Abstract
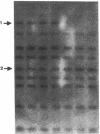
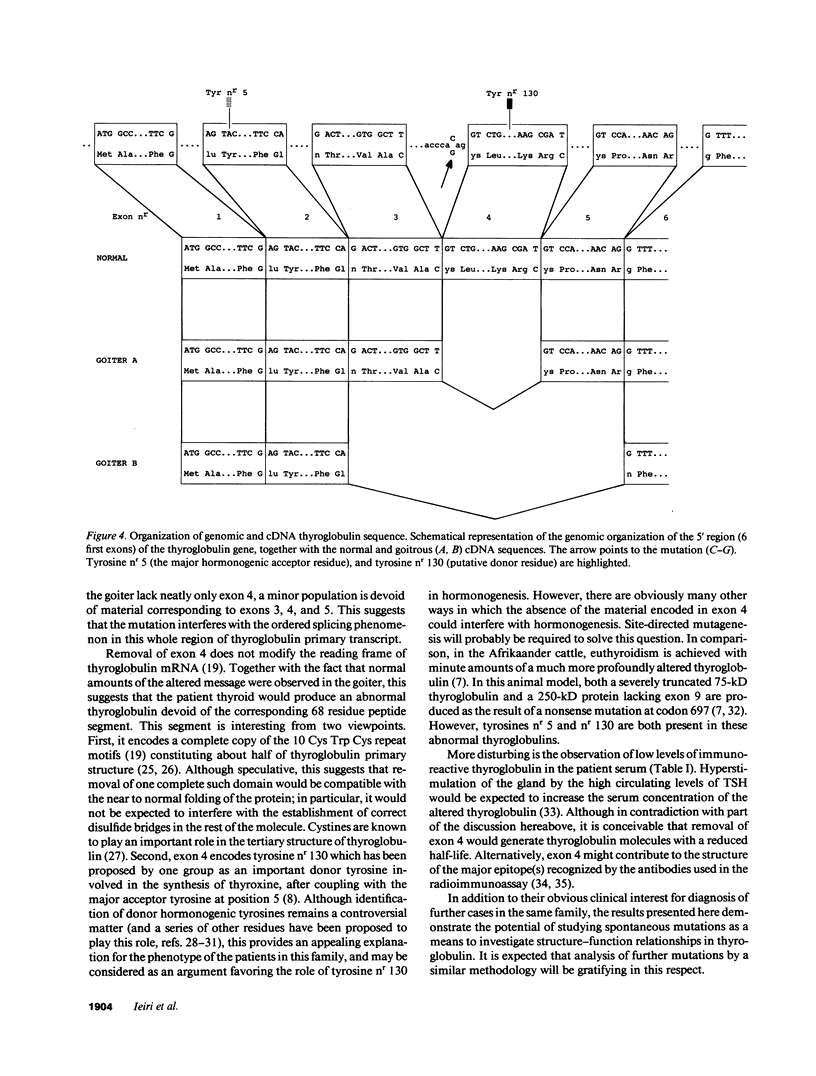
A case of congenital goiter with defective thyroglobulin synthesis has been studied in molecular terms. The patient is the fifth of a kindred of six, three of which have a goiter. The parents are first cousins. Segregation of thyroglobulin alleles in the family was studied by Southern blotting with a probe revealing a diallelic restriction fragment length polymorphism (RFLP). The results demonstrated that the three affected siblings were homozygous for the RFLP. Northern blotting analysis of the goiter RNA with a thyroglobulin probe suggested that thyroglobulin mRNA size was slightly reduced. Polymerase chain reaction amplification of the 8.5-kb thyroglobulin mRNA as overlapping cDNA fragments demonstrated that a 200-bp segment was missing from the 5' region of the goiter mRNA. Subcloning and sequencing of the cDNA fragments, and of the patient genomic DNA amplified from this region, revealed that exon 4 is missing from the major thyroglobulin transcript in the goiter, and that this aberrant splicing is due to a C to G transversion at position minus 3 in the acceptor splice site of intron 3. The presence in exon 4 of a putative donor tyrosine residue (Tyrosine nr 130) involved in thyroid hormone formation provides a coherent explanation to the hypothyroid status of the patient.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baas F., van Ommen G. J., Bikker H., Arnberg A. C., de Vijlder J. J. The human thyroglobulin gene is over 300 kb long and contains introns of up to 64 kb. Nucleic Acids Res. 1986 Jul 11;14(13):5171–5186. doi: 10.1093/nar/14.13.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocas H., Christophe D., Pohl V., Vassart G. Cloning of human thyroglobulin complementary DNA. FEBS Lett. 1982 Jan 25;137(2):189–192. doi: 10.1016/0014-5793(82)80346-3. [DOI] [PubMed] [Google Scholar]
- Chebath J., Chabaud O., Bergé-Lefranc J. L., Cartouzou G., Lissitzky S. Molecular weight of the thyroglobulin messenger RNA of sheep thyroid gland. Biochem Biophys Res Commun. 1977 Nov 7;79(1):267–273. doi: 10.1016/0006-291x(77)90090-0. [DOI] [PubMed] [Google Scholar]
- Dong Q., Ludgate M., Vassart G. Towards an antigenic map of human thyroglobulin: identification of ten epitope-bearing sequences within the primary structure of thyroglobulin. J Endocrinol. 1989 Jul;122(1):169–176. doi: 10.1677/joe.0.1220169. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Henry M., Malthièry Y., Zanelli E., Charvet B. Epitope mapping of human thyroglobulin. Heterogeneous recognition by thyroid pathologic sera. J Immunol. 1990 Dec 1;145(11):3692–3698. [PubMed] [Google Scholar]
