Abstract
Frontotemporal lobar degeneration (FTLD) often presents with asymmetric atrophy. We assessed whether premorbid occupations in FTLD patients were associated with these hemispheric asymmetries. In a multi-center chart review of 588 patients, occupation information was related to location of tissue loss or dysfunction. Patients with atrophy lateralized to the right had professions more dependent on verbal abilities than patients with left-lateralized or symmetrical atrophy. In a subgroup of 96 well-characterized patients with quantified neuroimaging data, the lateralization effect was localized to the temporal lobes and included verbal and mathematical ability. Patients whose professions placed high demands on language and mathematics had relatively preserved left temporal relative to right temporal volumes. Thus, occupation selection occurring in early adulthood is related to lateralized brain asymmetry in patients who develop FTLD decades later in the relatively deficient hemisphere. The finding suggests that verbal and mathematical occupations may have been pursued due to developmental right-lateralized functional impairment that precedes the neurodegenerative process. Alternatively, long-term engagement of activities associated with these occupations contributed to left-lateralized reserve, right-lateralized dysfunction, or both.
Keywords: Frontotemporal dementia, laterality, reserve
Introduction
Predisposition to dementia may be expressed prior to clinical symptomology, with significant implications for diagnosis and treatment. In Alzheimer’s Disease (AD), predisposition can be predicted decades prior to clinical manifestation from analysis of diary writings (Snowdon et al., 1996). Additionally, prodromal signs can be observed in the form of mild cognitive impairment years before those patients convert to dementia (Petersen et al., 2001). In keeping with the theory of cognitive reserve (Stern, 2006), certain life experiences have been attributed to protective effects that forestall the symptoms of dementia despite an ongoing degenerative process. The expression of AD may be attenuated by years of education (Stern et al., 1994), whereby individuals with higher levels of education present with few or no symptoms of Alzheimer’s despite levels of postmortem pathology that are similar in severity to those seen in lower education individuals who are symptomatic (Roe, Xiong, Miller, & Morris, 2007). Higher occupational attainment is also associated with reserve capacity in the attenuation of AD symptoms (Stern et al., 1994). Additionally, there is evidence for an association between specific occupational factors (interpersonal skills, physical demands) and Alzheimer’s-related parietal regional cerebral blood flow (Stern et al., 1995), further supporting the theory of cognitive reserve.
Frontotemporal lobar degeneration (FTLD) is as common a cause of dementia as AD in people under 65 years of age (Knopman, Petersen, Edland, Cha, & Rocca, 2004; Ratnavalli, Brayne, Dawson, & Hodges, 2002). This disease is characterized by either (a) early and progressive change in personality, characterized by difficulty in modulating behavior, often resulting in inappropriate responses or activities, or (b) early and progressive change in language, characterized by problems with expression of language or severe naming difficulty and problems with word meaning (McKhann et al., 2001). Atrophy in FTLD often begins asymmetrically, with the cognitive and behavioural changes associated with the lateralized origin of atrophy (Boone et al., 1999; Edwards-Lee et al., 1997; Thompson, Patterson, & Hodges, 2003).
There is a high degree of variability in the clinical manifestations of FTLD, dependent upon origin of the hemispheric degeneration, the extent of disease progression, and individual differences that may relate to cognitive reserve and cognitive style. Furthermore, the FTLD phenotype may manifest early in life, with one study reporting that healthy individuals carrying tau gene mutations were impaired on tests sensitive to frontal lobe function decades prior to potential onset of the disease (Geschwind et al., 2001). In support of cognitive reserve in FTLD patients, inverse relationships between years of education and job skill level with frontal pathology, as measured by regional cerebral metabolic rate and regional cerebral blood flow, have been observed (Perneczky, Diehl-Schmid, Drzezga, & Kurz, 2007; Borroni et al., 2009). There is some evidence to suggest a relationship between pre-symptomatic abilities and laterality of degeneration in FTLD. In a small case series, verbal learning disabilities were noted to be elevated in patients who later developed primary progressive aphasia, a subtype of FTLD with left-lateralized degeneration (Mesulam & Weintraub, 1992).
Case studies have described FLTD patients who chose professions dependent upon the activity of one hemisphere and eventually developed atrophy that was greatest in the contralateral hemisphere. Alajouanine (1948) reported a case of progressive cerebral atrophy with a progressive non-fluent aphasia in the composer Maurice Ravel. More recently, visual artists and musicians, individuals with professions that are heavily dependent on the right hemisphere, have been reported with aphasia due to left temporal atrophy (Mell, Howard, & Miller, 2003; Miller, Boone, Cummings, Read, & Mishkin, 2000; Seeley et al., 2008). These case studies suggest that FTLD patients who are highly skilled in music or the visual arts may have a propensity to left hemisphere degeneration, either due to premorbid brain vulnerability or to long-term effects of prolonged mental activity. In this study, we assessed whether this association would generalize to a large sample of FTLD patients whose occupations engaged varying degrees of capacity in different skill sets.
We assembled occupation and neuroimaging data from a sample of 588 FTLD patients from nine neurology clinics specialized in the diagnosis of this condition. The patients’ occupations were coded according to a standard database (United States Department of Labor, 2006) containing detailed information regarding the attributes of each occupation, reduced via principle component analysis to verbal, physical, mechanical, mathematic, and visuospatial components. We examined the relationship between these occupation attributes and localized brain abnormalities in two analyses. The first used visual ratings of relative atrophy or hypometabolism in all 588 FLTD patients and the second used quantitative cerebral volumes in a subset of 96 FTLD patients with high quality structural neuroimaging data and more detailed dementia severity information.
