodes: quadriceps and sartorius branches of the femoral nerve mounted in subcutaneous cuff electrodes; the posterior biceps and semitendinosus, anterior biceps and semimembranosus, gastrocnemius–soleus, plantaris, and deep peroneal, including extensor digitorum longus and tibialis anterior nerves.
Stimulation and recording
Peripheral nerves were stimulated with constant voltage stimuli at intensities expressed in multiples of threshold for the activation of the most excitable fibers. Axons of VSCT neurons running within the contralateral lateral funiculus at the level of the twelfth thoracic segment were stimulated extradurally, with a pair of silver ball electrodes in contact with its surface, using 0.2 ms long constant current stimuli of 200–500 μA. Axons of VSCT neurons within the cerebellum were stimulated via a tungsten electrode insulated except for its very tip (30–300 kOhm) by passing 0.2 ms long constant current stimuli of 5–100 μA. Axons of interneurons in motor nuclei were stimulated through tungsten electrodes placed at locations at which distinct antidromic field potentials were evoked by stimulation of the posterior biceps and semitendinosus nerves and/or gastrocnemius and soleus nerves using 0.2 ms long constant current stimuli of 4–50 μA. The nuclei were first localized on the basis of records obtained with a glass micropipette filled with 2 M NaCl. Thereafter the micropipette was replaced by a tungsten electrode and a second series of records of field potentials was obtained from different depths (see Fig. 4). The gastrocnemius and soleus and posterior biceps and semitendinosus motor nuclei were selected for the purposes of this study as representative extensor and flexor motor nuclei and because disynaptic IPSPs and excitatory postsynaptic potentials (EPSPs) from group I and group II afferents are evoked in high proportions of motoneurons in these nuclei (Eccles and Lundberg 1959; Eccles et al. 1957b), in parallel with disynaptic PSPs in VSCT and dorsal spinocerebellar tract neurons (Hongo et al. 1983a; Jankowska and Puczynska 2008; Lindström and Schomburg 1974; Lundberg and Weight 1970). The stimulators used were designed by E. Eide, D. Magnusson, and N. Pihlgren (University of Gothenburg; Eide 1973).They allow the use of square constant voltage or constant current pulses via inbuilt isolation units and setting the constant voltage stimuli in multiples of threshold for activation of nerve fibers via 15 output channels. Both intracellular and extracellular records from VSCT neurons were obtained using glass micropipettes (“sharp”; with tips broken to external diameters of 1.2 to 1.5 μm). The electrodes were mounted in the electrode holder attached to a step motor driven manipulator mounted on an arc that allowed positioning of the electrode to be adjusted in two planes with respect to the vertical plane and its movements to the left and right as well as rostrally and caudally, with an accuracy of about 2 μm (both constructed by Y. Källström, Gothenburg University; Eide and Källström 1968). Micropipettes used for extracellular and intracellular recording (against the reference electrode inserted in a back muscle) had an impedance of about 2–3 and 4–6 MOhms, respectively. Intracellular records were obtained using high-input resistance amplifiers, with inbuilt units that allowed not only the passing of constant current through the micropipettes but also recording and stimulating calibration current pulses and verification of the electrode resistance (constructed by E. Eide, D. Magnusson, and N. Pihlgren, Gothenburg University; Eide 1968). Records from the cord dorsum were obtained using a two-channel AC amplifier (constructed by N. Pihlgren, Gothenburg University).
Fig. 4.
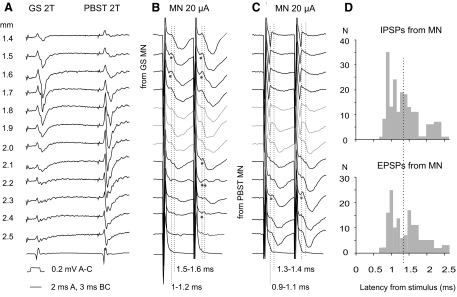
Examples of PSPs evoked from motor nuclei and distribution of their latencies. A: records of field potentials evoked at different depths from the surface along an electrode track traversing gastrocnemius–soleus (GS) and posterior biceps–semitendinosus (PBST) motor nuclei. B and C: intracellular records of PSPs evoked in 2 VSCT neurons by stimuli applied at the same depths as indicated in A. Vertical dotted lines indicate onset of various components of the EPSPs and IPSPs (with latencies to the right of these lines below B and C). Note that monosynaptic IPSPs (at latencies 0.9–1.4 ms) were evoked from within the GS MN in the first VSCT neuron and from the PBST MN in the second, whereas only longer latency IPSPs, or no IPSPs, were evoked from the other motor nuclei with the exception of IPSPs evoked within a 0.3 mm wide zone of overlap of field potentials from the 2 nuclei (in gray). Note also small EPSPs (indicated by dots below the records) at similar latencies. D: histograms of latencies of IPSPs and EPSPs evoked in VSCT neurons within ranges indicated along the abscissa. For each neuron, latencies of all distinct early and later components of PSPs evoked by stimuli applied at different depths along the electrode tracks and/or by various intensities of these stimuli were included, but only one for a given range of 0.1 ms per neuron. The dotted line indicates the likely border line between monosynaptically and disynaptically evoked PSPs. The distribution of latencies of both IPSPs and EPSPs was found to be statistically significantly different from normal (Shapiro–Wilk test).
Analysis
Both original data and averages of 10–40 single records (with the time resolution of 30 μs per address) were stored on-line using software for sampling and analysis developed by E. Eide, T. Holmström, and N. Pihlgren (Gothenburg University). The latencies of postsynaptic potentials were measured from afferent volleys recorded from the cord dorsum close to the recording electrode penetration site and/or from stimulus artifacts. They were measured from averaged traces, i.e., representing minimal latencies of 10–40 responses. The areas were measured within a time window of 1.2–1.6 ms, from the onset of the potentials to about one third of the declining phase (see shaded area in Fig. 6A). They were measured in arbitrary units using the above-cited software. Differences between data sets were assessed for statistical significance by using Student's t-test for unpaired samples, assuming equal variances and two-tail distribution.
Fig. 6.
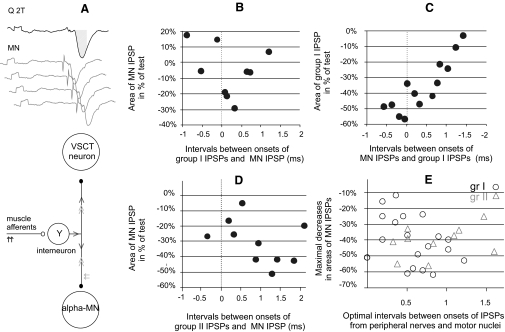
Effects of collision between synaptic actions evoked by stimulation of afferents in peripheral nerves and from motor nuclei. A: the basis of interactions between IPSPs evoked by the same interneuron by a collision of nerve impulses induced in axon collaterals of this interneuron by its synaptic activations following peripheral nerve stimulation (black arrows) and by stimuli applied in motor nuclei (gray arrows). The direction of nerve impulses initiated by stimulation of peripheral afferents is indicated by single black arrowheads and of those initiated by intraspinal stimuli by double gray arrowheads. Records above the diagram show examples of IPSPs evoked in this way in a VSCT neuron. Black trace: IPSP evoked by stimulation of the quadriceps nerve. Gray traces: IPSPs evoked at varying time intervals from the motor nuclei so that their onset would coincide with the onset of IPSPs evoked by nerve stimulation, precede it, or follow it. B–D: relative changes in the areas of the second IPSPs (ordinate; in percentages of the area of the test IPSP) as a function of intervals between onsets of the 2 IPSPs (abscissa) in the indicated combinations of stimuli. The areas were measured within time windows indicated by shadowing in A. The data points represent relative decreases in series of averages of 20 potentials evoked in single cells, each at a different time interval. E: maximal decreases in IPSPs recorded in 29 VSCT and SB neurons in which stimulation of motor nuclei followed stimulation of group I afferents (circles) or group II afferents (triangles) in fractions of amplitudes of test IPSPs from motor nuclei. They are plotted against the time intervals at which the maximal decreases occurred.
