Abstract
AIM: To investigate the expression of p53 and p21 and associations with possible risk factors, such as cigarette smoking, in esophageal squamous cell carcinoma (ESCC) in northeastern Iran, a region with a high incidence of ESCC.
METHODS: The expression of p53 and p21 proteins was investigated immunohistochemically in tumor tissue from 80 ESCC patients and in 60 available paraffin-embedded blocks of adjacent normal specimens from the cases, along with normal esophageal tissue from 80 healthy subjects.
RESULTS: Positive expression of p53 protein was detected in 56.2% (45/80) of ESCC cases, and in none of the normal esophageal tissue of the control group (P < 0.001). Furthermore, 73.8% (59/80) of ESCC cases and 43.8% (35/80) of controls had positive expression of p21 protein (P < 0.001). Cigarette smoking was significantly associated with p53 over-expression in ESCC cases (P = 0.010, OR = 3.64; 95% CI: 1.32-10.02). p21 over-expression was associated with poorer clinical outcome among the ESCC patients (P = 0.009).
CONCLUSION: Over-expression of p53 in association with cigarette smoking may play a critical role in ESCC carcinogenesis among this high-risk population of northeastern Iran. Furthermore, p21 over-expression was found to be associated with poor prognosis, specifically in the operable ESCC patients.
Keywords: Esophageal squamous cell carcinoma, p53, p21, Immunohistochemistry, Survival, Smoking
INTRODUCTION
Esophageal cancer has been reported as the eighth most common cancer worldwide, with a great variation in the incidence all around the world[1]. A high-risk area for this cancer is known as the so-called “Asian esophageal cancer belt”, which stretches from north central China into northern Iran, where esophageal squamous cell carcinoma (ESCC) predominates[2-4].
High incidence and mortality rates of ESCC have been reported in northeastern Iran, due to some distinct, but not well-known, environmental and genetic factors; however the complex network of molecular alterations underlying the development and progression of ESCC have not been clearly elucidated in this region[5,6].
Several studies have revealed that esophageal cancer, as with many other malignancies, is associated with cigarette smoking. However, the specific molecular targets affected by cigarette-derived carcinogens have not been thoroughly identified[7].
It has been shown that the p53 tumor suppressor gene is involved in the control of the cell cycle[8], and it is employed to protect cells exposed to DNA-damaging agents such as environmental risk factors including cigarette smoking[9]. The p53 inactivation in human cancer may result through binding to viral proteins, as a result of MDM2 or p19ARF gene alteration or indirectly by p53 protein localization in the cytoplasm[10]. Furthermore, it has been shown that p53 mutation is the most common aberration in human cancers, including esophageal carcinoma[11]. These mutations can lead to an increase in the stability of the protein, so it can accumulate in nuclei and be detected by immunohistochemistry methods. Therefore, it has been suggested that all cancer cells have some p53 aberrations resulting in over-expression of p53[12].
Furthermore, it has been shown that p53 over-expression appears to have a central role in the progression of esophageal cancer in patients who have a positive history of tobacco consumption[7,13-15].
It is generally accepted that esophageal cancer develops through a multi-step process of genetic and epigenetic changes leading to a sequence of histological changes in the epithelia, including esophagitis, basal cell hypertrophia, dysplasia, carcinoma in situ, and finally advanced ESCC[13,16-19].
In normal cells, wild-type p53 up-regulates the expression of several downstream genes to arrest the cell cycle so that damaged DNA either is repaired or apoptosis is promoted in response to DNA damaging agents[20,21]. The product of the mutated p53 gene has a much longer half-life compared to the wild-type protein. Because of some conformational changes, it is more stable; thus, the accumulation of this protein in the early steps of carcinogenesis is easily detected by immunohistochemical techniques. Previous reports have shown a significant association between p53 mutations and immunohistochemical p53 nuclear reactivity [19,22-24].
The p21 protein, which is encoded by the p21WAF1/Cip1 gene, is regulated by wild type, not the mutant, p53[25,26]. It inhibits DNA synthesis, as well as the G1/S phase transition, by forming a complex with proliferating cell nuclear antigen and cyclin dependent kinase[27,28]. However, in addition to p53-dependent expression, p21 can be regulated in a p53-independent manner[29,30]. Unlike p53, mutation of the p21 gene is a rare event in human cancers; therefore, alterations of this gene, involved in carcinogenesis, may be due to some abnormal changes at the expression level rather than genetic coding and epigenetic alterations[31]. Aberrant expression of p21, detected by immunohistochemical staining, has been shown in several cancers, including esophageal cancer, in which both the decrease and increase in p21 expression are reported to be associated with poor prognosis[32-34]. Concerning the clinical relevance of p21 and p53 expression in cancer patients, several studies have indicated that analyzing the combined immunohistochemical expression of these proteins may be more useful in interpreting the favorable and unfavorable clinical outcome than investigating each of them separately[35,36].
The current study was conducted to investigate the immunohistochemical expression of p53 and p21 in 80 ESCC patients in relation to possible risk factors, such as cigarette smoking, and to evaluate whether their expression is a prognostic factor with regard to p53-dependent and -independent pathways.
MATERIALS AND METHODS
Study population
A total of 80 consecutive patients with histologically-confirmed invasive squamous cell carcinoma of the esophagus (45 males and 35 females; mean age 61.39 ± 11.42 years, ranging from 35 to 83 years) were recruited from the two main referral oncology centers in northeastern Iran: Atrak clinic, the main specialized center for upper gastrointestinal (GI) disorders in Golestan province, and Omid Oncology Hospital, referral oncology hospital of northeastern Iran. All eligible subjects were recruited between September 2006 and September 2007 and written informed consents were obtained. The study was approved by the Ethics Committee of Tehran University of Medical Sciences.
The eligibility criteria for the enrolled ESCC patients were: (1) presence of a primary ESCC with no history of concurrent cancer in other organs or history of previous cancer in any organ; and (2) recent diagnosis of ESCC in the patients. Patients who had received any adjuvant therapy (radiotherapy or chemotherapy) were excluded.
Eighty eligible healthy subjects were randomly selected among individuals who were referred to Atrak clinic for upper GI health examination and diagnosed as normal based on physical examination and were histologically proven not to have a cancerous lesion. They were genetically unrelated to the cases and they had no previous cancer history. The control group was matched to the case group by age (± 6 years) and gender. According to a standard questionnaire, the demographic data of each patient and information about social habits, including cigarette smoking and opium use, were collected by an expert member of the research group.
With respect to social habits, never-users were defined as subjects who never or rarely used cigarettes or opium, and ever-users were defined as subjects who had used cigarettes or opium at least weekly for a period of 6 mo or more.