Methods
Participants
Chart reviews were conducted for 812 patients diagnosed with FTLD at dementia clinics specializing in FTLD assessment and research. Inclusion criteria were composed of a diagnosis of FTLD following the criteria of Neary and colleagues (1998), a primary occupation outside of the home, and abnormal findings on structural and/or functional diagnostic neuroimaging. One hundred and three patients were excluded due to the absence of occupation data, where no career was coded at intake, the patient was a homemaker. Patients who served in the military as the primary occupation were excluded because the United States Department of Labor Standard Occupational Classification Network (O*Net; United States Department of Labor, 2006) does not collect data on military occupations. An additional 121 patients were excluded due to the absence of neuroimaging data or failure to detect any abnormalities on diagnostic imaging.
Five-hundred eighty-eight patients (354 males) were included in this study (133 were contributed from the UCSF Memory & Aging Center; 107, Mayo Clinic, Jacksonville; 102, MRC Cognition & Brain Sciences Unit, Cambridge; 87, Department of Psychiatry of the Technische Universität München; 44, University of Texas Southwestern Medical Center; 39, Rancho Los Amigos/USC Alzheimer’s Disease Center, Los Angeles; 39, Sunnybrook Health Sciences Centre, Toronto; 24, West Los Angeles VA Medical Center; 13, Baycrest Centre, Toronto). Of the sample, 303 were diagnosed with frontotemporal dementia, 120 with primary progressive (non-fluent) aphasia, and 142 with semantic dementia (Neary et al., 1998). An additional 23 patients with disorders that are part of the spectrum of FTLD (McKhann et al., 2001) were studied, including 12 with a primary diagnosis of progressive supranuclear palsy, 8 with corticobasal degeneration, and 3 with Amyotrophic Lateral Sclerosis (ALS) with FTLD. Of these 588 patients, 32 had died and had autopsy-confirmation of pathology consistent with FTLD, including ubiquitin-positive, tau-negative inclusions with or without degeneration of the motor neurons, or tau-positive Pick bodies, or tau-positive inclusions associated with related disorders (progressive supranuclear palsy or cortical basal degeneration), or dementia lacking distinctive histology.
The charts of 30 patients did not indicate the number of years of education. To avoid exclusion of these cases due to listwise deletion in statistical analyses, these missing data values were replaced with the typical number of years of education for each respective profession as indicated by the O*Net database (United States Department of Labor, 2006). To confirm that this data replacement did not bias the results, we repeated the analyses excluding patients without education data. Because this did not significantly affect the results, we present data from the full sample. Four hundred forty-eight patients were right-handed, 37 left-handed, six ambidextrous; 97 charts contained no handedness information. In a preliminary analysis, we included handedness as a covariate in a subsample of 491 patients. Handedness was not a significant covariate, nor did its inclusion significantly affect the results. As such, we present data from the full sample without handedness as a covariate.
Data from 96 of the UCSF patients who had undergone high-resolution structural neuroimaging were subjected to more in-depth analyses. This sample, while smaller than the above sample, afforded the advantages of uniformly quantified neuroimaging, multiple measures of disease severity (disease duration, clinically-rated severity with the Clinical Dementia Rating scale, (CDR; Morris, 1993), and global atrophy) and a comparison group of matched AD patients from the same clinic. 42 of these 96 patients were diagnosed with frontotemporal dementia, 14 with primary progressive (non-fluent) aphasia, and 20 with semantic dementia (Neary et al., 1998). Eleven additional patients were included with a primary diagnosis of progressive supranuclear palsy, six with corticobasal degeneration, and three with ALS with FTLD. The duration of illness, calculated as the difference between age at MRI and clinically determined age of onset, was available for 86 patients. Dementia severity, measured by the CDR scale (Morris, 1993) within three days of acquiring brain imaging, was available for 89 patients. These data, along with demographic data, are presented in Table 1. As a comparison group, 30 patients diagnosed with probable AD according to the NINCDS-ADRDA criteria (McKhann et al., 1984) were included. These patients were comparable in age and years of education to the FTLD group (see Table 2). Structural neuroimaging data from 37 healthy comparison subjects, matched in age and education to the patients (see Table 2) was used to calculate the degree of atrophy. All patients and healthy controls were right handed.
Table 1.
Patient Group Means and Standard Deviations for Demographic and Neuropsychological Data
| Left Temporal |
Right Temporal |
Left Frontal |
Right Frontal |
Alzheimer’s |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean |
SD |
n |
Mean |
SD |
n |
Mean |
SD |
n |
Mean |
SD |
n |
Mean |
SD |
n |
|
| Age | 62.66 | 8.49 | 32 | 61.00 | 6.04 | 24 | 61.17 | 10.05 | 23 | 61.41 | 9.14 | 17 | 62.17 | 10.62 | 30 |
| Education | 16.44 | 3.32 | 32 | 15.71 | 2.66 | 24 | 16.04 | 2.23 | 23 | 15.47 | 2.45 | 17 | 15.10 | 3.88 | 30 |
| Onset Age | 59.33 | 9.48 | 26 | 55.76 | 6.65 | 23 | 57.33 | 10.34 | 21 | 57.82 | 10.43 | 16 | 57.54 | 10.52 | 24 |
| Duration | 4.12 | 3.10 | 26 | 5.48 | 3.62 | 23 | 4.71 | 3.73 | 21 | 3.69 | 1.78 | 16 | 5.17 | 3.83 | 24 |
| CDR | .77 | .50 | 31 | 1.21 | .73 | 21 | .60 | .34 | 21 | .78 | .41 | 16 | .95 | .48 | 30 |
| Global Atrophy | −1.05 | 0.97 | 32 | −1.38 | 0.89 | 24 | −1.25 | 1.01 | 23 | −1.72 | 1.04 | 17 | −1.42 | 0.88 | 30 |
| Gender(M/F) | 20/12 | 13/11 | 10/13 | 12/5 | 16/14 | ||||||||||
Table 2.