Criteria for monosynaptic and disynaptic PSPs evoked by intraspinal stimuli
The main criteria used to distinguish between monosynaptically and disynaptically evoked IPSPs were the latencies of ≤1.4 and >1.9 ms (from the onset of the stimuli). The latencies of IPSPs monosynaptically evoked from motor nuclei should include about 0.2 ms to account for the utilization time for generation of action potentials in fibers stimulated by electrical stimuli (Jankowska and Roberts 1972a; Roberts and Smith 1973); about 0.5 ms for the conduction time along stem axons of Ib interneurons [assuming 50 m/s conduction velocity over a distance of 20 mm (Hongo et al. 1983a,b) or about 70 m/s for Ia interneurons (Jankowska and Roberts 1972b)]; about 0.2 ms for the conduction time along the axonal terminal branches [at about 10 m/s (Alstermark et al. 1987; Jankowska and Roberts 1972b; Shinoda et al. 1979)]; and about 0.3 ms for the synaptic delay (Eccles 1964; Jankowska and Roberts 1972a). Together, this adds up to a minimum of 1.2 ms. Depending on the conduction distances and conduction velocities between motor nuclei and the location of individual VSCT neurons, however, latencies of monosynaptic IPSPs could be both shorter and longer.
Taking into account the minimal segmental latencies of IPSPs evoked in VSCT neurons from peripheral afferents [1.5–1.7 ms from the volleys (see Fig. 2, C, F, and I; Eccles et al. 1961)], disynaptic IPSPs following intraspinal stimuli would at the earliest be evoked about 1.9 ms from these stimuli because these are followed by nerve volleys at latencies of 0.4–0.6 ms. Thus the longest latencies of PSPs evoked monosynaptically by intraspinal stimuli might possibly overlap with the shortest latencies of disynaptically evoked IPSPs.
Fig. 2.
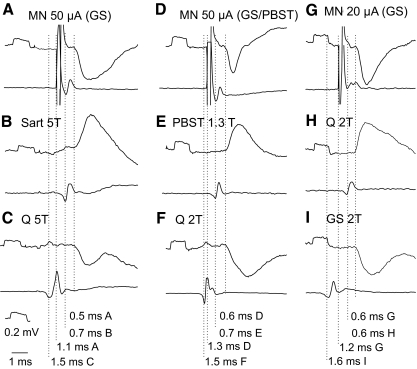
Examples of shortest latency inhibitory postsynaptic potentials (IPSPs) evoked from motor nuclei (MNs). In all panels top traces are microelectrode recordings and bottom traces are recordings from the cord dorsum (with the negativity downward and upward, respectively): 20 averaged records. A–C, D–F, and G–I: intracellular records of PSPs evoked in 2 SB-VSCT and in one Ib-VSCT neurons. IPSPs were evoked from gastrocnemius soleus (GS) motor nuclei or at the border between these nuclei and the posterior biceps–semitendinosus (PBST) nuclei and from the quadriceps (Q) and GS nerves, whereas excitatory postsynaptic potentials (EPSPs) were evoked from the sartorius (Sart), PBST, and Q nerves, as indicated. The records are aligned with respect to the onset of the PSPs. The rightmost dotted lines indicate the onset of the PSPs and those to the left indicate nerve volleys induced by intraspinal stimuli (in the top row A, D, G) and by stimulation of muscle nerves (in the middle and bottom rows). Latencies from the nerve volleys and from the intraspinal stimuli are indicated to the right of each dotted line. Note the much shorter time course of IPSPs in D, indicating that EPSPs were superimposed on the falling phase of the IPSPs. In this and in the following figures the rectangular pulses at the beginning of the records are calibration pulses of 0.2 mV and all records are at the same time base, unless indicated otherwise. The intensity of constant current intraspinal stimuli is in microamps (μA) and that of constant voltage stimuli applied to peripheral nerves in multiples of stimuli at threshold for the most sensitive nerve fibers in these nerves, as estimated on the basis of records from afferent volleys recorded from the cord dorsum.
RESULTS
Samples of VSCT neurons
The two samples of VSCT neurons investigated in this study included 92 Ib-VSCT neurons located in laminae VI–VII in the fifth lumbar segment, or at the border between the fourth and fifth segments, and 41 SB-VSCT neurons located just lateral or dorsal to motor nuclei in the third and fourth segments. Both were identified by antidromic activation by stimuli applied within the cerebellum (at locations illustrated in Fig. 1, B and C), as well as by stimulation of the contralateral lateral funiculus at the level of the eleventh to twelfth thoracic segment (at latencies ranging between 2.9 and 6.0 and 0.7 and 1.7 ms, respectively). In addition, Ib-VSCT neurons were identified by their location at depths (2.4–3.9 mm from the surface of the dorsal columns at an angle of about 0° from the vertical, at which focal field potentials were evoked from both group I and group II, or only group II muscle afferents; see Hammar et al. 2002) and by their typical input: excitatory from group Ib afferents and inhibitory from group I and high-threshold muscle afferents (Eccles et al. 1961). SB-VSCT neurons were identified in addition by their location within a 100–200 μA wide strip at the border between the lateral funiculus and the ventral horn (usually at depths 1.8–2.2 from the surface of the lateral funiculus at an angle of 10–20°) and by their typical excitatory input from group Ia afferents and inhibitory input from group I and high-threshold muscle afferents (Burke et al. 1971; Lundberg and Weight 1971). The samples included all neurons of the two VSCT populations successfully penetrated, with action potentials of ≥50 mV, in which IPSPs with latencies indicating disynaptic coupling were evoked from group I and/or group II muscle afferents (1.1–1.5 ms at thresholds <twofold threshold for the most sensitive fibers in the nerve and 1.8–3.5 ms at higher thresholds, respectively) during the whole period of recording. No other selection criteria were used.
Synaptic actions evoked by stimuli applied in motor nuclei
IPSPs.
Stimuli applied in motor nuclei evoked IPSPs in 88% of the total sample of VSCT and SB neurons. As illustrated in Fig. 2, the shortest latencies of these IPSPs (measured from the volleys following the stimuli) were practically the same as the segmental latencies of monosynaptic EPSPs from group I afferents (from afferent volleys recorded at the same spinal level) and about 1 ms shorter than segmental latencies of disynaptic IPSPs. However, volleys evoked by intraspinal stimuli were most often superimposed on shock artifacts that prevented relating all of the PSPs evoked from motor nuclei to the volleys. Measurements of latencies of PSPs were therefore routinely made from the beginning of the stimuli. However, allowing about 0.5 ms between the beginning of the stimuli and the volleys induced by them at the level of location of VSCT neurons, latencies of ≤1.4 ms from the stimulus (see methods) would correspond to latencies <0.9 ms from the volleys, as required for monosynaptic coupling.