All patients were staged with radiological contrast (barium swallow) and computed tomography (CT) scans of the chest, abdomen, and pelvis. Endoscopic ultrasound and magnetic resonance imaging were performed when available. After the initial staging, patients with potentially operable conditions (defined as stages II or IIIA) proceeded directly to esophageal resection. The rest were categorized as an inoperable subgroup of patients based on their clinicopathological conditions. For those patients who were candidates for surgical resection, pathological stage was determined by histopathology at the time of esophagectomy, on the basis of the Union International Cancer TNM classification guidelines[37].
All 80 ESCC cases were followed up every 3 mo by office visits for clinical evaluation or via telephone contact. Deaths caused by ESCC were taken as outcome events, whereas others were considered censored. Survival duration was defined as the time interval from diagnosis to either death or the time of the last clinical evaluation of the patients. The cause of death was determined from the patient’s records and death certificate.
Tissue collection
Tumor tissue and corresponding adjacent normal esophageal tissue specimens were obtained from the ESCC patients. All untreated specimen-proven carcinoma of the esophagus in ESCC cases, as well as esophageal normal epithelia of healthy controls, were obtained by esophagectomy or endoscopy procedure. All specimens were fixed and stored in 70% ethanol and embedded in paraffin. Esophageal squamous tumors were comprised of > 70% malignant cells with minimal necrosis, and normal esophageal specimens with no contaminating tumor cells were confirmed as noncancerous tissue by histological examination of a representative hematoxylin and eosin stained slide. Tumors were histologically verified as ESCC and sub-typed based on the grade of differentiation as well differentiated, moderately differentiated or poorly differentiated. Tumor tissue samples were selected so that all adjacent normal esophageal tissues were obtained from the macroscopically normal esophageal epithelium, distant from the cancerous lesion.
Immunohistochemical staining
Tissue sections 4-μm in thickness were obtained from archival alcohol-fixed paraffin-embedded tissues of the esophageal squamous tumor and normal esophageal specimens and mounted on poly-L-lysine-coated slides for immunohistochemistry study. After being dewaxed in xylene and rehydrated in a series of graded alcohols, they were placed in 10 mmol/L citrate buffer pH 6.0 to unmask the epitopes. After microwave antigen retrieval (20 min, 120 W; 3 × 5 min, 450 W), the sections were allowed to cool down to room temperature (approximately 20 min), and then incubated with 3% H2O2 for 10 min to quench the endogenous peroxidase activity. After blocking the nonspecific protein binding with serum-free protein block (Dako, Inc.) for 5 min, slides were incubated for 45 min at 37°C with either anti-human p21waf1/cip1 monoclonal antibody (clone SX118, DAKO, CA, USA; dilution 1:50) or anti-p53 monoclonal antibody (clone DO-7; DAKO, CA, USA; dilution 1:50) DO-7 which was raised against an epitope between amino acids 1 and 45 in the C-terminal domain of human wild-type and mutant p53 recognizing both mutant and wild-type p53 protein, followed by phosphate buffered saline wash. Finally the primary antibody was detected, using EnVision™ + System/HRP, rabbit/mouse (DAB+) (Dako, Denmark), a secondary antibody. Staining was visualized using 3,3’-diaminobenzidine chromogen for 10 min, followed by acidified hematoxylin counterstaining for 1 min. Thereafter, the sections were mounted with mounting medium.
Control sections of known p53-positive and p21-positive cases of ESCC were included in each run, and the negative control section was carried out by omitting the primary antibody. Two expert pathologists who were blinded to the clinical and molecular results evaluated the tissue slides, independently. The final result was obtained through the consensus between the pathologists. Only staining of the cell nucleus was considered as a positive reaction for both p21WAF1/CIP1 and p53 proteins (Figures 1 and 2). For p21 protein, the expression of p21 was graded as negative staining, < 10%; intermediate staining or low-expression, 10%-49%; high staining or over-expression, ≥ 50%. The p53-negative expression was defined as less than 5% of p53 immunoreactivity, and p53-positive expression was classified into two groups according to the percentage of positive nuclei (5%-49%, intermediate staining or low-expression; strong staining or over-expression, ≥ 50%). The median value for each p53 or p21 immunostaining (50%) was used as the cut off point for over-expression[24,38-40]. We also considered the adjacent non-neoplastic squamous epithelia to compare the positive staining in tumors.
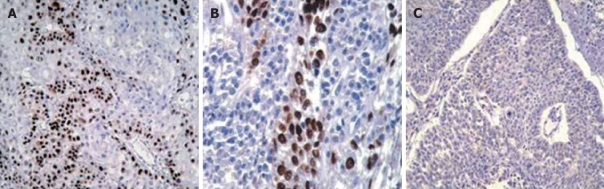
Figure 1.
Immunohistochemical staining for p53 in an esophageal squamous cell carcinoma exhibiting expression. A: With primary antibody, showing reactivity (brown nuclear staining of some tumor cells) (× 100); B: With primary antibody, showing reactivity (× 400); C: With primary antibody, no reactivity (× 100). Sections were counter-stained with hematoxylin.
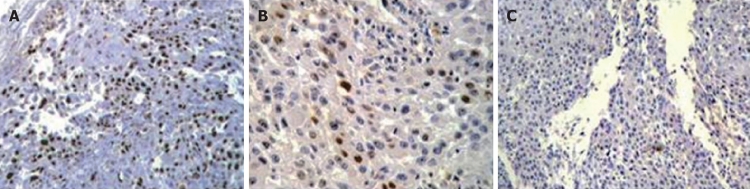
Figure 2.
Immunohistochemical staining for p21 in an esophageal squamous cell carcinoma exhibiting expression. A: With primary antibody, showing reactivity (brown nuclear staining of some tumor cells) (× 100); B: With primary antibody, showing reactivity (× 400); C: With primary antibody, no reactivity (× 100). Sections were counter-stained with hematoxylin.
Statistical analysis
The Statistical Package for the Social Sciences software version 16.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses. The associations between p53 or p21 expression, clinicopathological parameters, and related risk factors were evaluated by the χ2 test and Fisher’s exact test in univariate analysis, and by logistic regression modeling in multivariate analysis. Prognostic factors were evaluated at the univariate level using the Kaplan-Meier method with log-rank test, and in multivariate analysis using the Cox’s proportional hazards model of relevant prognostic variables. A 2-sided P value < 0.05 was considered as significant statistically.
RESULTS
A total of 80 ESCC cases (45 males and 35 females; mean age 61.39 ± 11.42 years, ranging from 35 to 83 years) and 80 healthy controls (48 males and 32 females; mean age 62.81 ± 10.36 years, ranging from 32 to 84 years) were examined in this study. The distribution of demographic variables for the cases and controls are summarized in Table 1.
Table 1.