Age and Education by Gender for Healthy Controls, FTLD and AD Patients
| Healthy Controls |
FTLD |
Alzheimer’s Disease |
|||||
|---|---|---|---|---|---|---|---|
| Mean |
SD |
Mean |
SD |
Mean |
SD |
||
| Male | Age | 63.91 | 10.31 | 61.73 | 8.84 | 66.06 | 11.38 |
| Education | 17.27 | 2.10 | 16.56 | 2.67 | 15.93 | 4.86 | |
| N | 22 | 55 | 16 | ||||
| Female | Age | 64.00 | 8.64 | 61.59 | 7.84 | 57.71 | 7.87 |
| Education | 15.60 | 1.76 | 15.22 | 2.72 | 14.14 | 2.11 | |
| N | 15 | 41 | 14 | ||||
Coding of occupation and derivation of occupation component scores
Patients’ occupations were coded according to O*Net, the United States Department of Labor Standard Occupational Classification Network (United States Department of Labor, 2006). This database provides a classification for all workers into one of over 796 occupations according to their occupational definition. For patients engaged in more than one occupation, the occupation with the longest duration was included in the analysis, consistent with most studies of occupation and dementia (e.g., Helmer et al., 2001; Potter, Plassman, Helms, Foster, & Edwards, 2006; Stern et al., 1995; Sutedja et al., 2007).
O*Net offers a common language for communication across the diversity of occupations with definitions and concepts for describing worker attributes and job characteristics that are broadly understood, easily accepted, and applied in many industrialized countries. An alternative international standard occupation classification system (the ISCO-88) was ill-suited for comparing occupation characteristics across occupations. While a cultural bias in occupation attributes due to our use of the O*Net cannot be ruled out, we have no basis to expect this from patients attending academic dementia clinics in Canada, Germany, the United Kingdom and the United States.
O*Net contains 128 descriptor variables concerning abilities, skills, and general work activities comparable across all occupations derived from surveys of workers and job analysts. We used principle component analysis to reduce the 128 variables to composite scores describing broad characteristics of occupations that were in turn related to the degree of hemispheric abnormalities in FLTD patients (see below). A principle component analysis was conducted on the occupation ratings variables with all 796 O*Net occupations treated as “participants”. A scree test (Cattell, 1966) revealed that the sixth component was nearly indistinguishable in slope from the subsequent components, supporting an interpretation of a five-component solution. The five components account for approximately 70% of the variance. As such, five components were retained and an orthogonal rotation was then performed to maximize the variance of the squared loadings within the components. The five components comprised verbal (accompanied by social capacities and general intellectual demands), physical, mechanical, mathematical, and visuospatial capacities (see Supplemental Table 1). On the basis of component saturation
(the absolute magnitude of the loadings), the five components are considered a stable representation of the population parameter (Guadagnoli & Velicer, 1988; Stevens, 2002).
The five components derived from the principal component analysis are largely consistent with the Dictionary of Occupational Titles (US Employment Service, 1991) factor analyses of worker functions and worker characteristics that has also been used in studies of dementia (Cain, 1981; Link, 1993; Potter et al., 2006; Smyth et al., 2004; Stern et al., 1994). One major difference between the Dictionary of Occupational Titles factor scores is the division of mental, managerial and interpersonal factors. The present analysis combines these attributes along the common thread of verbal behavior. Although our verbal component contains items related to social capacities, these have a common pathway through verbal behavior. In the workplace, occupations with high verbal demands as characterized by O*Net (e.g., administration) are inherently interpersonal, and vice versa. Accordingly, occupations rated low on this component (e.g., manufacturing worker) have little or no interpersonal requirements (see Supplemental Table 2) Both analyses also found a physical component, although the present analysis does not distinguish the physical from the motor (e.g., Link, 1993). The mathematical component was unique to the component structure of occupations from the O*Net database. Overall, the commonalities between the analyses are remarkable considering the component scores were derived from a set of data that differed in the number and type of descriptor variables, method of measurement, and occupation classification.
In order to calculate the component scores for each occupation, each of the 128 occupation attributes were multiplied by the corresponding loading and summed for each of the five components. These values were then standardized, each with a mean of zero and standard deviation of one. Scaling of the computed scores was such that higher values indicated greater levels of engagement in the parameter. As a result, each patient had five occupation scores, reflecting each of the occupational dimensions. Examples of the occupational scores that were highest and lowest for each of the five components can be found in Supplemental Tables 2A and 2B.
Imaging
In order to estimate the degree of imaging abnormalities for the larger sample of patients, imaging data were derived from different imaging platforms depending on availability in this multi-centre study, including SPECT, fluoro-deoxy-glucose PET, or structural MRI. While different platforms and analysis methods are differentially sensitive to pathology and may have increased the noise in the data, there is no reason to expect that the combination of such methods would produce a systematic bias into the investigation of the relationship between occupation and brain imaging data.
Degeneration was coded from the earliest scan in which abnormalities were detected as included in radiologists’ reports where available (n = 455), as rated by a neurologist with expertise in FTLD (n = 37), or, for those patients included in the second set of analyses (n = 96; see below), volumetric measures of MRIs. All coding was accomplished blind to occupation data. Patients were classified according to location of greatest abnormality in terms of hemisphere and lobe (right, left, frontal, temporal). When abnormalities were bilateral, but with evidence of asymmetry, this was reflected in the coding (e.g., if a patient was characterized with frontal atrophy, left greater than right, they were coded as left frontal). When atrophy was judged to be symmetrical across hemisphere or lobes (e.g., bifrontal atrophy), patients were classified as showing bilateral atrophy. Although this method is coarse, it was the only way to harmonize the imaging data for the present study across centers. Two-hundred seventy-three patients were coded as having left-lateralized degeneration, 122 right and 193 bilateral. These same patients were also rated on lobe of degeneration: 229 patients were classified as frontal, 210 as temporal, and 149 as frontotemporal. There were no differences in gender or years of education between these groups (all p’s > .30).