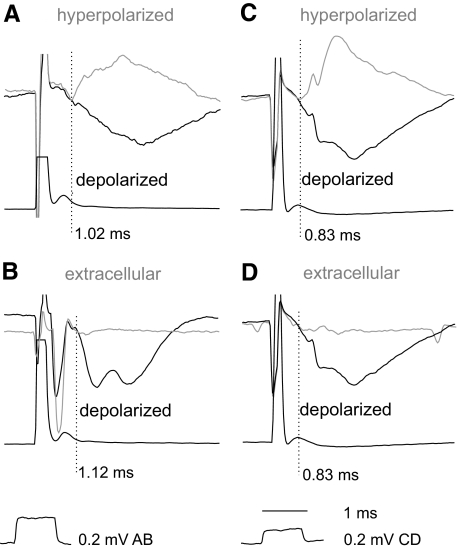
When the onset of IPSPs evoked from motor nuclei was not sharp, especially when IPSPs overlapped with shock artifacts and were preceded by EPSPs, two procedures were used to increase the accuracy in estimating their latency. First, we superimposed IPSPs recorded while passing depolarizing and hyperpolarizing currents (10–15 nA) that resulted in an increased amplitude and in a reversal, respectively. The point of deviation between these records (as illustrated in Fig. 3, A and C) was taken as the onset of the IPSPs. The second procedure involved comparison of intracellular and extracellular records as in Fig. 3, B and D, with the point of deviation between them similarly used to define the onset of the IPSPs.
Fig. 3.
Onset of IPSPs evoked from motor nuclei defined by comparing intracellular records in depolarized and hyperpolarized neurons as well as intracellular and extracellular records. Records from 3 VSCT neurons (A, B, and C, D) and cord dorsum potentials. Black traces: during depolarization by 10–15 nA. Gray traces in A and C, during hyperpolarization by 5 and 15 nA and the ensuing reversal of the IPSPs; gray traces in B and D, extracellular records. The pairs of traces in each panel were superimposed so that both stimulus artifacts and the traces directly preceding the IPSPs overlapped. The points of deviation between them are indicated by the vertical dotted lines and show the onset of the IPSPs otherwise not sharp enough to be estimated.
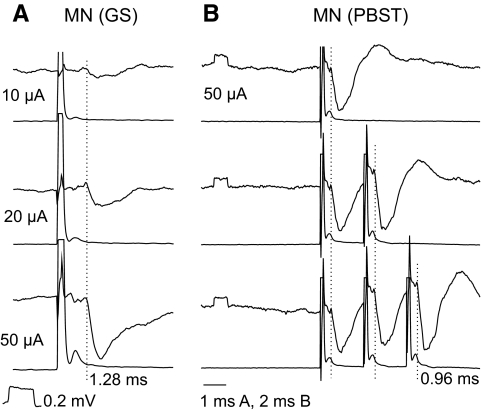
Based on these measurements latencies of IPSPs evoked from motor nuclei were found to range between 0.7 and 3.5 ms, with the majority evoked at latencies not exceeding 2 ms (Fig. 4D). However, the onset of IPSPs varied even in individual neurons when the intraspinal stimuli were applied at different depths along the electrode track and at different intensities. As illustrated in Fig. 4, B and C, latencies of the earliest components of IPSPs evoked from different sites within the motor nuclei varied between 0.9 and 1.4 ms and were found to change stepwise, even within distances as short as 100–200 μm. This variability is well in keeping with effects of stimulation of either terminal or larger axonal branches of interneurons, the latter conducting much faster (Jankowska and Roberts 1972b) and thus inducing shorter latency PSPs. However, the variability of these latencies could also reflect effects of stimulation of axons of different interneurons.
The distribution of latencies of PSPs evoked from motor nuclei (Fig. 4D) suggests that there are two peaks, one at around or just below 1 ms and another about 0.5 ms later, although there is no sharp division between them. This distribution pattern indicates that even though PSPs evoked at latencies <1.4 ms may be reliably classified as evoked monosynaptically, PSPs evoked at longer latencies may include both monosynaptically and disynaptically, or even trisynaptically, evoked responses. The division line between monosynaptic and disynaptic PSPs set at 1.4 ms may thus be considered as the dividing line between reliably and unreliably defined monosynaptic PSPs rather than as between monosynaptically and disynaptically evoked PSPs. Latencies of IPSPs and EPSPs were distributed in a similar manner.
Using temporal facilitation to differentiate between disynaptically and monosynaptically evoked PSPs (see Jankowska et al. 2003) proved unreliable in the case of PSPs evoked from motor nuclei because increases of IPSPs evoked by successive stimuli occurred not only for IPSPs evoked at relatively long latencies but also for their earliest components that could not be mediated by interposed interneurons (see Fig. 5B).
Fig. 5.
Changes in amplitudes of IPSPs evoked from motor nuclei depending on stimulus intensity and effects of successive stimuli. In all panels top traces are microelectrode recordings and bottom traces are recordings from the cord dorsum. A and B: intracellular records of PSPs evoked in 2 VSCT neurons by stimuli applied in GS and PBST MNs, respectively. Dotted lines indicate onset of PSPs. Note that the latencies of these IPSPs defined them as evoked monosynaptically and that the increase in amplitudes of IPSPs occurred without shortening the latencies and without an obvious addition of later components.
As shown in Table 1, responses at short latencies (0.7–1.4 ms) were consistently evoked in both subpopulations of VSCT neurons, with IPSPs being more frequently encountered (73%) than EPSPs (48%). The mean latencies of PSPs evoked in Ib-VSCT and SB-VSCT neurons showed only minor differences (<0.15 ms, albeit statistically significant), or no differences, and IPSPs and EPSPs were found in similar proportions. Threshold intensities of effective stimuli varied depending on the stimulation site in the motor nuclei but were most often as low as 10–20 μA, as illustrated in Figs. 2G, 4, B and C, and 5A. However, the amplitudes increased considerably when stronger stimuli were used (Fig. 5A) and in some neurons stimuli exceeding 50 μA were needed, showing that some VSCT neurons of our sample might have been affected by premotor interneurons projecting further away from the electrode tip.
Table 1.