Distribution of the demographic variables for esophageal squamous cell carcinoma cases and healthy subjects n (%)
| Case | Control | P value1 | |
| Age (yr) | |||
| < 60 | 36 (45) | 27 (33.8) | NS |
| ≥ 60 | 44 (55) | 53 (66.2) | |
| Gender | |||
| Male | 45 (56.2) | 48 (60) | NS |
| Female | 35 (43.8) | 32 (40) | |
| Smoking | |||
| Never | 52 (65) | 28 (35) | 0.03 |
| Ever | 64 (80) | 16 (20) | |
| Opium use | |||
| Ever | 21 (26.2) | 18 (22.5) | NS |
| Never | 59 (73.8) | 62 (77.5) | |
| p53 expression | |||
| Positive | 45 (56.2) | 0 (0) | < 0.001 |
| Negative | 35 (43.8) | 80 (100) | |
| p21 expression | |||
| Positive | 59 (73.8) | 37 (46.2) | < 0.001 |
| Negative | 21 (26.2) | 43 (53.8) |
Not statistically significant by χ2 test. NS: Not significant.
Analysis of protein expression by immunohistochemical staining
Expression of p53 protein in ESCC: Positive expression of p53 protein (Figure 1) was detected in 56.2% (45/80) of ESCC cases, and in none of the normal esophageal tissues of the control group (P < 0.001). Among the 35 ESCC tumors with p53-negative expression, 31 tumors showed no expression at all. The percentage of p53-positive cells ranged from 0% to 100%, with a mean of 54.6% and a median value of 50%. Of the p53-positive specimens, low-expression of p53 was detected in 21 of 80 cases (26.2%) and p53 over-expression was found in 24 of 80 cases (30%).
In the group of normal adjacent tissue, positive expression of p53 was observed only in the nuclei of basal cells. p53-negative expression was detected in 90% (54/60) of the normal adjacent tumor tissues; whereas, we detected the positive expression of p53 in 10% (6/60) of the normal adjacent tumor tissue samples including 50% (3/6) with dysplastic lesions, one graded as esophageal tissue with moderate to severe esophagitis, and the remaining two were histopathologically normal adjacent tissues.
There was no significant association between tumor and normal adjacent tissues based on p53-positive expression.
Expression of p21 protein in ESCC: Of the 80 ESCC cases assessed in this study, positive expression of p21 protein (Figure 2) was detected in 73.8% (59/80) of ESCC cases, whereas only 43.8% (35/80) of controls had positive expression for p21 protein (P < 0.001). In the group of esophageal tumors, the percentage of cells within a section showing definite immunoreactivity varied from 0%-90%. Positive expression of p21 was detected with the average of 42.5% of cells in cases, and 17.5% of cells in controls. The corresponding median values of positive cells were 50% and 15% in the case and control groups, respectively. Twenty-one cases (26.2%) were detected as p21-negative, 29 of 80 (36.3%) cases had intermediate staining (low-expression) of p21 and in 30 of 80 (37.5%) cases, we detected p21 over-expression (high staining). Conversely, the corresponding values were 45 of 80 (56.2%), 35 of 80 (43.8%), and none (0%), for the control group, respectively (P < 0.001).
p21-positive nuclei were detected in 45% (27/60) of normal adjacent tissue in ESCC cases. This was significantly lower than in the tumor tissues in the case group (P = 0.001).
Comparison of p21 and p53 protein expression in ESCC: Immunohistochemical expression of p53 and p21 varied in the proportion of stained cells and the distribution of positive cells was heterogeneous between cancer nests. Overall, there was no significant correlation between p21 and p53 expression, at all cut off values, among ESCC cases (neither in tumors, nor in the normal adjacent tissues). Combined analysis of p21 and p53 expression has been summarized in Table 2.
Table 2.
Correlation between p53 and p21 expression in esophageal squamous cell carcinoma cases n (%)
| p53 expression |
p21 expression |
Total number | ||
| Negative (< 10%) |
Positive |
|||
| Low-expression (10%-49%) | Over-expression (≥ 50%) | |||
| Negative (< 5%) | 10/80 (12.5) | 15/80 (18.7) | 9/80 (11.2) | 34 |
| Positive | ||||
| Low-expression (5%-49%) | 7/80 (8.75) | 7/80 (13.6) | 9/80 (11.2) | 23 |
| Over-expression (≥ 50%) | 4/80 (5) | 7/80 (13.6) | 12/80 (15) | |
| Total number | 21 | 29 | 30 | 80 |
There was no significant association between p53 and p21 expression among esophageal squamous cell carcinoma cases.
Relationship between the expression of p53 and p21 proteins and clinicopathological parameters, including cigarette smoking
The relationship between p53 and p21 protein expression (at any cut off value) and different demographic and clinicopathologic parameters has been analyzed in the whole series of patients, in the p53-negative and p53-positive subgroups, and in the subgroups of patients who did or did not undergo esophagectomy, separately.
In the whole series of ESCC patients, our results showed that p53 or p21 expression was not related to age category, opium use, tumor location, histology of the tumor, depth of tumor invasion, lymph node involvement, or disease stage, when they were simply dichotomized to positive and negative groups, whereas over-expression of the p53 protein was observed in 46.4% (13/28) of ever-smokers but in only 19.2% (10/52) of never-smokers; the difference was statistically significant (P = 0.01, OR = 3.64; 95% CI: 1.32-10.02). After controlling for the potential confounding effects of age, sex, opium use, tumor size, tumor location, depth of tumor, lymph node involvement, disease stage, histology of the tumor, and p21 expression, multiple logistic regression analysis showed similar results (P = 0.03, OR = 3.89; 95% CI: 1.09-13.89). We did not find any statistically significant association between cigarette smoking and p21 protein expression at any cut off value of 10% or 50%.
In addition, combined analysis of p53 and p21 expression showed that there was no significant correlation between p21/p53 expression and pathological stages or other parameters when we used different cut off values; however, the esophageal tumors only expressing high levels of p21 protein (≥ 50%) (without p53 over-expression), were significantly associated with deep invasion (P = 0.01).
The relationship between clinicopathological findings and p53/p21 over-expression is shown in Table 3. We did not detect any significant association between p53 or p21 over-expression with different parameters among ESCC patients, when we compared all the cut off values.
Table 3.