For the subset of 96 UCSF patients, AD patients, and healthy comparison subjects, MRI scans were acquired from a 1.5-T Magnetom VISION system (Siemens Inc., Iselin, NJ) equipped with a standard quadrature head coil. Sequences of the structural MRI included: (i) 2D FLASH MRI along three orthogonal directions, 3mm slices, approximately 15 slices in each direction to acquire scout views of the brain for positioning subsequent MRI slices, (ii) A double spin echo sequence [repetition time/echo time 1/echo time 2 (TR/TE1/TE2) = 5000/20/80 ms] to acquire proton density and T2-weighted MRIs, 51 contiguous axial slices (3 mm) covering the entire brain and angulated −10° from the AC-PC line; 1.0 × 1.25 mm2 in-plane resolution, (iii) Volumetric magnetization prepared rapid gradient echo MRI [MPRAGE, repetition time/echo time/inversion time (TR/TE/TI) = 10/4/300 ms] to obtain T1-weighted images of the entire brain, 15° flip angle, coronal orientation perpendicular to the double spin echo sequence, 1.0 × 1.0 mm2 in-plane resolution and 1.5 mm slab thickness.
Images were processed using the BRAINS2 software package using a standard algorithm to produce lobar volumes (Magnotta et al., 2002). The T1-weighted images were spatially normalized and resampled to 1.0 mm3 voxels so that the anterior-posterior axis of the brain was realigned parallel to the anterior commissure-posterior commissure line and the inter-hemispheric fissure aligned on the other two axes. Next, the outermost boundaries of the cortex, as well as the anterior commissure and posterior commissure, identified in order to warp the Talairach grid (Talairach & Tournoux, 1988) onto the current brain. The T2- and PD-weighted images were then realigned to the spatially normalized T1-weighted image using an automated image registration program (Woods, Cherry, & Mazziotta, 1992). The resampled images were then segmented into grey matter, white matter, and CSF using the co-registered images and a discriminate analysis method based on automated training class selection (Harris et al., 1999). This tissue classification algorithm uses a Bayesian classifier based on discriminate analysis in order to reduce the variability in signal intensity across individual image sets and correct for partial voluming. This step requires the manual tracing of venous blood and is subsequently able to perform “plug” selection for grey matter, white matter, and cerebrospinal fluid automatically. Lobar volumes are calculated using an automated Talairach-based method of regional classification that designates individual brain voxels as belonging to a particular lobe based on their location within this standardized space (Harris et al., 1999; Magnotta et al., 2002). This method of lobar classification in Tailarach space has been validated for use in atrophied brains (Krueger et al., 2009; Magnotta et al., 2002). Total brain volumes were corrected for head size using the total intercranial volume (Arndt, Cohen, Alliger, Swayze, & Andreasen, 1991), and then converted to z-scores based on the mean and standard deviation derived from healthy controls. Measures of total brain volume are reported as standardized scores, where lower scores are indicative of greater atrophy. Site of greatest atrophy (left frontal, right frontal, left temporal, right temporal) was determined to be the lobe with the largest z-score deviation from normal healthy age and education matched healthy adults. Thus, we were able to identify the specific region of greatest atrophy in each patient. The ability to assign patients to discrete groups according to atrophy reflected a major advantage afforded by the precision of quantified MRI data over and above the clinical ratings as done for the larger sample.
There were no significant differences in age, years of education, age of onset, duration of dementia, or degree of global atrophy (all p’s > .15; see Table 2) between any of the regional imaging abnormality groups. Differences were found among groups for dementia severity, F (4, 114) = 5.21; p < .001. The left frontal group had significantly lower CDR scores than the right temporal, right frontal and AD patient groups, and the left temporal patients had significantly lower CDR scores than the right temporal and AD patient groups. These effects likely reflect the earlier clinical presentation of patients with language deficits due to left-lateralized degeneration (Rosen et al., 2004).
Statistical analysis
Multivariate analysis of covariance (MANCOVA) was conducted to determine the association between site of greatest imaging abnormality and occupation component scores while statistically controlling for potentially confounding factors (for the larger sample, differences in gender and education, for the smaller sample, differences in gender, education, and CDR scores). Follow-up comparisons for occupation scores across groups were adjusted for multiple comparisons with Tukey’s Least Significant Difference (LSD) at the .05 level.
Results
As seen in Figure 1, verbal scores for patients with right-lateralized degeneration were higher than for patients with left- and bilateral degeneration, which were not different from each other. The reliability of these findings was supported by a main effect of laterality on verbal occupation scores adjusted for gender and years of education (F (2, 577) = 4.95; p < .01). Across occupation scores, the omnibus MANCOVA statistic showed a trend towards significance for a main effect of laterality (Wilks’ Lambda F (10, 1146) = 1.59; p = .10) but there were no effects of lobe or laterality by lobe interactions. There were no significant effects involving other occupation component scores. See Supplemental Tables 3 and 4 for occupation scores by laterality and region.
Figure 1.
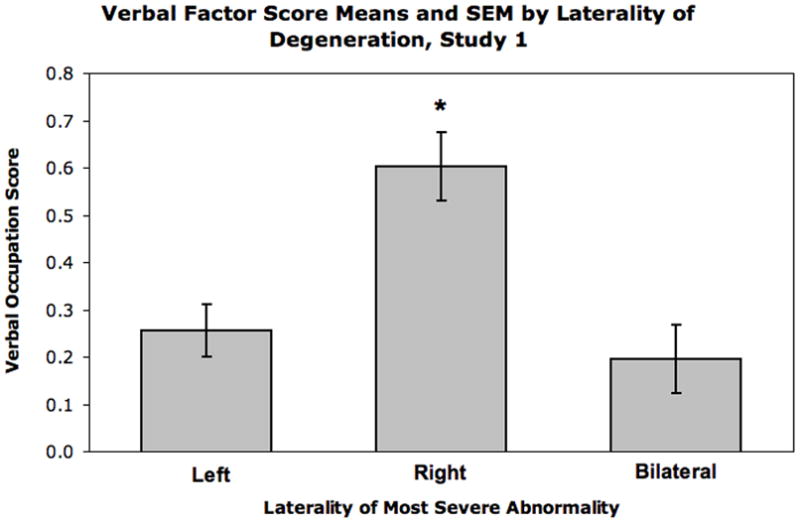
Verbal occupation component scores are significantly higher for the right lateralized group than the left and bilateral group scores (which were not different from each other).