Comparison of latencies of IPSPs and EPSPs evoked from motor nuclei in Ib-VSCT and SB-VSCT neurons
| Total VSCT (n = 133) |
Ib-VSCT (n = 92) |
SB-VSCT (n = 41) |
||||
|---|---|---|---|---|---|---|
| Latency Range | Latency | Incidence | Latency | Incidence | Latency | Incidence |
| IPSPs | ||||||
| 0.7–1.4 ms | 1.06 ± 0.02 | 73% | 1.02 ± 0.02* | 75% | 1.17 ± 0.02** | 68% |
| 1.4–3.0 ms | 1.74 ± 0.04 | 65% | 1.76 ± 0.05† | 60% | 1.71 ± 0.06†† | 76% |
| 0.7–3.0 ms | 88% | 88% | 88% | |||
| EPSPs | ||||||
| 0.7–1.4 ms | 1.03 ± 0.02 | 48% | 0.99 ± 0.02‡ | 50% | 1.11 ± 0.03‡‡ | 44% |
| 1.4–3.0 ms | 1.69 ± 0.05 | 43% | 1.62 ± 0.05§ | 46% | 1.87 ± 0.12§§ | 37% |
Values are means ± SE of minimal latencies of PSPs evoked by 20- to 50-μA stimuli within ranges of 07–1.4 and 1.4–3.0 ms. These two ranges would correspond to latencies of monosynaptically evoked PSPs and of PSPs evoked disynaptically, but most likely including some evoked not only monosynaptically, but also tri- and polysynaptically (see text). Data are given for the whole sample of 133 VSCT neurons tested and for the two subpopulations of these neurons, together with proportions of neurons in which the PSPs were found. Note that the sums of these proportions exceed 100% because different kinds of PSPs were often evoked in the same neurons (see Figs. 2 and 9). Differences between latencies of monosynaptic PSPs evoked in Ib-VSCT and SB-VSCT neurons (‡ − ‡‡; * − **) were found to be statistically significant (P < 0.01), whereas those for longer latencies only for EPSPs (§ − §§). Differences between latencies of EPSPs and IPSPs were found to be statistically significant (P < 0.005) only for the shortest ones (‡ − *; ‡‡ − **).
Origin of IPSPs evoked by intraspinal stimuli.
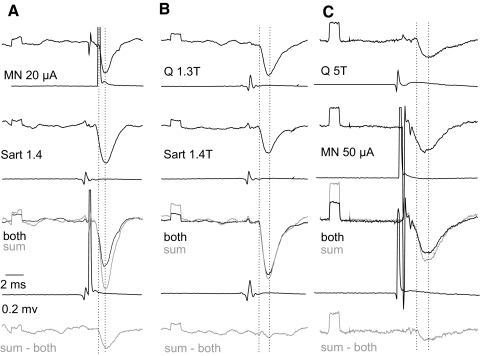
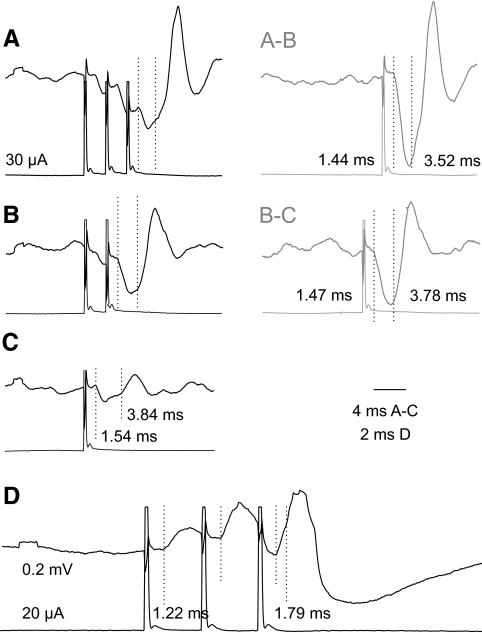
Demonstration of short-latency IPSPs evoked in VSCT neurons by stimuli applied in motor nuclei shows that these IPSPs are mediated by premotor interneurons, but does not specify which kind of premotor interneurons they are. To identify them we verified that the same interneurons were involved in inducing monosynaptic IPSPs evoked by intraspinal stimuli and in mediating disynaptic IPSPs from group Ia afferents from muscle spindles, group Ib afferents from tendon organs, or group II afferents in muscle nerves. This was done by examining the interactions between effects evoked by stimulation of group Ia, group Ib, or group II afferents in peripheral nerves and by stimuli applied in the motor nuclei. The principle behind this test (explained in Fig. 6A) presumes that nerve impulses induced following synaptic activation of interneurons invade all axon collaterals of these interneurons, without a block at their branching points (Cattaert et al. 1999; Smith 1980a,b). Axon collaterals of premotor interneurons within motor nuclei would thus be refractory for about 1 ms during and after the passage of synaptically generated action potentials. Provided that intraspinal stimuli giving rise to IPSPs in VSCT neurons activated axons of the same synaptically activated interneurons, they would fail to initiate any action potentials, or initiate them in a smaller number of axon collaterals, during an interval of about 1 ms between the peripheral and intraspinal stimuli. Consequently, IPSPs evoked from motor nuclei would then be significantly reduced. Records in Figs. 7A and 8A and plots in Fig. 6, A and C show that such interactions were indeed found.
Fig. 7.
Collision between synaptic actions evoked by stimulation of group I afferents in peripheral nerves and from motor nuclei. A and C: examples of records from the 2 VSCT neurons on which plots B and C in Fig. 6 are based. The records show IPSPs evoked from motor nuclei (MNs) alone, IPSPs evoked by stimulation of Sart or Q nerves alone, and of IPSPs appearing when the 2 stimuli were applied one just after another with a time delay (labeled “both”). The areas were measured between the 2 vertical dotted lines. Note that the sums of IPSPs evoked by individual stimuli (gray) were larger than IPSPs following the joint application (black). The differences between them are shown in bottom traces. They were related to the areas of IPSPs evoked from the MNs (top records in C) or by group I afferents (top records in E). B: control records illustrating that IPSPs evoked from one peripheral nerve during IPSPs evoked from another peripheral nerve were decreased to a much smaller extent or not at all.
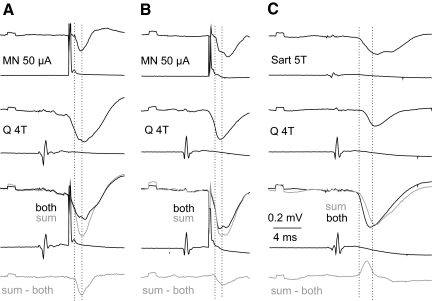
Fig. 8.
Collision between synaptic actions evoked by stimulation of group II afferents in peripheral nerves and from motor nuclei. A: examples of records from the VSCT neuron on which the plot in Fig. 6D was based, arranged as in Fig. 6C. B and C: records from another VSCT neuron comparing effects of joint stimulation of group II afferents in a muscle nerve and of motor nuclei (B) and of joint stimulation of group II afferents in 2 muscle nerves (C). Note that IPSPs evoked by stimuli applied in motor nuclei decreased following stimulation of a peripheral nerve, whereas IPSPs evoked by stimulation of 2 peripheral nerves (C) were larger than the sum of IPSPs evoked from these nerves separately. Records in B were taken when the amplitude of the IPSP was smaller.
The maximal decreases in the areas of IPSPs evoked from motor nuclei following IPSPs evoked by stimulation of group I or group II afferents (represented by bottom traces in Figs. 7A and 8, A and B) amounted to −40.5 ± 3.5 and to −40.9 ± 3.1%, respectively, of areas of test IPSPs evoked from the motor nucleus (top records in Figs. 7A and 8, A and C). When the stimuli were applied in reversed order (i.e., IPSPs evoked from motor nuclei preceded IPSPs evoked by stimulation of group I afferents, with an example in Fig. 7B) the resulting decreases were smaller, by −30.7 ± 9.1% of areas of test IPSPs from group I afferents. This would in fact be expected for actions mediated by a larger population of interneurons activated by primary afferents than of interneurons projecting to a fraction of hindlimb motor nuclei. The time delays at which the most potent depression was observed appeared to be slightly longer for IPSPs evoked from motor nuclei with respect to IPSPs evoked from group II afferents (0.86 ± 0.14 ms) than that with respect to IPSPs evoked from group I afferents (0.59 ± 0.08 ms) or for IPSPs from group I afferents following IPSPs from motor nuclei (0.45 ± 0.13 ms), as indicated by distribution of data points in Fig. 6, B, C, D, and E. However, none of these differences has been found to be statistically significant.