- Lamas L., Anderson P. C., Fox J. W., Dunn J. T. Consensus sequences for early iodination and hormonogenesis in human thyroglobulin. J Biol Chem. 1989 Aug 15;264(23):13541–13545. [PubMed] [Google Scholar]
- Lissitzky S., Bismuth J., Codaccioni J. L., Cartouzou G. Congenital goiter with iodoalbumin replacing thyroglobulin and defect of deiodination of iodotyrosines. Serum origin of the thyroid iodoalbumin. J Clin Endocrinol Metab. 1968 Dec;28(12):1797–1806. doi: 10.1210/jcem-28-12-1797. [DOI] [PubMed] [Google Scholar]
- Malthièry Y., Marriq C., Bergé-Lefranc J. L., Franc J. L., Henry M., Lejeune P. J., Ruf J., Lissitzky S. Thyroglobulin structure and function: recent advances. Biochimie. 1989 Feb;71(2):195–209. doi: 10.1016/0300-9084(89)90057-6. [DOI] [PubMed] [Google Scholar]
- Malthiéry Y., Lissitzky S. Primary structure of human thyroglobulin deduced from the sequence of its 8448-base complementary DNA. Eur J Biochem. 1987 Jun 15;165(3):491–498. doi: 10.1111/j.1432-1033.1987.tb11466.x. [DOI] [PubMed] [Google Scholar]
- Marriq C., Lejeune P. J., Venot N., Vinet L. Hormone synthesis in human thyroglobulin: possible cleavage of the polypeptide chain at the tyrosine donor site. FEBS Lett. 1989 Jan 2;242(2):414–418. doi: 10.1016/0014-5793(89)80513-7. [DOI] [PubMed] [Google Scholar]
- Mercken L., Simons M. J., Brocas H., Vassart G. Alternative splicing may be responsible for heterogeneity of thyroglobulin structure. Biochimie. 1989 Feb;71(2):223–226. doi: 10.1016/0300-9084(89)90059-x. [DOI] [PubMed] [Google Scholar]
- Mercken L., Simons M. J., Swillens S., Massaer M., Vassart G. Primary structure of bovine thyroglobulin deduced from the sequence of its 8,431-base complementary DNA. Nature. 1985 Aug 15;316(6029):647–651. doi: 10.1038/316647a0. [DOI] [PubMed] [Google Scholar]
- Musti A. M., Avvedimento E. V., Polistina C., Ursini V. M., Obici S., Nitsch L., Cocozza S., Di Lauro R. The complete structure of the rat thyroglobulin gene. Proc Natl Acad Sci U S A. 1986 Jan;83(2):323–327. doi: 10.1073/pnas.83.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmiya Y., Hayashi H., Kondo T., Kondo Y. Location of dehydroalanine residues in the amino acid sequence of bovine thyroglobulin. Identification of "donor" tyrosine sites for hormonogenesis in thyroglobulin. J Biol Chem. 1990 Jun 5;265(16):9066–9071. [PubMed] [Google Scholar]
- Ohmori T., Ieiri T., Asakura Y., Ohki Y., Teshirogi T., Terasaki A., Hosoya T. Application of improved coupling assay method for peroxidase of diseased thyroids: report of three cases. Endocrinol Jpn. 1991 Feb;38(1):113–117. doi: 10.1507/endocrj1954.38.113. [DOI] [PubMed] [Google Scholar]
- Palumbo G. Thyroid hormonogenesis. Identification of a sequence containing iodophenyl donor site(s) in calf thyroglobulin. J Biol Chem. 1987 Dec 15;262(35):17182–17188. [PubMed] [Google Scholar]