Correlation between clinicopathological parameters and p53 and p21 over-expression in esophageal squamous cell carcinoma patients n (%)
| Number |
p53 over-expression |
p21 over-expression |
|||||
| Yes | No | P value | Yes | No | P value1 | ||
| Age (yr) | |||||||
| < 60 | 36 | 10 (27.8) | 26 (72.2) | NS | 14 (38.9) | 22 (61.1) | NS |
| ≥ 60 | 44 | 13 (29.5) | 31 (70.5) | 16 (36.4) | 28 (63.6) | ||
| Gender | |||||||
| Male | 45 | 15 (33.3) | 30 (66.7) | NS | 20 (44.4) | 25 (55.6) | NS |
| Female | 35 | 8 (22.9) | 27 (77.1) | 10 (28.6) | 25 (71.4) | ||
| Smoking | |||||||
| Ever-user | 28 | 13 (46.4) | 15 (53.6) | 0.01 | 14 (50) | 14 (50) | 0.09 |
| Never-user | 52 | 10 (19.2) | 42 (80.8) | 16 (30.8) | 36 (69.2) | ||
| Differentiation | |||||||
| Well | 46 | 12 (26.1) | 34 (73.9) | 20 (43.5) | 26 (56.5) | ||
| Moderate | 23 | 8 (34.8) | 15 (65.2) | 6 (26.1) | 17 (73.9) | NS | |
| Poor | 11 | 3 (27.3) | 8 (72.7) | NS | 4 (36.4) | 7 (63.6) | |
| Tumor site | |||||||
| Middle | 59 | 19 (32.2) | 40 (67.8) | NS | 26 (44.1) | 33 (55.9) | NS |
| Lower | 20 | 4 (20) | 16 (80) | 4 (20) | 16 (80) | ||
| Size of tumor (cm) | |||||||
| < 3 | 23 | 8 (34.8) | 15 (65.2) | 5 (21.7) | 18 (78.3) | 0.07 | |
| ≥ 3 | 53 | 15 (28.3) | 38 (71.7) | NS | 23 (43.4) | 30 (56.6) | |
| Operability | |||||||
| Operable | 45 | 12 (26.7) | 33 (73.3) | NS | 17 (37.8) | 28 (62.2) | NS |
| Inoperable | 35 | 11 (31.4) | 24 (68.6) | 13 (37.1) | 22 (62.9) | ||
Not statistically significant by χ2 test. NS: Not significant.
Clinical outcome
All patients were followed up, and survival analysis was performed at the end of the study period in September 2009 (Figure 3). Among the entire patient population, mean survival was 8.21 ± 4.92 mo, with a median of 7.5 mo; ranging from 4 to 24 mo. Of the 80 ESCC patients, 56.2% (45/80) underwent curative esophagectomy, including 73.3% and 26.7% with stage II and IIIA of ESCC, respectively (mean survival, 9.49 ± 5.02 mo; median, 8 mo; ranging from 4 to 24 mo). On the other hand, 43.8% (35/80) of the cases were categorized as inoperable ESCC patients (mean survival, 6.57 ± 4.33 mo; median, 5 mo; ranging from 4 to 17 mo).
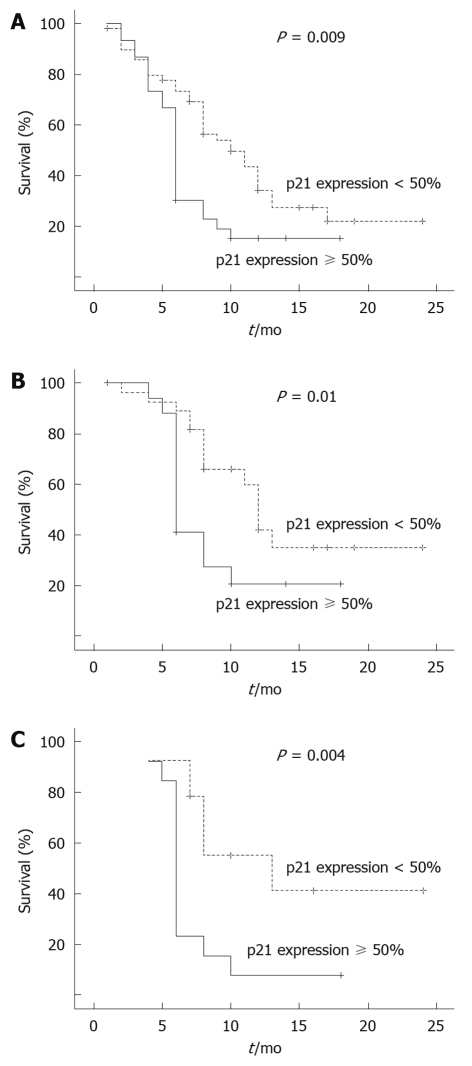
Figure 3.
Kaplan-Meier survival curves for esophageal squamous cell carcinoma patients with or without p21 over-expression. A: Overall survival curves were classified by p21 over-expression in the whole series of ESCC patients; B: Survival curves in the operable group of ESCC patients, stratified according to p21 over-expression; C: Survival curves in the operable group of patients with positive expression of p53, stratified according to p21 over-expression.
Prognosis of ESCC patients according to clinicopathological parameters and p21 and/or p53 protein expression
The overall 6-mo, 1- and 2-year survival rates of the entire group (80 ESCC patients) were 56.7%, 26.7% and 18.6%, respectively.
Results of the univariate analysis for the whole series of patients showed no influence of p53 protein expression on survival duration, even if different cut off values were considered (5% and 50%). Similarly, no significant association was found between p21 expression and survival duration, using the cut off value of 10%, whereas the 50% cut off value revealed a significant association between p21 over-expression and poor clinical outcome (P = 0.009). In a univariate survival analysis for the entire group of cancer patients, there was no significant survival effect for all available clinicopathologic factors for ESCC patients, except for the patients who were aged above 60 years (P = 0.006), or those who underwent surgical operation (P = 0.001); factors which were significantly associated with poorer prognosis. Our findings also revealed a significantly reduced survival period among the cases with both p21 and p53 over-expressing tumors compared to patients with p21 over-expressing tumors alone (without p53 over-expression), or in those without over-expression of both p21 and p53 proteins (P < 0.001).
Furthermore, to analyze the factors related to prognosis according to p53 protein expression (for both 5% and 50% cut off values), univariate and multivariate analysis were performed separately. Among the p53-positive cases, the factors related to poorer clinical outcome consisted of patients with p21 over-expressing tumors (P = 0.009) and those who were aged above 60 years (P = 0.03).
Additionally, when analyzing clinical outcome according to p53 and p21 expression in 45 patients who underwent surgery, patients with p21 over-expressing tumors showed poorer clinical outcome (P = 0.01). This adverse effect was still significant when the study population was restricted to the operable patients with p53 over-expression (P = 0.004).
The Cox proportional hazards regression model showed that age categories, surgical operation status (operable or inoperable), and p21 over-expression were independent prognostic factors (Table 4).
Table 4.
Log-rank and proportional hazard regression analysis (Cox method) for clinicopathological parameters in esophageal squamous cell carcinoma patients
| Mean survival time (mo) | Log rank P value |
Cox-regression |
|||
| HR | 95% CI | P value1 | |||
| Operability | |||||
| Operable | 12.68 ± 2.56 | 0.001 | 2.27 | 1.30-3.97 | 0.004 |
| Inoperable | 7.08 ± 1.72 | ||||
| p21 over-expression | |||||
| Yes | 7.41 ± 1.78 | 0.009 | 1.82 | 1.02-3.25 | 0.04 |
| No | 11.71 ± 2.36 | ||||
| p53 over-expression | |||||
| Yes | 8.00 ± 2.06 | 0.30 | 1.23 | 0.67-2.26 | NS |
| No | 10.69 ± 2.08 | ||||
| Tumor size (cm) | |||||
| < 3 | 12.58 ± 3.28 | 0.06 | 1.75 | 0.92-3.32 | 0.08 |
| ≥ 3 | 9.43 ± 2.18 | ||||
| Age (yr) | |||||
| < 60 | 12.61 ± 2.46 | 0.006 | 2.30 | 1.29-4.09 | 0.005 |
| ≥ 60 | 8.40 ± 2.14 | ||||
Not statistically significant by χ2 test. NS: Not significant; HR: Hazard ratio; CI: Confidence interval.