Considering that the depression might depend not so much on interactions between nerve volleys initiated in different axon collaterals of the same interneurons, as on effects due to hyperpolarization of VSCT neurons associated with the first IPSP, additional tests were performed to verify this possibility. To this end we compared interactions between IPSPs evoked by stimulation of two different peripheral nerves rather than a nerve and a motor nucleus. As illustrated in Figs. 7B and 8C, two IPSPs of peripheral origin (of similar amplitudes and similarly timed) showed a much smaller depression or even mutual facilitation. Neither were any decreases of IPSP areas found when longer-latency IPSPs, most likely disynaptic IPSPs from motor nuclei, followed IPSPs evoked from group I afferents. Taken together, this leads to the conclusion that IPSPs evoked by stimuli applied in motor nuclei were evoked by interneurons in inhibitory pathways from group Ib and/or group II afferents to motoneurons.
The regions of origin of monosynaptic IPSPs within the motor nuclei were defined using intraspinal stimuli of 20 μA, with an estimated radius of actions of about 0.2 mm (Gustafsson and Jankowska 1976). These stimuli were applied along single electrode tracks at depths 0.1 mm apart, at six to eight locations within both the gastrocnemius–soleus and posterior biceps–semitendinosus motor nuclei. The effects were attributed to stimuli applied within only one of these nuclei when evoked at latencies ≤1.4 ms from most of the stimulation sites within one nucleus, but failing to be evoked from stimulation sites within the other nucleus, with the exception of one to two stimulation sites located within the border region between the two motor nuclei. Using these criteria it appeared that monosynaptic IPSPs from one of these nuclei were evoked in 5 of 37 and 10 of 37, respectively, of VSCT neurons analyzed in more detail. However, in 4 of these neurons the same stimuli also evoked somewhat longer (1.41–1.47 ms) latency IPSPs from the other nucleus. These IPSPs were classified as evoked disynaptically but they might have been evoked monosynaptically (see previous text and the discussion), further decreasing the proportion of VSCT neurons targeted by premotor interneurons projecting to either flexor or extensor motor nuclei.
These data suggest that a widespread origin of monosynaptic IPSPs evoked from motor nuclei might be a general feature of VSCT neurons. This would mean that individual VSCT neurons receive feedback information on actions of inhibitory interneurons projecting to flexor motor nuclei as well as those projecting to extensor motor nuclei. It would also mean that, in contrast to motoneurons, IPSPs evoked in individual VSCT neurons may be relayed by interneurons mediating reciprocal as well as nonreciprocal inhibition of motoneurons from group Ia afferents, group Ia and group Ib afferents, and/or group II afferents. This is illustrated in Figs. 7 and 8 by the origin of monosynaptic IPSPs evoked from motor nuclei in VSCT neurons with disynaptic IPSPs of various afferent sources.
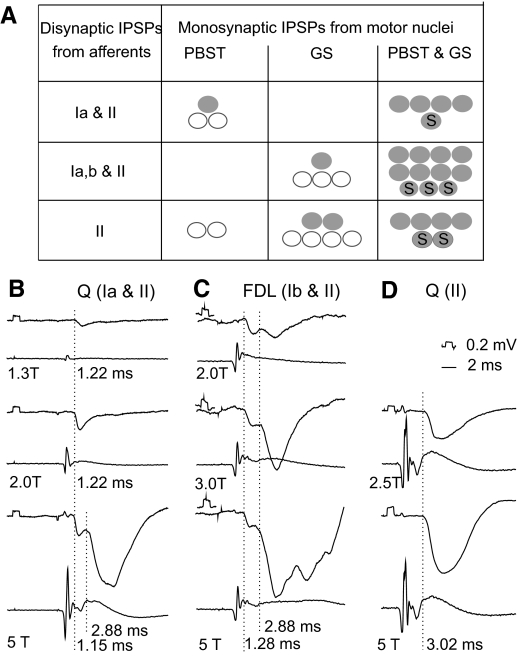
The summary diagram in Fig. 9A shows that similar origin of monosynaptic IPSPs from gastrocnemius–soleus and/or posterior biceps–semitendinosus motor nuclei was found in both fractions of VSCT neurons (Ib-VSCT and SB neurons). Effects of intraspinal stimuli differed only in neurons in which IPSPs were evoked from both group I and group II or only group II afferents (illustrated in Fig. 9, BC and D, respectively) in that IPSPs evoked in a larger proportion of the latter were not matched by monosynaptic IPSPs from motor nuclei.
Fig. 9.
Relationships between sources of inhibitory afferent input to VSCT neurons and the origin of monosynaptic IPSPs from motor nuclei. A: proportions of VSCT and SB neurons in which monosynaptc IPSPs (at latencies ≤1.4 ms) were evoked from within GS and/or PBST motor nuclei. The data are grouped for neurons in which IPSPs were evoked from lowest threshold (group Ia) afferents without or with group II afferents (n = 8, all from Q), the whole range of group I afferents (most likely group Ia and Ib) and group II afferents (n = 15 most often from extensors Q, GS, FDL, Pl but also from Sart, PBST, and DP), or from only group II afferents of any of the tested nerves (n = 14). Filled circles are for neurons in which monosynaptic IPSPs were evoked from PBST only, from GS only, or from both PBST and GS motor nuclei. Open circles are for neurons in which monosynaptic IPSPs were evoked from only one of the indicated nuclei but for which it could not be excluded that they were likewise evoked from the other nucleus (see text). Circles with “S” are for spinal border neurons. B–D: examples of IPSPs evoked from group Ia and group II afferents, group I and group II afferents, and only group II afferents of the dissected nerves and afferent volleys recorded from the cord dorsum. The nerves of origin are indicated above and stimulus intensities below the records. Dotted lines indicate onset of the IPSPs and the figures to the right of these lines their latencies with respect to the volleys.
EPSPs.
Stimuli applied in motor nuclei evoked not only IPSPs but also EPSPs. These were found in smaller proportions of VSCT neurons than IPSPs (see Table 1) and at latencies that were only marginally (by <0.1 ms), although statistically significantly different. Examples of small monosynaptically evoked EPSPs are shown in Fig. 4B (e.g., at the depth 1.6 mm from gastrocnemius–soleus motor nucleus), Fig. 4C (at the depth 2.3 ms from posterior biceps–semitendinosus motor nucleus), and of larger EPSPs evoked at longer latencies in Fig. 10. As shown in these figures the EPSPs either preceded or followed IPSPs evoked by the same stimuli and showed a similar degree of temporal facilitation (compare e.g., increases in IPSPs and EPSPs after the first, second, and third stimuli in Fig. 10).
Fig. 10.