- Parma J., Christophe D., Pohl V., Vassart G. Structural organization of the 5' region of the thyroglobulin gene. Evidence for intron loss and "exonization" during evolution. J Mol Biol. 1987 Aug 20;196(4):769–779. doi: 10.1016/0022-2836(87)90403-7. [DOI] [PubMed] [Google Scholar]
- Ricketts M. H., Simons M. J., Parma J., Mercken L., Dong Q., Vassart G. A nonsense mutation causes hereditary goitre in the Afrikander cattle and unmasks alternative splicing of thyroglobulin transcripts. Proc Natl Acad Sci U S A. 1987 May;84(10):3181–3184. doi: 10.1073/pnas.84.10.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987 Sep 11;15(17):7155–7174. doi: 10.1093/nar/15.17.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon P., Brocas H., Rodesch C., Vassart G. RFLP detected at the 8924 locus by a thyroglobulin cDNA probe. Nucleic Acids Res. 1987 Jan 12;15(1):373–373. doi: 10.1093/nar/15.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterk A., van Dijk J. E., Veenboer G. J., Moorman A. F., de Vijlder J. J. Normal-sized thyroglobulin messenger ribonucleic acid in Dutch goats with a thyroglobulin synthesis defect is translated into a 35,000 molecular weight N-terminal fragment. Endocrinology. 1989 Jan;124(1):477–483. doi: 10.1210/endo-124-1-477. [DOI] [PubMed] [Google Scholar]
- Tassi V. P., Di Lauro R., Van Jaarsveld P., Alvino C. G. Two abnormal thyroglobulin-like polypeptides are produced from Afrikander cattle congenital goiter mRNA. J Biol Chem. 1984 Aug 25;259(16):10507–10510. [PubMed] [Google Scholar]
- Taylor B. A., Rowe L. The congenital goiter mutation is linked to the thyroglobulin gene in the mouse. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1986–1990. doi: 10.1073/pnas.84.7.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Herle A. J., Vassart G., Dumont J. E. Control of thyroglobulin synthesis and secretion. (First of two parts). N Engl J Med. 1979 Aug 2;301(5):239–249. doi: 10.1056/NEJM197908023010504. [DOI] [PubMed] [Google Scholar]
- Vassart G., Verstreken L., Dinsart C. Molecular weight of thyroglobulin 33 S messenger RNA as determined by polyacrylamide gel electrophoresis in the presence of formamide. FEBS Lett. 1977 Jul 1;79(1):15–18. doi: 10.1016/0014-5793(77)80340-2. [DOI] [PubMed] [Google Scholar]
- Wong C., Antonarakis S. E., Goff S. C., Orkin S. H., Forget B. G., Nathan D. G., Giardina P. J., Kazazian H. H., Jr Beta-thalassemia due to two novel nucleotide substitutions in consensus acceptor splice sequences of the beta-globin gene. Blood. 1989 Mar;73(4):914–918. [PubMed] [Google Scholar]
- de Crombrugghe B., Edelhoch H. The properties of thyroglobulin. XIV. The structure of reoxidized thyroglobulin. Biochemistry. 1966 Jul;5(7):2238–2245. doi: 10.1021/bi00871a012. [DOI] [PubMed] [Google Scholar]