DISCUSSION
The significant positive expression of p53 and p21 in the ESCC patients of this studied population, compared with the healthy subjects, revealed that these proteins play an important role in ESCC development in northeastern Iran. Furthermore, we found that p53 over-expression, but not p21, was associated with cigarette smoking habit in the ESCC patients. Contradictory results have been reported regarding the association of p53 protein expression and cigarette smoking. Our finding is consistent with the studies published by Mizobuchi et al[7], Montesano et al[41] and Cruz et al[39], but discordant with the observations of Lam et al[42]. Recent studies have shown that various kinds of carcinogens produced by smoked cigarettes might be responsible for different p53 gene mutations and p53 over-expression; thus, they may play a role in carcinogenesis, including esophageal cancer development[7,43-45]. In this regard, recent evidence from Golestan province (in northeastern Iran) inhabitants showed that moderate to high exposure to polycyclic aromatic hydrocarbon (PAH) components, one of the substances related to cigarette smoke, may be associated with esophageal carcinogenesis[46]. Therefore, it has been hypothesized that continuous exposure to specific carcinogenic components of tobacco smoke in the studied area, such as PAH, may cause mutations in some important cell cycle genes, such as p53, leading to over-expression and abnormal accumulation of the translated proteins. This specific mutation may result in the formation of a dysfunctional protein, which is sequestered and accumulated in the cell, leading to cancer development. These observations provide support for further studies to evaluate the effect of possible carcinogen components of tobacco smoke, such as PAH, in ESCC patients residing in this region.
In the present study, positive expression of p53 protein in the peripheral layers of cancer nests, which are representative of the most proliferative and invasive cells in the esophageal SCC (due to the inactivation by mutation or deregulation of other cancer-related genes in the cell cycle), may suggest that the p53-positive expression is a frequent genetic alteration and plays an important role in the carcinogenesis of esophageal carcinoma in the studied population.
Recent studies have indicated that alteration in the p53 gene, as well as p53 protein accumulation, is frequently detected in dysplastic or precancerous lesions adjacent to ESCC tumors[47]. In the present study, 10% (6/60) of morphologically normal esophageal specimens adjacent to tumors showed p53 positive staining, including 3 samples with dysplastic lesions, one sample with moderate to severe esophagitis, and another two normal samples with no pathologic change. Although all of these specimens were positive for p21 immunostaining, the frequency and intensity of p21 expression were greater in dysplastic lesions than in the others. The observation of p53-positive expression in the adjacent dysplastic lesions (with or without p53 positivity in corresponding tumor) in this study supports the concept that potentially multiple origins, through similar or independent genetic alterations, may result in the development or recurrence of esophageal tumor in this site (either from the same clone or a different one as a consequence of other involved molecular alterations and pathways). Therefore, it is important to note that in patients, who have had the primary tumor removed, p53 accumulation may be a risk factor for tumor recurrence. This finding may be an important factor for the screening of the ESCC patients in a high-risk population. Therefore, immunohistochemical staining of p53 protein in the remaining unresected normal-appearing esophagus, beyond the normal margin, may be a valuable tool in these patients, to evaluate the risk of developing a secondary ESCC after an esophagectomy. Further prospective, large-scale studies are required as a validation set to support this concept.
Regarding the association between p21 and p53 protein expression in cancer patients, several studies have shown that one of the important ways to investigate the functional status of p53 is to evaluate some of its downstream effectors such as p21Waf1/Cip1[48]. Unlike p53, the positive expression of p21 is most often representative of the wild-type protein since no mutations in this gene have been detected in a large number of human tumors[38,49]. p21 protein may be regulated either in a p53-dependent or -independent manner. In our study we found no significant correlation between p53 and p21 proteins, and co-expression of p21 and p53 proteins in a proportion of ESCC cases supports the hypothesis that activation of p21 was regulated through a p53-independent pathway in this series of esophageal tumor samples, in agreement with a previous report by Seta et al[50], who showed there was no correlation between the expression of these proteins in esophageal or gastric cancer. Similar results were also reported by Yasui et al[51] and Gomyo et al[52] for gastric cancer.
Several studies have investigated the significant prognostic impact of p21 over-expression in different cancers, including esophageal carcinoma[35,36,52,53]. However, the results are contradictory[54,55]. The discrepancy in the findings might be due to lack of a standard classification for p21 immunostaining interpretation, or it may depend on different characteristics of malignant cells or different molecular markers regulating p21 expression in a specific tissue or tumor type. In the present study, we adopted the cut off value of 50% nuclear staining to indicate p21 over-expression, as it was applied in some of the previous studies[53]. Using these criteria, our results showed that the prognosis of esophageal cancer patients deteriorates with p21 over-expression (in both univariate and multivariate survival analysis). This is consistent with the study by Sarbia et al[53] who showed an adverse survival effect of p21 over-expressing esophageal tumors when they considered the cut off value of 50% as p21 over-expression. Goan et al[36] also indicated that p21 over-expression was associated with adverse prognosis in ESCC patients. However, this result contradicts the result of Shimada et al[56]. In addition, the adverse survival effect of p21 over-expression in the present study was still significant when the study population was restricted to the patients with p53-positive expression who underwent surgical operation. Some studies have shown that combined analysis of p53 and p21 expression may provide more prognostic information than evaluation of either variable alone[57,58]. In this regard, our findings also revealed a significantly reduced survival period among the cases with both p21 and p53 over-expressing tumors than in patients with p21 over-expressed tumors alone (without p53 over-expression), or than in those without over-expression of both p21 and p53 proteins. This may suggest a possibly more malignant behavior of the tumors when they over-express both p53 and p21 proteins. In other words, patients who harbor p21 over-expressing tumor have a compromised survival that could be superimposed by the adverse effect of non-functional accumulated p53, leading to poorer prognosis.
Concerning the adverse prognostic effect of p21 over-expression, recent studies have shown that despite the role of p21 in cell cycle arrest, this protein could contribute to the inhibition of DNA repair and mitotic control. In the presence of p53 mutation, the adverse survival effect of p21 over-expression could be increased, leading to uncontrolled high expression of p21, as well as sustained genomic instability, leading to facilitation of the progression of the tumor[36,59]. Therefore, this phenomenon may also be responsible for the adverse survival effect of p21 over-expression among the studied population in the present study.