Examples of EPSPs evoked from motor nuclei and their temporal facilitation. A–C and D: intracellular records from 2 Ib-VSCT neurons and cord dorsum potentials. A–B and B–C: differences between records in AB and BC, showing net records of potentials evoked by the second and third stimuli. Dotted lines in A–C indicate onset of disynaptic IPSPs and most likely trisynaptic EPSPs (at latencies below). Dotted lines in D indicate onset of monosynaptic and disynaptic EPSPs. Note marked temporal facilitation of di- and trisynaptic PSPs and difficult to estimate increase of amplitudes of monosynaptic EPSPs, but with a sharper increase in their rising slope.
DISCUSSION
The results of the present study show that inhibitory premotor interneurons in reflex pathways from group Ib tendon organs and group II muscle spindle afferents to motoneurons have potent collateral actions on VSCT neurons. They also show that feedback information provided by these premotor interneurons may be used jointly with feedback information provided by Ia inhibitory interneurons and Renshaw cells to signal the degree of inhibition and/or excitability of alpha motoneurons.
Monosynaptic IPSPs evoked by premotor interneurons
Stimuli applied within hindlimb motor nuclei evoked IPSPs in parallel with IPSPs evoked by group I and/or group II muscle afferents in a total of 88% of our sample of VSCT and SB neurons and in the majority of these neurons (73%) the IPSPs were evoked at latencies that would not allow enough time for actions to be relayed by additional interneurons (see methods).
Unfortunately, we were unable to use the test of temporal facilitation to differentiate between monosynaptic and disynaptic PSPs evoked at somewhat longer latencies (see Jankowska et al. 2003) because of effects of previous stimuli. Because even the earliest components of IPSPs evoked by the second or third stimulus were somewhat larger than those evoked by the first stimulus (see e.g., Fig. 5B), the effect could not be attributed to facilitation of activation of interposed interneurons. However, it could be accounted for by an increase in the excitability of nerve fibers following generation of spike potentials in these fibers and the resulting increases in nerve volleys evoked by the stimuli (P. Krutki, S. Jelen, and E. Jankowska, unpublished data). Intraspinal stimuli could therefore activate increasing numbers of axon collaterals of interneurons projecting to motor nuclei, thereby causing an apparent temporal facilitation.
Considering that stimuli applied along an electrode track crossing motor nuclei could activate only a fraction of terminal axonal branches of any interneurons, even the conservative estimate of 73% of VSCT neurons with monosynaptic IPSPs evoked by these stimuli leads to the conclusion that actions of inhibitory premotor interneurons projecting to motor nuclei are widely spread among VSCT neurons. These results would therefore indicate that premotor interneurons may influence the majority if not all VSCT neurons. Whether IPSPs evoked in VSCT neurons by peripheral afferents are mediated exclusively by premotor interneurons, or also by other interneurons, cannot be estimated on the basis of our results. Records in Figs. 2 and 7 show that the amplitudes of monosynaptic IPSPs evoked from motor nuclei and disynaptic IPSPs evoked by peripheral afferents were sometimes comparable, which would be compatible with both IPSPs being mediated by the same premotor interneurons. However, amplitudes of IPSPs evoked from motor nuclei were often <0.5 mV (see Figs. 4 and 5), i.e., several times smaller than those of IPSPs evoked from peripheral nerves.
Which interneurons provide inhibitory input to VSCT neurons?
Interactions between monosynaptic IPSPs evoked from motor nuclei and disynaptic IPSPs evoked by group Ib and/or group II afferents provide evidence that some premotor interneurons mediate inhibition of both hindlimb motoneurons and VSCT neurons of group Ib and/or group II origin. As described in results, IPSPs evoked by intraspinal stimuli were reduced within a time window of 1 ms following the onset of synaptically evoked IPSPs, which is compatible with a collision of nerve volleys in axons of the same interneurons (as outlined in Fig. 6A). IPSPs evoked from peripheral nerves were also reduced when delayed with respect to monosynaptic IPSPs evoked from motor nuclei, although most often to a lesser degree. The weaker depression of IPSPs evoked by peripheral nerves than by intraspinal stimuli could be explained by stimuli applied within a limited area in the motor nuclei being more likely to activate a smaller proportion of last-order interneurons than a maximal stimulation of peripheral afferents.
In a subset of VSCT neurons monosynaptic IPSPs from the posterior–biceps semitendinosus motor nucleus were found in parallel with IPSPs apparently selectively evoked by group Ia afferents in the quadriceps nerve (see Fig. 8A). They are thus most likely mediated by Ia inhibitory interneurons, especially because such IPSPs were always (although not exclusively) evoked from these motor nuclei, which are the main target of Ia inhibitory interneurons activated by quadriceps Ia afferents (Eccles and Lundberg 1957; Eccles et al. 1956) and were shown to be depressed by activation of Renshaw cells as effectively as Ia IPSPs in motoneurons (Lindström 1973; Lindström and Schomburg 1973, 1974).
However, in the majority of VSCT neurons of the present sample, IPSPs were evoked from both group Ia and group Ib afferents and/or group II afferents. Premotor interneurons mediating these IPSPs should therefore be coexcited by either group Ia and group Ib afferents or by both group I and group II afferents (see Jankowska and Edgley 2010). The finding that IPSPs were evoked from both gastrocnemius–soleus and posterior biceps–semitendinosus motor nuclei, albeit more frequently from the former, was thus consistent with actions of premotor interneurons in pathways from group Ib and group II afferents to motoneurons (Eccles and Lundberg 1959; Eccles et al. 1957b; Laporte and Lloyd 1952). In addition, the terminal projection areas of these interneurons were found to extend over lamina VII as well as motor nuclei (see Fig. 7 in Bannatyne et al. 2009), which would allow individual interneurons to synapse with both VSCT neurons and motoneurons. The variability in projection areas of individual interneurons revealed by Bannatyne et al. (2009) would nevertheless leave room for the possibility that different subpopulations of these interneurons act exclusively on motoneurons or on VSCT neurons, whereas other subpopulations form connections with both.
Analysis of the peripheral origin of IPSPs evoked from group II afferents in VSCT neurons with concurrent monosynaptic IPSPs from motor nuclei provides additional information as to which populations of premotor interneurons might mediate these actions. Because these IPSPs were most often evoked from the quadriceps, sartorius, flexor digitorum longus, and deep peroneal nerves, the involvement of midlumbar interneurons, in which convergence from these nerves is most marked, seems highly probable.
Similarities and differences in effects of premotor interneurons on subpopulations of VSCT neurons
No marked differences in effects of inhibitory premotor interneurons on Ib-VSCT and SB-VSCT neurons were found in the present series of experiments. As shown in Table 1 and in Fig. 8 monosynaptic IPSPs were found in both populations. Nevertheless, the proportion of neurons with IPSPs from group II afferents in which we failed to evoke monosynaptic IPSPs from motor nuclei was somewhat larger (see Table 1) in SB neurons than that in VSCT neurons. This might indicate that a greater proportion of SB neurons are affected by interneurons that do not project to motor nuclei. Although these interneurons might project to other motor nuclei not investigated in the present series of experiments, this might also reflect functional differences in the information forwarded to the cerebellum between SB and VSCT neurons in terms of monitoring premotor interneuronal activity in the spinal cord.