There were significant effects of gender and years of education (Wilks’ Lambda F’s (5, 573) = 20.25 and 27.19, respectively; p’s < .001). Men had significantly higher physical scores, mechanical scores, and mathematical scores, t’s (586) = −3.97, −3.88, and −8.20, respectively, p ‘s < .001). Years of education positively correlated with verbal scores and mathematical scores, r’s (586) = .41 and .13, p’s < .001 and .01, respectively). Physical scores were negatively associated with years of education (r (586) = −.17, p < .001).
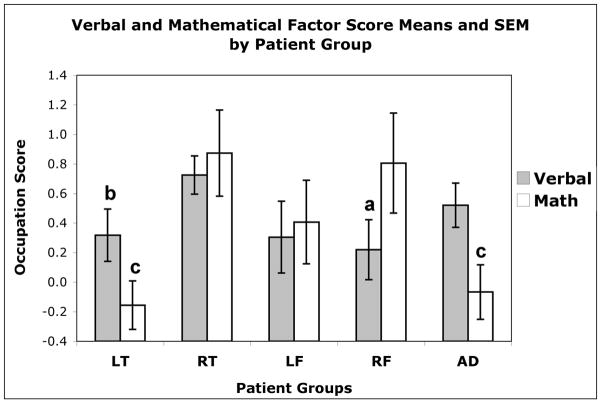
Whereas the analysis of the larger sample revealed lateralization but not lobar effects, the analysis of the smaller sample, where regional changes were more accurately measured, revealed specific lobar effects. For the MANCOVA omnibus test, there was a significant effect of group (i.e. left frontal, right frontal, left temporal, right temporal, AD) when controlling for the influence of gender, years of education and dementia severity (Wilks’ Lambda F (20, 356) = 1.91; p < .01; Table 3). The effect of verbal occupation scores approached significance, F (4, 111) = 2.25; p < .07. There was a significant effect for mathematical scores, F (4, 111) = 4.27; p < .01. As seen in Figure 2, patients with atrophy that was most severe in the left temporal lobe had significantly lower verbal and mathematical occupation scores than patients with atrophy originating in the right temporal lobe. Their verbal occupation scores were lower than for patients with AD. AD patients shared low mathematical scores with the left temporal group. Significant effects were also noted for patients with right frontal lobe atrophy, who had lower verbal scores than the right temporal atrophy group, but shared the right temporal group’s advantage in mathematical scores over the left temporal and AD groups.
Table 3.
Occupation Factor Scores by Patient Group
| Left Temporal |
Right Temporal |
Left Frontal |
Right Frontal |
Alzheimer’s |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean |
SD |
Mean |
SD |
Mean |
SD |
Mean |
SD |
Mean |
SD |
|
| Verbal | 0.31 | 1.00 | 0.72 | 0.63 | 0.25 | 1.14 | 0.22 | 0.85 | 0.53 | 0.82 |
| Physical | −0.46 | 0.51 | −0.51 | 0.64 | −0.37 | 0.51 | −0.39 | 0.64 | −0.08 | 1.03 |
| Mechanical | −0.69 | 0.72 | −0.04 | 0.98 | −0.41 | 0.82 | −0.24 | 0.81 | −0.25 | 1.01 |
| Mathematical | −0.17 | 0.94 | 0.88 | 1.41 | 0.36 | 1.23 | 0.85 | 1.38 | −0.03 | 0.99 |
| Visuospatial | −0.21 | 0.75 | −0.15 | 0.80 | 0.10 | 1.08 | −0.04 | 0.68 | 0.09 | 0.99 |
Figure 2.
FTLD patient groups indicate region with greatest abnormality. LT = Left temporal lobe; RT = Right temporal lobe; LF = Left frontal lobe; RF = Right frontal lobe; AD = Alzheimer’s Disease.
a Lower than RT
b Lower than RT, AD
c Lower than RF, RT
These effects were significant after controlling for gender, education, and dementia severity, Wilks’ Lambda F’s (5, 107) = 5.44, 13.74, and 2.85 respectively; p’s < .001, .001, and .05, respectively. The gender effect was due to higher mathematical scores for men than women (t(130) = 3.55, p < .001). Years of education positively correlated with verbal occupation scores (r(130) = .44, p < .001) and negatively correlated with physical scores (r(130) = −.24, p < .01). Dementia severity was positively correlated with mechanical workmanship (r(126) = .27, p < .01) and negatively with years of education (r(126) = −.32, p < .001).
Discussion
Occupation selection provides a unique view into cognitive style and practice of long-term behaviors predating the onset of symptoms. In this study, we used quantitatively derived occupation scores to measure long-term engagement in specific cognitive activities. We then related these occupation scores to relative degree of hemispheric and lobar pathology in subsequently developing FTLD. Two analyses were conducted. The first used clinically-derived estimates of the site of most severe abnormality from heterogeneous imaging platforms that permitted assessment in a large sample of patients. The second was conducted in a subset of patients whose regional cerebral volumes were quantitatively measured. Although smaller, this sample permitted additional analysis of disease progression factors as well as comparison with a group of AD patients.
Both analyses demonstrated an association between verbal occupation attributes and the site of most severe pathology based on imaging: FTLD patients with right-lateralized degeneration engaged in occupations more reliant on verbal abilities than patients with left-lateralized degeneration. The quantitative neuroimaging data available for the second analysis allowed for more precise localization of this effect to the temporal lobe, accompanied by a similar effect for mathematical occupations. The right temporal atrophy patients were drawn towards occupations that placed demands on verbal and mathematical ability, managerial positions and complex problem solving, while left temporal patients were drawn away from such professions, or they may not have been promoted to positions requiring high verbal and mathematical skills (e.g., Schooler, Mulatu, & Oates, 1999). For verbal professions, the left temporal group’s disadvantage was robust when they were compared with the AD group, although this was not the case for mathematical occupations.