In conclusion, this is the first study focused on evaluating the prognostic effect of p21 and p53 protein expression, as well as their role as a target for cigarette smoking, in ESCC patients in northeastern Iran which is a high-incidence area for this type of cancer. Our results showed that (1) p53 and p21 expression play an important role in ESCC development in northeastern Iran; (2) p53 as a target of cigarette smoking plays a critical role in ESCC development among this high-risk population; (3) the presence of abnormally accumulated p53 in the morphologically normal tissue adjacent to the resected tumor may be a predictor of future recurrence of tumor, thus evaluating the remaining normal esophageal tissue after resection of tumor could help to indicate a population who are at higher risk for tumor recurrence at this site; and finally (4) we indicated the adverse survival effect of p21 over-expression in the ESCC patients of northeastern Iran. Therefore, our data suggest that the immunohistochemical assessment of p21 over-expression, in relation to p53 over-expression, in esophageal cancer patients may provide useful prognostic markers for identifying the subgroup of high risk patients with poor clinical outcome who need closer postoperative follow up, and probably a more intensive therapeutic protocol.
COMMENTS
Background
The incidence of esophageal squamous cell carcinoma (ESCC) is high in northeastern Iran. This is due to environmental and genetic risk factors. p53 gene mutation appears to have a central role in the progression of esophageal cancer in patients who have a positive history of cigarette smoking. The current study was conducted to investigate the immunohistochemical expression of p53 and p21 among ESCC patients from northeastern Iran in relation to possible risk factors, such as cigarette smoking, and to evaluate whether their expression is a prognostic factor according to p53-dependent and -independent pathways.
Research frontiers
This study showed that the presence of abnormally accumulated p53 in the morphologically normal tissue adjacent to a resected tumor may be a predictor of future recurrence of the tumor. Also, results show the adverse survival effect of p21 over-expression on the ESCC patients of northeastern Iran.
Innovations and breakthroughs
This is the first study describing progression of ESCC in patients who are resident in northeastern Iran with a positive history of cigarette smoking.
Applications
The results of this study may provide useful prognostic markers, such as p53 and p21 over-expression, for identifying the subgroup of high risk patients with poor clinical outcome who need closer follow up, and probably more intensive therapeutic protocol, during postoperative management.
Peer review
As this author mentioned, over-expression of p53 in immunohistochemistry is generally thought to be mutated p53. Sometimes it is difficult to say whether these all originate from the mutation, because there are other ways of inactivation of p53. The author should try to clarify how the p53 overexpression happened or give more scientific evidence or reference about this point.
Acknowledgments
This manuscript is dedicated to Ms. Mansoureh Rezaei Manesh for her patience during the research work. We also appreciate the contribution of colleagues at the Atrak clinic: Dr. Karim Aghcheli, Dr. Ramin Shakeri, Ms. Safora Kor, Ms. Bita Mohammadi, Ms. Eskandarnezhad, and Mr. Ashor Yolmeh; and the support of Bu-Ali Research Institute of Mashhad University of Medical Sciences and the National Institute of Genetic Engineering and Biotechnology.
Footnotes
Supported by The National Institute of Genetic Engineering and Biotechnology, Projects No. 291, 316; and the Digestive Diseases Research Center of Tehran University of Medical Sciences
Peer reviewer: Sang Kil Lee, MD, Assistant Professor, Department of Gastroenterology, Yonsei University College of Medicine, #134 Shinchon-dong, Seodaemun-gu, Seoul 120-752, South Korea
S- Editor Wang YR L- Editor Logan S E- Editor Zheng XM
References
- 1.Gibson MK, Brock M. Esophageal cancer: adenocarcinoma in populations dominated by squamous cell histology. Esophagus. 2008;5:1–4. [Google Scholar]
- 2.Kmet J, Mahboubi E. Esophageal cancer in the Caspian littoral of Iran: initial studies. Science. 1972;175:846–853. doi: 10.1126/science.175.4024.846. [DOI] [PubMed] [Google Scholar]
- 3.Lam AK. Molecular biology of esophageal squamous cell carcinoma. Crit Rev Oncol Hematol. 2000;33:71–90. doi: 10.1016/s1040-8428(99)00054-2. [DOI] [PubMed] [Google Scholar]
- 4.The World Cancer Report--the major findings. Cent Eur J Public Health. 2003;11:177–179. [PubMed] [Google Scholar]
- 5.Islami F, Kamangar F, Aghcheli K, Fahimi S, Semnani S, Taghavi N, Marjani HA, Merat S, Nasseri-Moghaddam S, Pourshams A, et al. Epidemiologic features of upper gastrointestinal tract cancers in Northeastern Iran. Br J Cancer. 2004;90:1402–1406. doi: 10.1038/sj.bjc.6601737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ke L. Mortality and incidence trends from esophagus cancer in selected geographic areas of China circa 1970-90. Int J Cancer. 2002;102:271–274. doi: 10.1002/ijc.10706. [DOI] [PubMed] [Google Scholar]
- 7.Mizobuchi S, Furihata M, Sonobe H, Ohtsuki Y, Ishikawa T, Murakami H, Kurabayashi A, Ogoshi S, Sasaguri S. Association between p53 immunostaining and cigarette smoking in squamous cell carcinoma of the esophagus. Jpn J Clin Oncol. 2000;30:423–428. doi: 10.1093/jjco/hyd114. [DOI] [PubMed] [Google Scholar]
- 8.Doak SH, Jenkins GJ, Parry EM, Griffiths AP, Shah V, Baxter JN, Parry JM. Characterisation of p53 status at the gene, chromosomal and protein levels in oesophageal adenocarcinoma. Br J Cancer. 2003;89:1729–1735. doi: 10.1038/sj.bjc.6601323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woods DB, Vousden KH. Regulation of p53 function. Exp Cell Res. 2001;264:56–66. doi: 10.1006/excr.2000.5141. [DOI] [PubMed] [Google Scholar]
- 10.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 11.Biramijamal F, Allameh A, Mirbod P, Groene HJ, Koomagi R, Hollstein M. Unusual profile and high prevalence of p53 mutations in esophageal squamous cell carcinomas from northern Iran. Cancer Res. 2001;61:3119–3123. [PubMed] [Google Scholar]