Monosynaptic IPSPs in SB neurons were also evoked at slightly (0.15 ms) longer latencies than those in VSCT neurons. It is difficult to assess whether these somewhat longer latencies are of any significant functional consequence, even though the differences in latencies were statistically significant. They could merely reflect the more rostral location of SB neurons of our sample and thus longer distances between the stimulated motor nuclei and these neurons.
Interneuronally mediated EPSPs
In contrast to IPSPs evoked by intraspinal stimuli, monosynaptically evoked EPSPs might have been evoked by collateral actions of either premotor interneurons, or of group Ia or group II afferents providing monosynaptic input to motoneurons (Eccles and Lundberg 1958; Eccles et al. 1957a; Hongo 1992; Stauffer et al. 1976). Latencies as short as 0.7–1.4 ms appear to be compatible with direct actions of afferents as well as of interneurons activated by intraspinal stimuli, considering that the intraspinal conduction velocity of the afferents is about half (∼50 m/s; Eccles et al. 1961; Lloyd and Ak 1950) that along peripheral nerves. EPSPs evoked at longer latencies (ranging between 1.4 and 3.0 ms) would likewise be compatible with collateral actions of either slower conducting afferents or of excitatory interneurons stimulated within motor nuclei on interneurons affecting VSCT neurons. Furthermore, monosynaptic EPSPs evoked from gastrocnemius–soleus and/or posterior biceps–semitendinosus motor nuclei were most often matched by EPSPs following stimulation of peripheral nerves providing the main monosynaptic input to motoneurons in these nuclei and, even in cases when they were not matched, we could not exclude possible effects of afferents from other nerves projecting to these motor nuclei (such as the gracilis, adductor femoris, plantaris, or peroneus nerves). For all these reasons we cannot exclude that not only inhibitory but also excitatory premotor interneurons provide feedback information to VSCT neurons and via them to the cerebellum, but lack sufficiently strong indications to make this claim.
Functional consequences of collateral actions of premotor interneurons on VSCT neurons
Functional consequences of collateral information on operation of spinal neuronal networks provided to the cerebellum have been repeatedly discussed (see Lundberg 1971) and there is a general consensus that this information should be of utmost importance for the corrective functions of the cerebellum.
By weighting information on the degree of inhibition of motoneurons by premotor interneurons in pathways from group Ia and group Ib afferents, as well as other afferents, against information on excitatory reflex actions of these afferents, VSCT neurons in particular could enable the cerebellum to adjust these reflex actions (Lundberg 1971). Another purpose of inhibition of VSCT neurons by premotor interneurons could be to decouple spinal interneuronal networks from cerebellar control based on information from muscle receptors. Such decoupling might be useful under some behavioral conditions, such as during sequences of rhythmic locomotor or scratching movements steered by autonomous spinal interneuronal networks; once these sequences have been initiated, premotor inhibitory interneurons should effectively block the activation of motoneurons (and most likely also VSCT neurons) during certain phases of these movements.
Nevertheless, results of the present study draw attention to a further important consequence of inhibition of VSCT and SB neurons, especially those with a main excitatory input from the descending tract neurons (Baldissera and Roberts 1975; Fu et al. 1977). Specifically, they indicate that inhibition by premotor interneurons may be used to inform the cerebellum that the effectiveness of descending commands is likely to be lower under conditions of strong inhibitory input to motoneurons and that the descending actions on alpha motoneurons should be strengthened.
GRANTS
This study was supported by National Institute of Neurological Disorders and Stroke Grant R01 NS-040863 and Swedish Research Council Grants 15393-01 to E. Jankowska and 522-2005-7255 to I. Hammar.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. H. Drzymala-Celichowska for participation in one of the experiments and E. Nilsson for assistance with histological control.
REFERENCES
- Alstermark et al., 1987.Alstermark B, Kummel H, Pinter MJ, Tantisira B. Branching and termination of C3–C4 propriospinal neurones in the cervical spinal cord of the cat. Neurosci Lett 74: 291–296, 1987 [DOI] [PubMed] [Google Scholar]
- Baldissera and Roberts, 1975.Baldissera F, Roberts WJ. Effects on the ventral spinocerebellar tract neurones from Deiters' nucleus and the medial longitudinal fascicle in the cat. Acta Physiol Scand 93: 228–249, 1975 [DOI] [PubMed] [Google Scholar]
- Bannatyne et al., 2009.Bannatyne BA, Liu TT, Hammar I, Stecina K, Jankowska E, Maxwell DJ. Excitatory and inhibitory intermediate zone interneurons in pathways from feline group I and II afferents: differences in axonal projections and input. J Physiol 587: 379–399, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke et al., 1971.Burke R, Lundberg A, Weight F. Spinal border cell origin of the ventral spinocerebellar tract. Exp Brain Res 12: 283–294, 1971 [DOI] [PubMed] [Google Scholar]
- Cattaert et al., 1999.Cattaert D, El Manira A, Bevengut M. Presynaptic inhibition and antidromic discharges in crayfish primary afferents. J Physiol (Paris) 93: 349–358, 1999 [DOI] [PubMed] [Google Scholar]
- Cavallari et al., 1987.Cavallari P, Edgley SA, Jankowska E. Post-synaptic actions of midlumbar interneurones on motoneurones of hind-limb muscles in the cat. J Physiol 389: 675–689, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles, 1964.Eccles JC. The Physiology of Synapses. Berlin: Springer Verlag, 1964 [Google Scholar]
- Eccles et al., 1957a.Eccles JC, Eccles RM, Lundberg A. The convergence of monosynaptic excitatory afferents onto many different species of alpha motoneurones. J Physiol 137: 22–50, 1957a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles et al., 1957b.Eccles JC, Eccles RM, Lundberg A. Synaptic actions in motoneurones caused by impulses in Golgi tendon afferents. J Physiol 138: 227–252, 1957b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles et al., 1956.Eccles JC, Fatt P, Landgren S. Central pathway for direct inhibitory action of impulses in largest afferent nerve fibres to muscle. J Neurophysiol 19: 75–98, 1956 [DOI] [PubMed] [Google Scholar]
- Eccles et al., 1961.Eccles JC, Hubbard JI, Oscarsson O. Intracellular recording from cells of the ventral spinocerebellar tract. J Physiol 158: 486–516, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles and Lundberg, 1957.Eccles R, Lundberg A. Integrative pattern of reflex actions by impulses in large muscle spindle afferents on motoneurones to hip muscles. Experientia 13: 414–415, 1957 [DOI] [PubMed] [Google Scholar]
- Eccles and Lundberg, 1959.Eccles R, Lundberg A. Synaptic actions in motoneurones by afferents which may evoke the flexion reflex. Arch Ital Biol 97: 199–221, 1959 [Google Scholar]