This analysis also revealed effects specific to patients who had a predominance of right frontal atrophy, who were similar to the right temporal group in their bias towards mathematical occupations, but dissociated from the right temporal group in that they tended to select occupations with low verbal attributes. This suggests that the lateralized effect observed in the first set of analyses may not generalize to the frontal lobe, at least for verbal occupations.
Considering the numerous intervening variables likely to determine occupation selection and lateralization effects in FTLD, it is striking that statistically significant effects emerged. The influence of occupational engagement is likely to be distributed throughout the brain, with lateralized specialization contributing to the some cognitive components. We emphasize the lateralized effects that were consistent across the two analyses. These convergent effects are not attributable to the inclusion of the subset of 96 well-characterized patients in the larger sample. An ancillary analysis of the larger sample excluding these patients did not significantly alter the pattern of results. Furthermore, results from both analyses indicate that differential effects of gender, years of education, age, disease duration, global atrophy, and symptom severity across groups cannot explain our effects.
Our findings that patients developed degeneration contralateral to the hemisphere putatively supporting their occupational skills are consistent with the pattern of findings from artists with left-lateralized volume loss due to FTLD (Miller et al., 2000). We did not observe elevated visuospatial scores in patients with left-lateralized damage specifically, however (although all five visual artists in our sample had left-lateralized atrophy), possibly due to the heterogeneity of functionally localizable skills among artistic professions, their low prevalence in the overall sample, or the poor characterization of artistic professions by the occupation components. Unlike the previous studies involving artists (e.g. Mell, Howard, & Miller, 2003; Miller, Boone, Cummings, Read, & Mishkin, 2000; Seeley et al., 2008), the present work demonstrates an association between region of most severe pathology and occupation in more prevalent, less specialized careers.
While functional localization at the lobar level is coarse by contemporary standards, occupational attributes as defined here may not demonstrate a finer grain of functional localization. The association of verbal occupation attributes with the left temporal neocortex is consistent with this region’s specificity to phonological linguistic operations (Lamdon Ralph et al., 2001). Mathematical occupation attributes were also associated with the left temporal neocortex. Indeed individuals with high mathematical competence may rely on linguistic representations to attain high arithmetic precision (Grabner et al., 2007; Dehaene, Spelke, Pinel, Stanescu, & Tsivkin, 1999). Furthermore, early left hemisphere dysfunction has been associated with verbal and mathematical problem-solving difficulties (Hynd, Semrud-Clikeman, Lorys, Novey, & Eliopulos, 1990; Isaacs, Edmonds, Lucas, & Gadian, 2001; Larsen, Hoien, Lundberg, & Odegaard, 1990).
It is unclear whether the findings are specific to FTLD neuropathology or whether they may generalize to asymmetric temporal lobe damage due to other etiologies. In order to test this, a sample of patients capable of engaging the workforce for 20+ years with progressive unilateral disease other than FTLD would be needed. This may be possible to assess in patients with unilateral AD. Our sample of AD patients, however, had relatively symmetrical changes.
The finding of an association between occupation and regions of most severe pathology are differentiated from those related to prodromal signs (Snowdon et al., 1996) or mild cognitive impairment preceding AD (Petersen et al., 2001), as indicated by the relative remoteness of occupational engagement. Rather, these findings suggest that occupational activities within a normal spectrum of behavior may relate to factors that ultimately influence the regions most affected in neurodegenerative disease. The determinants of lateralization of neurodegeneration in FTLD are unclear (Geschwind & Miller, 2001; Kertesz et al., 2000). Genetic influences may contribute to selective vulnerability, susceptibility to pathology resulting from an unknown early neurological insult or, possibly, cognitive style; yet individuals with similar genotypes do not necessarily develop the same lateralization of degeneration in FTLD (Kertesz et al., 2000).
An association between most severe pathology and occupation attributes may reflect a causal effect of occupation on lateralized brain degeneration, a premorbid bias towards occupations with certain characteristics among those vulnerable to FLTD, or an interaction of the two. Long-term practice effects of verbal behavior in the course of an occupation may offer neural protection to the left hemisphere by building reserve status. More generally, cognitive performance spanning decades may strengthen resistance to pathology within the supporting neuroanatomy, thereby building localizable neurocognitive reserve. Functional reorganization may extend across cortical representations reflecting patterns of work activity as observed with highly skilled musicians (Pantev et al., 2003) and taxi drivers (Maguire et al., 2000).
Alternately, occupational selection may be optimized to cognitive and physical predispositions, including, in the case of highly verbal and mathematical occupations, incipient right temporal dysfunction. The evidence from artists (Mell et al., 2003; Miller et al., 2000; Seeley et al., 2008) and from the present study suggests an enhancement of function associated with FTLD, especially in the temporal lobe. This process may be indicative of ‘compensatory augmentation’ (Kapur, 1996) by which left-lateralized functions excel in the context of reduced competition/interference from the right hemisphere. Accordingly, patients with primary progressive aphasia also had reading, spelling and arithmetic difficulties as children (Mesulam & Weintraub, 1992). These findings indicate a “tardative expression of a genetic or acquired vulnerability focused upon the left hemisphere language network” (Mesulam & Weintraub, 1992). This vulnerability could interact with other factors, such as the developmental organization of large-scale brain networks that are associated with vulnerability to disease (Seeley, Crawford, Shou, Miller & Greicius, 2009), which in turn support occupation attributes, and determine a site of least resistance in an emergent pathological process.