- 12.Guimaraes DP, Hainaut P. TP53: a key gene in human cancer. Biochimie. 2002;84:83–93. doi: 10.1016/s0300-9084(01)01356-6. [DOI] [PubMed] [Google Scholar]
- 13.Wang LD, Hong JY, Qiu SL, Gao H, Yang CS. Accumulation of p53 protein in human esophageal precancerous lesions: a possible early biomarker for carcinogenesis. Cancer Res. 1993;53:1783–1787. [PubMed] [Google Scholar]
- 14.Gao H, Wang LD, Zhou Q, Hong JY, Huang TY, Yang CS. p53 tumor suppressor gene mutation in early esophageal precancerous lesions and carcinoma among high-risk populations in Henan, China. Cancer Res. 1994;54:4342–4346. [PubMed] [Google Scholar]
- 15.Brennan JA, Boyle JO, Koch WM, Goodman SN, Hruban RH, Eby YJ, Couch MJ, Forastiere AA, Sidransky D. Association between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:712–717. doi: 10.1056/NEJM199503163321104. [DOI] [PubMed] [Google Scholar]
- 16.Correa P. Precursors of gastric and esophageal cancer. Cancer. 1982;50:2554–2565. [PubMed] [Google Scholar]
- 17.Qiu SL, Yang GR. Precursor lesions of esophageal cancer in high-risk populations in Henan Province, China. Cancer. 1988;62:551–557. doi: 10.1002/1097-0142(19880801)62:3<551::aid-cncr2820620319>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 18.Mandard AM, Hainaut P, Hollstein M. Genetic steps in the development of squamous cell carcinoma of the esophagus. Mutat Res. 2000;462:335–342. doi: 10.1016/s1383-5742(00)00019-3. [DOI] [PubMed] [Google Scholar]
- 19.Bennett WP, Hollstein MC, Metcalf RA, Welsh JA, He A, Zhu SM, Kusters I, Resau JH, Trump BF, Lane DP. p53 mutation and protein accumulation during multistage human esophageal carcinogenesis. Cancer Res. 1992;52:6092–6097. [PubMed] [Google Scholar]
- 20.Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- 21.Yang G, Zhang Z, Liao J, Seril D, Wang L, Goldstein S, Yang CS. Immunohistochemical studies on Waf1p21, p16, pRb and p53 in human esophageal carcinomas and neighboring epithelia from a high-risk area in northern China. Int J Cancer. 1997;72:746–751. doi: 10.1002/(sici)1097-0215(19970904)72:5<746::aid-ijc7>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 22.Qiao GB, Han CL, Jiang RC, Sun CS, Wang Y, Wang YJ. Overexpression of P53 and its risk factors in esophageal cancer in urban areas of Xi'an. World J Gastroenterol. 1998;4:57–60. doi: 10.3748/wjg.v4.i1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wynford-Thomas D. P53 in tumour pathology: can we trust immunocytochemistry? J Pathol. 1992;166:329–330. doi: 10.1002/path.1711660402. [DOI] [PubMed] [Google Scholar]
- 24.Kropveld A, Rozemuller EH, Leppers FG, Scheidel KC, de Weger RA, Koole R, Hordijk GJ, Slootweg PJ, Tilanus MG. Sequencing analysis of RNA and DNA of exons 1 through 11 shows p53 gene alterations to be present in almost 100% of head and neck squamous cell cancers. Lab Invest. 1999;79:347–353. [PubMed] [Google Scholar]
- 25.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 26.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 27.Villwock Mde M, Meurer L, Cavazzola LT, Gurski RR, Edelweiss MI, Schirmer CC. Prevalence of p21 immunohistochemical expression in esophageal adenocarcinoma. Arq Gastroenterol. 2006;43:212–218. doi: 10.1590/s0004-28032006000300011. [DOI] [PubMed] [Google Scholar]
- 28.el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 29.Ding Z, Parchment RE, LoRusso PM, Zhou JY, Li J, Lawrence TS, Sun Y, Wu GS. The investigational new drug XK469 induces G(2)-M cell cycle arrest by p53-dependent and -independent pathways. Clin Cancer Res. 2001;7:3336–3342. [PubMed] [Google Scholar]
- 30.Sato T, Koseki T, Yamato K, Saiki K, Konishi K, Yoshikawa M, Ishikawa I, Nishihara T. p53-independent expression of p21(CIP1/WAF1) in plasmacytic cells during G(2) cell cycle arrest induced by Actinobacillus actinomycetemcomitans cytolethal distending toxin. Infect Immun. 2002;70:528–534. doi: 10.1128/IAI.70.2.528-534.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox LS. Multiple pathways control cell growth and transformation: overlapping and independent activities of p53 and p21Cip1/WAF1/Sdi1. J Pathol. 1997;183:134–140. doi: 10.1002/(SICI)1096-9896(199710)183:2<134::AID-PATH960>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 32.Yuen PW, Chow V, Choy J, Lam KY, Ho WK, Wei WI. The clinicopathologic significance of p53 and p21 expression in the surgical management of lingual squamous cell carcinoma. Am J Clin Pathol. 2001;116:240–245. doi: 10.1309/WKA0-AXP2-B8J5-DUJ3. [DOI] [PubMed] [Google Scholar]
- 33.Wakasugi E, Kobayashi T, Tamaki Y, Ito Y, Miyashiro I, Komoike Y, Takeda T, Shin E, Takatsuka Y, Kikkawa N, et al. p21(Waf1/Cip1) and p53 protein expression in breast cancer. Am J Clin Pathol. 1997;107:684–691. doi: 10.1093/ajcp/107.6.684. [DOI] [PubMed] [Google Scholar]
- 34.Chen YQ, Cipriano SC, Arenkiel JM, Miller FR. Tumor suppression by p21WAF1. Cancer Res. 1995;55:4536–4539. [PubMed] [Google Scholar]
- 35.Natsugoe S, Nakashima S, Matsumoto M, Xiangming C, Okumura H, Kijima F, Ishigami S, Takebayashi Y, Baba M, Takao S, et al. Expression of p21WAF1/Cip1 in the p53-dependent pathway is related to prognosis in patients with advanced esophageal carcinoma. Clin Cancer Res. 1999;5:2445–2449. [PubMed] [Google Scholar]
- 36.Goan YG, Hsu HK, Chang HC, Chou YP, Chiang KH, Cheng JT. Deregulated p21(WAF1) overexpression impacts survival of surgically resected esophageal squamous cell carcinoma patients. Ann Thorac Surg. 2005;80:1007–1016. doi: 10.1016/j.athoracsur.2005.03.050. [DOI] [PubMed] [Google Scholar]
- 37.Sobin LH, Fleming ID. TNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer. 1997;80:1803–1804. doi: 10.1002/(sici)1097-0142(19971101)80:9<1803::aid-cncr16>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 38.Ikeguchi M, Saito H, Katano K, Gomyo Y, Tsujitani S, Maeta M, Kaibara N. Relationship between the long-term effects of intraperitoneal chemotherapy and the expression of p53 and p21 in patients with gastric carcinoma at stage IIIa and stage IIIb. Int Surg. 1997;82:170–174. [PubMed] [Google Scholar]