- Eccles and Lundberg, 1958.Eccles RM, Lundberg A. Integrative pattern of Ia synaptic actions on motoneurones of hip and knee muscles. J Physiol 144: 271–298, 1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley and Jankowska, 1987.Edgley SA, Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. J Physiol 389: 647–674, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide, 1968.Eide E. Input amplifier for intracellular potential and conductance measurements. Acta Physiol Scand 73: 1A, 1968. 5672447 [Google Scholar]
- Eide, 1973.Eide E. An electrically isolated stimulator unit for neurophysiological research. Acta Physiol Scand 84: 3A, 1973 [Google Scholar]
- Eide and Källström, 1968.Eide E, Källström Y. Remotely controlled micromanipulator for neurophysiological use. Acta Physiol Scand 73: 2A, 1968 [Google Scholar]
- Fu et al., 1977.Fu TC, Jankowska E, Tanaka R. Effects of volleys in cortico-spinal tract fibres on ventral spino-cerebellar tract cells in the cat. Acta Physiol Scand 100: 1–13, 1977 [DOI] [PubMed] [Google Scholar]
- Gustafsson and Jankowska, 1976.Gustafsson B, Jankowska E. Direct and indirect activation of nerve cells by electrical pulses applied extracellularly. J Physiol 258: 33–61, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammar et al., 2002.Hammar I, Chojnicka B, Jankowska E. Modulation of responses of feline ventral spinocerebellar tract neurons by monoamines. J Comp Neurol 443: 298–309, 2002 [DOI] [PubMed] [Google Scholar]
- Hongo, 1992.Hongo T. Patterns of spinal projection of muscle spindle group II fibres. In: Muscle Afferents and Spinal Control of Movement, edited by Jami L, Pierrot-Deseilligny E, Zytnicki D. New York: Pergamon Press, 1992, p. 389–394 [Google Scholar]
- Hongo et al., 1983a.Hongo T, Jankowska E, Ohno T, Sasaki S, Yamashita M, Yoshida K. Inhibition of dorsal spinocerebellar tract cells by interneurones in upper and lower lumbar segments in the cat. J Physiol 342: 145–159, 1983a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo et al., 1983b.Hongo T, Jankowska E, Ohno T, Sasaki S, Yamashita M, Yoshida K. The same interneurones mediate inhibition of dorsal spinocerebellar tract cells and lumbar motoneurones in the cat. J Physiol 342: 161–180, 1983b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn et al., 1971.Hultborn H, Jankowska E, Lindström S. Recurrent inhibition from motor axon collaterals of transmission in the Ia inhibitory pathway to motoneurones. J Physiol 215: 591–612, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska and Edgley, 2010.Jankowska E, Edgley SA. Functional subdivision of feline spinal interneurons in reflex pathways from group Ib and II muscle afferents; an update. Eur J Neurosci (August18, 2010). doi:10.1111/j.1460-s9568.2010.07376.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska et al., 2003.Jankowska E, Hammar I, Slawinska U, Maleszak K, Edgley SA. Neuronal basis of crossed actions from the reticular formation upon feline hindlimb motoneurons. J Neurosci 23: 1867–1878, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska and Puczynska, 2008.Jankowska E, Puczynska A. Interneuronal activity in reflex pathways from group II muscle afferents is monitored by dorsal spinocerebellar tract neurons in the cat. J Neurosci 28: 3615–3622, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska and Roberts, 1972a.Jankowska E, Roberts W. Synaptic actions of single interneurones mediating reciprocal Ia inhibition of motoneurones. J Physiol 222: 623–642, 1972a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska and Roberts, 1972b.Jankowska E, Roberts WJ. An electrophysiological demonstration of the axonal projections of single spinal interneurones in the cat. J Physiol 222: 597–622, 1972b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte and Lloyd, 1952.Laporte Y, Lloyd DPC. Nature and significance of the reflex connections established by large afferent fibres of muscles. Am J Physiol 169: 609–621, 1952 [DOI] [PubMed] [Google Scholar]
- Lindström, 1973.Lindström S. Recurrent control from motor axon collaterals of Ia inhibitory pathways in the spinal cord of the cat. Acta Physiol Scand Suppl 392: 1–43, 1973 [PubMed] [Google Scholar]
- Lindström and Schomburg, 1973.Lindström S, Schomburg ED. Recurrent inhibition from motor axon collaterals of ventral spinocerebellar tract neurones. Acta Physiol Scand 88: 505–515, 1973 [DOI] [PubMed] [Google Scholar]
- Lindström and Schomburg, 1974.Lindström S, Schomburg ED. Group I inhibition in Ib excited ventral spinocerebellar tract neurones. Acta Physiol Scand 90: 166–185, 1974 [DOI] [PubMed] [Google Scholar]
- Lloyd and Ak, 1950.Lloyd DP, Ak M. Dorsal column conduction of group I muscle efferent impulses and their relay through Clarke's column. J Neurophysiol 13: 39–54, 1950 [DOI] [PubMed] [Google Scholar]
- Lundberg, 1971.Lundberg A. Function of the ventral spinocerebellar tract. A new hypothesis. Exp Brain Res 12: 317–330, 1971 [DOI] [PubMed] [Google Scholar]
- Lundberg et al., 1987a.Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 1. Distribution and linkage of reflex actions to alpha-motoneurones. Exp Brain Res 65: 271–281, 1987a [DOI] [PubMed] [Google Scholar]
- Lundberg et al., 1987b.Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 3. Secondary spindle afferents and the FRA: a new hypothesis. Exp Brain Res 65: 294–306, 1987b [DOI] [PubMed] [Google Scholar]
- Lundberg and Weight, 1970.Lundberg A, Weight F. Signalling of reciprocal Ia inhibition by the ventral spinocerebellar tract. Brain Res 23: 109–111, 1970 [DOI] [PubMed] [Google Scholar]
- Lundberg and Weight, 1971.Lundberg A, Weight F. Functional organization of connexions to the ventral spinocerebellar tract. Exp Brain Res 12: 295–316, 1971 [DOI] [PubMed] [Google Scholar]
- Oscarsson, 1973.Oscarsson O. Functional organization of spinocerebellar paths. In: Handbook of Sensory Physiology, edited by Iggo I. Berlin: Springer Verlag, 1973, p. 339–380 [Google Scholar]
- Roberts and Smith, 1973.Roberts WJ, Smith DO. Analysis of threshold currents during microstimulation of fibres in the spinal cord. Acta Physiol Scand 89: 384–394, 1973 [DOI] [PubMed] [Google Scholar]
- Shinoda et al., 1979.Shinoda Y, Zarzecki P, Asanuma H. Spinal branching of pyramidal tract neurons in the monkey. Exp Brain Res 34: 59–72, 1979 [DOI] [PubMed] [Google Scholar]
- Smith, 1980a.Smith DO. Mechanisms of action potential propagation failure at sites of axon branching in the crayfish. J Physiol 301: 243–259, 1980a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, 1980b.Smith DO. Morphological aspects of the safety factor for action potential propagation at axon branch points in the crayfish. J Physiol 301: 261–269, 1980b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer et al., 1976.Stauffer EK, Watt DG, Taylor A, Reinking RM, Stuart DG. Analysis of muscle receptor connections by spike-triggered averaging. II. Spindle group II afferents. J Neurophysiol 39: 1393–1402, 1976 [DOI] [PubMed] [Google Scholar]
- ten Bruggencate and Lundberg, 1974.ten Bruggencate GT, Lundberg A. Facilitatory interaction in transmission to motoneurones from vestibulospinal fibres and contralateral primary afferents. Exp Brain Res 19: 248–270, 1974 [DOI] [PubMed] [Google Scholar]
- Vanderhorst and Holstege, 1997.Vanderhorst VG, Holstege G. Organization of lumbosacral motoneuronal cell groups innervating hindlimb, pelvic floor, and axial muscles in the cat. J Comp Neurol 382: 46–76, 1997 [PubMed] [Google Scholar]