Supplementary Material
Acknowledgments
This study was supported by Canadian Institutes of Health Research (MGP–62963) and the National Institute of Child Health and Human Development (HD42385–01) grants to B. Levine. The authors declare no actual or potential conflicts of interest. All appropriate approval and procedures were followed concerning human subjects.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alajouanine T. Aphasia and artistic realization. Brain. 1948;71:229–241. doi: 10.1093/brain/71.3.229. [DOI] [PubMed] [Google Scholar]
- Arndt S, Cohen G, Alliger RJ, Swayze VW, II, Andreasen NC. Problems with ratio and proportion measures of imaged cerebral structures. Psychiatry Research: Neuroimaging. 1991;40:79–89. doi: 10.1016/0925-4927(91)90031-k. [DOI] [PubMed] [Google Scholar]
- Boone KB, Miller BL, Lee A, Berman N, Sherman D, Stuss DT. Neuropsychological patterns in right versus left frontotemporal dementia. J Int Neuropsychol Soc. 1999;5(7):616–622. doi: 10.1017/s1355617799577047. [DOI] [PubMed] [Google Scholar]
- Borroni B, Premi E, Agosti C, Alberici A, Garibotto V, Bellelli G, Paghera B, Lucchini S, Giubbini R, Perani D, Padovani A. Revisiting brain reserve hypothesis in frontotemporal dementia: evidence from a brain perfusion study. Dementia and Geriatric Cognitive Disorders. 2009;28:130–135. doi: 10.1159/000235575. [DOI] [PubMed] [Google Scholar]
- Cain P. The dictionary of occupational titles as a source of occupational data. American Sociological Review. 1981;46:253–278. [Google Scholar]
- Cattell RB. The scree test for the number of factors. Multivariate Behavioral Research. 1966;1:245–276. doi: 10.1207/s15327906mbr0102_10. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Spelke E, Pinel P, Stanescu R, Tsivkin S. Sources of mathematical thinking: behavioral and brain-imaging evidence. Science. 1999;284(5416):970–974. doi: 10.1126/science.284.5416.970. [DOI] [PubMed] [Google Scholar]
- Edwards-Lee T, Miller BL, Benson DF, Cummings JL, Russell GL, Boone K, Mena I. The temporal variant of frontotemporal dementia. Brain. 1997;120(Pt 6):1027–1040. doi: 10.1093/brain/120.6.1027. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Miller BL. Molecular approaches to cerebral laterality: development and neurodegeneration. Am J Med Genet. 2001;101(4):370–381. [PubMed] [Google Scholar]
- Geschwind DH, Robidoux J, Alarcon M, Miller BL, Wilhelmsen KC, Cummings JL, Nasreddine ZS. Dementia and neurodevelopmental predisposition: cognitive dysfunction in presymptomatic subjects precedes dementia by decades in frontotemporal dementia. Annals of Neurology. 2001;50(6):741–746. doi: 10.1002/ana.10024. [DOI] [PubMed] [Google Scholar]
- Grabner RH, Ansari D, Reishofer G, Stern E, Ebner F, Neuper C. Individual differences in mathematical competence predict parietal brain activation during mental calculation. Neuroimage. 2007;38(2):346–356. doi: 10.1016/j.neuroimage.2007.07.041. [DOI] [PubMed] [Google Scholar]
- Guadagnoli E, Velicer WF. Relation of sample size to the stability of component patterns. Psychol Bull. 1988;103(2):265–275. doi: 10.1037/0033-2909.103.2.265. [DOI] [PubMed] [Google Scholar]
- Harris G, Andreasen NC, Cizadlo T, Bailey JM, Bockholt HJ, Magnotta VA, Arndt S. Improving tissue classification in MRI: a three-dimensional multispectral discriminant analysis method with automated training class selection. J Comput Assist Tomogr. 1999;23(1):144–154. doi: 10.1097/00004728-199901000-00030. [DOI] [PubMed] [Google Scholar]
- Helmer C, Letenneur L, Rouch I, Richard-Harston S, Barberger-Gateau P, Fabrigoule C, Orgogozo J, Dartigues J. Occupation during life and risk of dementia in French elderly community residents. J Neurol Neurosurg Psychiatry. 2001;71(3):303–309. doi: 10.1136/jnnp.71.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynd GW, Semrud-Clikeman M, Lorys AR, Novey ES, Eliopulos D. Brain morphology in developmental dyslexia and attention deficit disorder/hyperactivity. Arch Neurol. 1990;47(8):919–926. doi: 10.1001/archneur.1990.00530080107018. [DOI] [PubMed] [Google Scholar]
- Isaacs EB, Edmonds CJ, Lucas A, Gadian DG. Calculation difficulties in children of very low birthweight: a neural correlate. Brain. 2001;124(Pt 9):1701–1707. doi: 10.1093/brain/124.9.1701. [DOI] [PubMed] [Google Scholar]
- Kapur N. Paradoxical functional facilitation in brain-behaviour research. A critical review. Brain. 1996;119(Pt 5):1775–1790. doi: 10.1093/brain/119.5.1775. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Kawarai T, Rogaeva E, St George-Hyslop P, Poorkaj P, Bird TD, Munoz DG. Familial frontotemporal dementia with ubiquitin-positive, tau-negative inclusions. Neurology. 2000;54(4):818–827. doi: 10.1212/wnl.54.4.818. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Petersen RC, Edland SD, Cha RH, Rocca WA. The incidence of frontotemporal lobar degeneration in Rochester, Minnesota, 1990 through 1994. Neurology. 2004;62(3):506–508. doi: 10.1212/01.wnl.0000106827.39764.7e. [DOI] [PubMed] [Google Scholar]
- Krueger CE, Dean DL, Rosen HJ, Halabi C, Weiner M, Miller BL, Kramer JH. Longitudinal Rates of Lobar Atrophy in Frontotemporal Dementia, Semantic Dementia, and Alzheimer’s Disease. Alzheimer Dis Assoc Disord. 2009 doi: 10.1097/WAD.0b013e3181a6f101. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, McClelland JL, Patterson K, Galton CJ, Hodges JR. No right to speak? The relationship between object naming and semantic impairment: neuropsychological evidence and a computational model. Journal of Cognitive Neuroscience. 2001;13(3):341–356. doi: 10.1162/08989290151137395. [DOI] [PubMed] [Google Scholar]
- Larsen JP, Hoien T, Lundberg I, Odegaard H. MRI evaluation of the size and symmetry of the planum temporale in adolescents with developmental dyslexia. Brain Lang. 1990;39(2):289–301. doi: 10.1016/0093-934x(90)90015-9. [DOI] [PubMed] [Google Scholar]