- 39.Cruz I, Snijders PJ, Van Houten V, Vosjan M, Van der Waal I, Meijer CJ. Specific p53 immunostaining patterns are associated with smoking habits in patients with oral squamous cell carcinomas. J Clin Pathol. 2002;55:834–840. doi: 10.1136/jcp.55.11.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarbia M, Stahl M, zur Hausen A, Zimmermann K, Wang L, Fink U, Heep H, Dutkowski P, Willers R, Müller W, et al. Expression of p21WAF1 predicts outcome of esophageal cancer patients treated by surgery alone or by combined therapy modalities. Clin Cancer Res. 1998;4:2615–2623. [PubMed] [Google Scholar]
- 41.Montesano R, Hollstein M, Hainaut P. Genetic alterations in esophageal cancer and their relevance to etiology and pathogenesis: a review. Int J Cancer. 1996;69:225–235. doi: 10.1002/(SICI)1097-0215(19960621)69:3<225::AID-IJC13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 42.Lam KY, Loke SL, Chen WZ, Cheung KN, Ma L. Expression of p53 in oesophageal squamous cell carcinoma in Hong Kong Chinese. Eur J Surg Oncol. 1995;21:242–247. doi: 10.1016/s0748-7983(95)91228-2. [DOI] [PubMed] [Google Scholar]
- 43.Puisieux A, Lim S, Groopman J, Ozturk M. Selective targeting of p53 gene mutational hotspots in human cancers by etiologically defined carcinogens. Cancer Res. 1991;51:6185–6189. [PubMed] [Google Scholar]
- 44.Spruck CH 3rd, Rideout WM 3rd, Olumi AF, Ohneseit PF, Yang AS, Tsai YC, Nichols PW, Horn T, Hermann GG, Steven K. Distinct pattern of p53 mutations in bladder cancer: relationship to tobacco usage. Cancer Res. 1993;53:1162–1166. [PubMed] [Google Scholar]
- 45.Habuchi T, Takahashi R, Yamada H, Ogawa O, Kakehi Y, Ogura K, Hamazaki S, Toguchida J, Ishizaki K, Fujita J. Influence of cigarette smoking and schistosomiasis on p53 gene mutation in urothelial cancer. Cancer Res. 1993;53:3795–3799. [PubMed] [Google Scholar]
- 46.Kamangar F, Strickland PT, Pourshams A, Malekzadeh R, Boffetta P, Roth MJ, Abnet CC, Saadatian-Elahi M, Rakhshani N, Brennan P, et al. High exposure to polycyclic aromatic hydrocarbons may contribute to high risk of esophageal cancer in northeastern Iran. Anticancer Res. 2005;25:425–428. [PubMed] [Google Scholar]
- 47.Wang LD, Zhou Q, Hong JY, Qiu SL, Yang CS. p53 protein accumulation and gene mutations in multifocal esophageal precancerous lesions from symptom free subjects in a high incidence area for esophageal carcinoma in Henan, China. Cancer. 1996;77:1244–1249. [PubMed] [Google Scholar]
- 48.Caffo O, Doglioni C, Veronese S, Bonzanini M, Marchetti A, Buttitta F, Fina P, Leek R, Morelli L, Palma PD, et al. Prognostic value of p21(WAF1) and p53 expression in breast carcinoma: an immunohistochemical study in 261 patients with long-term follow-up. Clin Cancer Res. 1996;2:1591–1599. [PubMed] [Google Scholar]
- 49.Shiohara M, el-Deiry WS, Wada M, Nakamaki T, Takeuchi S, Yang R, Chen DL, Vogelstein B, Koeffler HP. Absence of WAF1 mutations in a variety of human malignancies. Blood. 1994;84:3781–3784. [PubMed] [Google Scholar]
- 50.Seta T, Imazeki F, Yokosuka O, Saisho H, Suzuki T, Koide Y, Isono K. Expression of p53 and p21WAF1/CIP1 proteins in gastric and esophageal cancers: comparison with mutations of the p53 gene. Dig Dis Sci. 1998;43:279–289. doi: 10.1023/a:1018889818855. [DOI] [PubMed] [Google Scholar]
- 51.Yasui W, Akama Y, Kuniyasu H, Yokozaki H, Semba S, Shimamoto F, Tahara E. Expression of cyclin-dependent kinase inhibitor p21WAF1/CIP1 in non-neoplastic mucosa and neoplasia of the stomach: relationship with p53 status and proliferative activity. J Pathol. 1996;180:122–128. doi: 10.1002/(SICI)1096-9896(199610)180:2<122::AID-PATH647>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 52.Gomyo Y, Ikeda M, Osaki M, Tatebe S, Tsujitani S, Ikeguchi M, Kaibara N, Ito H. Expression of p21 (waf1/cip1/sdi1), but not p53 protein, is a factor in the survival of patients with advanced gastric carcinoma. Cancer. 1997;79:2067–2072. doi: 10.1002/(sici)1097-0142(19970601)79:11<2067::aid-cncr3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 53.Sarbia M, Stahl M, zur Hausen A, Zimmermann K, Wang L, Fink U, Heep H, Dutkowski P, Willers R, Müller W, et al. Expression of p21WAF1 predicts outcome of esophageal cancer patients treated by surgery alone or by combined therapy modalities. Clin Cancer Res. 1998;4:2615–2623. [PubMed] [Google Scholar]
- 54.Nita ME, Nagawa H, Tominaga O, Tsuno N, Hatano K, Kitayama J, Tsuruo T, Domene CE, Muto T. p21Waf1/Cip1 expression is a prognostic marker in curatively resected esophageal squamous cell carcinoma, but not p27Kip1, p53, or Rb. Ann Surg Oncol. 1999;6:481–488. doi: 10.1007/s10434-999-0481-x. [DOI] [PubMed] [Google Scholar]
- 55.Güner D, Sturm I, Hemmati P, Hermann S, Hauptmann S, Wurm R, Budach V, Dörken B, Lorenz M, Daniel PT. Multigene analysis of Rb pathway and apoptosis control in esophageal squamous cell carcinoma identifies patients with good prognosis. Int J Cancer. 2003;103:445–454. doi: 10.1002/ijc.10850. [DOI] [PubMed] [Google Scholar]
- 56.Shimada Y, Imamura M, Watanabe G, Uchida S, Harada H, Makino T, Kano M. Prognostic factors of oesophageal squamous cell carcinoma from the perspective of molecular biology. Br J Cancer. 1999;80:1281–1288. doi: 10.1038/sj.bjc.6990499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yanamoto S, Kawasaki G, Yoshitomi I, Mizuno A. p53, mdm2, and p21 expression in oral squamous cell carcinomas: relationship with clinicopathologic factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:593–600. doi: 10.1067/moe.2002.127404. [DOI] [PubMed] [Google Scholar]
- 58.Xie X, Clausen OP, Boysen M. Prognostic significance of p21WAF1/CIP1 expression in tongue squamous cell carcinomas. Arch Otolaryngol Head Neck Surg. 2002;128:897–902. doi: 10.1001/archotol.128.8.897. [DOI] [PubMed] [Google Scholar]
- 59.Chang BD, Watanabe K, Broude EV, Fang J, Poole JC, Kalinichenko TV, Roninson IB. Effects of p21Waf1/Cip1/Sdi1 on cellular gene expression: implications for carcinogenesis, senescence, and age-related diseases. Proc Natl Acad Sci USA. 2000;97:4291–4296. doi: 10.1073/pnas.97.8.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]