Abstract
The genetic contributions to common disease and complex disease phenotypes are pleiotropic, multifactorial, and combinatorial. Gene set analysis is a computational approach used in the analysis of microarray data to rapidly query gene combinations and multifactorial processes. Here we use novel gene sets based on population-based human genetic associations in common human disease or experimental genetic mouse models to analyze disease-related microarray studies. We developed a web-based analysis tool that uses these novel disease- and phenotype-related gene sets to analyze microarray-based gene expression data. These gene sets show disease and phenotype specificity in a species-specific and cross-species fashion. In this way, we integrate population-based common human disease genetics, mouse genetically determined phenotypes, and disease or phenotype structured ontologies, with gene expression studies relevant to human disease. This may aid in the translation of large-scale high-throughput datasets into the context of clinically relevant disease phenotypes.
Keywords: genome-wide association, microarray, common disease, disease ontology, data integration
microarray-based gene expression studies as well as the public repositories of microarray-based gene expression experiments such as National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) (4) or European Bioinformatics Institute (EBI) Array Express (21) are increasing in content, size, and experimental diversity. This includes a growing number of studies relevant to human disease or those related to mouse genetic models of human disease. Gene set analysis is an approach in the analysis of microarray data that considers aggregate changes in relative expression levels of groups of genes, or “gene sets,” rather than in individual genes (14, 15, 18, 19). Collections of gene sets used in this approach are typically based on common biological themes such as biological pathways, chromosomal position, transcription factor binding sites, or individually contributed sets of genes previously shown to be differentially expressed under different experimental conditions (24). However, publicly available gene sets do not necessarily systematically incorporate large amounts of genetic data from either mouse or human.
The human disease and mouse phenotype-based gene sets used here were derived from the Genetic Association Database (GAD) (5, 26) in the case of human, or the phenotypes section of the Mouse Genome Informatics (MGI) database (7) for mouse. These databases archive gene-based disease and phenotype information based on either human genetic association studies or mouse genetic models. Unlike commonly used gene sets, the novel gene sets used here are based on population-based genetic association studies in common human disease and from mouse genetic models. These were recently described and shown to have relevance to common human disease and disease models by direct comparison and through multiple clustering techniques (26). These two new gene set collections are not related to the gene set enrichment analysis (GSEA)-MSig (24), KEGG (Kyoto Encyclopedia of Genes and Genomes) (20), or Gene Ontology (GO) (1)-based gene sets and are among the largest collections of gene sets publicly available.
Importantly, these gene sets are constructed with two unique features; 1) each gene in each gene set has some published genetic evidence of the assigned phenotype and 2) all disease or phenotype annotations use a controlled vocabulary, either MeSH (Medical Subject Heading) disease headings in human or mammalian phenotype terms (7) in mouse.
Here we demonstrate the utility and disease specificity of these human disease and mouse phenotype gene sets in large-scale gene set analysis of independent archival microarray studies of many different types. These disease and phenotype gene sets show marked disease or phenotype specificity in a species-specific way as well as in a cross-species manner. This approach enables rapid systematic integration of population-based genetic information or experimentally based mouse phenotype information with a growing archive of microarray-based gene expression information. This should aid in the interpretation, cross-validation, and clinically relevant translation of disease-based gene expression studies.
METHODS
Disease/phenotype web-PAGE.
Disease/phenotype web-parametric analysis of gene expression (D/P webPAGE) is a publicly available web utility we developed for PAGE using the PAGE algorithm developed by Kim and Volsky in 2005 (15). D/P webPAGE rapidly and systematically analyzes microarray-based gene expression data, using our recently identified novel human disease or mouse phenotype gene sets (26). The D/P webPAGE is a cgi web server written in PERL with embedded R and interpreted with a Mysql database. This web tool can be found freely available at: http://dpwebpage.nia.nih.gov.