- Link BG, Lennon MC, Dohrenwewnd BP. Socioeconomic status and depression: The role of occupations involving direction, control and planning. American Journal of Sociology. 1993;98:1351–1387. [Google Scholar]
- Magnotta VA, Harris G, Andreasen NC, O’Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26(4):251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD. Navigation-related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(8):4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol. 2001;58(11):1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mell JC, Howard SM, Miller BL. Art and the brain: the influence of frontotemporal dementia on an accomplished artist. Neurology. 2003;60(10):1707–1710. doi: 10.1212/01.wnl.0000064164.02891.12. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Weintraub S. Spectrum of primary progressive aphasia. Baillieres Clin Neurol. 1992;1(3):583–609. [PubMed] [Google Scholar]
- Miller BL, Boone K, Cummings JL, Read SL, Mishkin F. Functional correlates of musical and visual ability in frontotemporal dementia. Br J Psychiatry. 2000;176:458–463. doi: 10.1192/bjp.176.5.458. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Pantev C, Ross B, Fujioka T, Trainor LJ, Schulte M, Schulz M. Music and learning-induced cortical plasticity. Ann N Y Acad Sci. 2003;999:438–450. doi: 10.1196/annals.1284.054. [DOI] [PubMed] [Google Scholar]
- Perneczky R, Diehl-Schmid J, Drzezga A, Kurz A. Brain reserve capacity in frontotemporal dementia: a voxel-based 18F-FDG PET study. Eur J Nucl Med Mol Imaging. 2007;34(7):1082–1087. doi: 10.1007/s00259-006-0323-z. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Potter GG, Plassman BL, Helms MJ, Foster SM, Edwards NW. Occupational characteristics and cognitive performance among elderly male twins. Neurology. 2006;67(8):1377–1382. doi: 10.1212/01.wnl.0000240061.51215.ed. [DOI] [PubMed] [Google Scholar]
- Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58(11):1615–1621. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- Roe CM, Xiong C, Miller JP, Morris JC. Education and Alzheimer disease without dementia: support for the cognitive reserve hypothesis. Neurology. 2007;68(3):223–228. doi: 10.1212/01.wnl.0000251303.50459.8a. [DOI] [PubMed] [Google Scholar]
- Roland PE, Friberg L. Localization of cortical areas activated by thinking. J Neurophysiol. 1985;53(5):1219–1243. doi: 10.1152/jn.1985.53.5.1219. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Narvaez JM, Hallam B, Kramer JH, Wyss-Coray C, Gearhart R, Johnson JK, Miller BL. Neuropsychological and functional measures of severity in Alzheimer disease, frontotemporal dementia, and semantic dementia. Alzheimer Dis Assoc Disord. 2004;18(4):202–207. [PubMed] [Google Scholar]
- Schooler C, Mulatu MS, Oates G. The continuing effects of substantively complex work on the intellectual functioning of older workers. Psychol Aging. 1999;14(3):483–506. doi: 10.1037//0882-7974.14.3.483. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Matthews BR, Crawford RK, Gorno-Tempini ML, Foti D, Mackenzie IR, Miller BL. Unravelling Bolero: progressive aphasia, transmodal creativity and the right posterior neocortex. Brain. 2008;131(Pt 1):39–49. doi: 10.1093/brain/awm270. [DOI] [PubMed] [Google Scholar]
- Simon O, Mangin JF, Cohen L, Le Bihan D, Dehaene S. Topographical layout of hand, eye, calculation, and language-related areas in the human parietal lobe. Neuron. 2002;33(3):475–487. doi: 10.1016/s0896-6273(02)00575-5. [DOI] [PubMed] [Google Scholar]
- Smyth KA, Fritsch T, Cook TB, McClendon MJ, Santillan CE, Friedland RP. Worker functions and traits associated with occupations and the development of AD. Neurology. 2004;63(3):498–503. doi: 10.1212/01.wnl.0000133007.87028.09. [DOI] [PubMed] [Google Scholar]
- Snowdon DA, Kemper SJ, Mortimer JA, Greiner LH, Wekstein DR, Markesbery WR. Linguistic ability in early life and cognitive function and Alzheimer’s disease in late life. Findings from the Nun Study.[see comment] JAMA. 1996;275(7):528–532. [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20(3 Suppl 2):S69–74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- Stern Y, Alexander GE, Prohovnik I, Stricks L, Link B, Lennon MC, Mayeux R. Relationship between lifetime occupation and parietal flow: implications for a reserve against Alzheimer’s disease pathology. Neurology. 1995;45(1):55–60. doi: 10.1212/wnl.45.1.55. [DOI] [PubMed] [Google Scholar]
- Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. Jama. 1994;271(13):1004–1010. [PubMed] [Google Scholar]
- Stevens J. Applied multivariate statistics for the social sciences. 4. L. Erlbaum Associates; 2002. [Google Scholar]
- Sutedja NA, Veldink JH, Fischer K, Kromhout H, Wokke JH, Huisman MH, Heederik DJ, Van den Berg LH. Lifetime occupation, education, smoking, and risk of ALS. Neurology. 2007;69(15):1508–1514. doi: 10.1212/01.wnl.0000277463.87361.8c. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical; 1988. [Google Scholar]
- Thompson SA, Patterson K, Hodges JR. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology. 2003;61(9):1196–1203. doi: 10.1212/01.wnl.0000091868.28557.b8. [DOI] [PubMed] [Google Scholar]
- United States Department of Labor, National O*Net Consortium. Occupational information network: O*Net 10.0 database (O*Net-SOC 2006), National Center for O*Net Development [online] 2006 Available at: http://online.O*Netcenter.org/
- United States Employment Service. Dictionary of occupational titles. Washington, DC: U.S. Government Printing Office; 1991. [Google Scholar]
- Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr. 1992;16(4):620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.