The human gene sets used with this tool are based on the GAD (5) derived from human genetic association studies from common disease. This includes both candidate gene studies and GWAS studies, as well as additional independent gene sets from the OMIM (Online Mendelian Inheritance in Man) database. Mouse phenotype gene sets are based on the MGI database of mouse genetic phenotypes (7). All gene sets described here have been described recently (26). Each gene assignment in each gene set, human or mouse, is based on genetic evidence in the context of disease or phenotype. All gene sets can be found at this website: http://www.grc.nia.nih.gov/branches/rrb/dna/gas_data.htm.
We then identified multiple independent gene expression studies in the NCBI GEO or EBI ArrayExpress microarray databases that were relevant to either human disease or mouse disease models. Table 1 lists these studies including the reference, cells or tissues used, as well as the accession number for access to the individual experimental design and raw microarray data. Using the D/P webPAGE we analyzed individual studies in both a species-specific and cross-species manner.
Table 1.
Microarray gene expression studies
| Reference | PubMed ID # | Species | Disease/Model | Cell/Tissue | GEO/ArrayExpress Acc. # |
|---|---|---|---|---|---|
| Mootha Vamsi K et al. 2003 | 12808457 | human | Type II diabetes | skeletal muscle | E-CBIL-30 |
| Shanley TP et al. 2007 | 17932561 | human | sepsis | whole blood | GSE8121 |
| Blalock EM et al. 2004 | 14769913 | human | Alzheimer's | hippocampus | GSE1297 |
| Spira A et al. 2004 | 15210990 | human | smoking | airway epithelial cell | GSE994 |
| Lovegrove FE et al. 2007 | 17991715 | mouse | cerebral malaria | brain | GSE7814 |
| McDunn JE et al. 2006 | 16931309 | mouse | sepsis | CD4+ splenocytes | GSE4479 |
| Albino D 2008 | (unpub.) | mouse | developmental | neural crest cells | GSE11356 |
| Conrad CG et al. 2006 | (unpub.) | mouse | α-synuclein KO | brain | GSE4758 |
GEO, Gene Expression Omnibus; Acc., accession; unpub., unpublished; KO, knockout.
PAGE analysis of microarray data.
PAGE analysis of the microarray results was calculated according to Kim and Volsky (15) with the following modifications. The disease phenotype (i) Z-score is calculated by
in which the phenotype index I = 1,2,…, K. K is the total number of the disease phenotypes we included in our data set; ni is the number of genes in the subgroup of phenotype i in the current sample array; σa is the standard deviation of the current gene expression changes of the sample. Diff(i) is the difference between the mean value of gene expression changes in the subgroup disease phenotype (i) (GCi) and the mean value of the gene expression changes on the whole sample (GCa): diffi = ḠC̄i − ḠC̄a.
The empirical P-value of the disease phenotype i changes is described by:
in which Φ(x) is the standard normal distribution function with the variable as x = diffi/σ(diffi). σ(diffi) is the standard deviation of the difference for gene expression changes between phenotype subgroup (i) and the whole array (25)
σi is the standard deviation of the average gene expression changes in the disease phenotype (i). na is the total number of genes in the whole sample set. The plots were drawn with R-statistical programming language (R Development Core Team 2005) using either calculated or absolute z-score values.
In addition to the parametric-based P-value produced by the D/P webPAGE tool, we also performed a permutation test in parallel using 1,000 permutations on a representative sample. Results of this randomization provided similar results to the empirical parametric test and are shown in Supplemental Fig. S3.1
RESULTS
In this study we investigated the use of gene set analysis using novel human disease and mouse phenotype-based gene sets on gene expression to identify significantly altered gene sets that were enriched for disease relevance and biological meaning in the context of each specific gene expression study. To accomplish this we developed a publicly available web utility, Disease/Phenotype webPAGE (D/P webPAGE), which uses these novel gene sets relevant to human disease and disease models. D/P webPAGE accepts properly formatted microarray data and provides output of over- or underrepresented human disease or mouse phenotype groups. The gene set files used contain either 1,317 human or 5,142 mouse gene sets from common human disease or mouse phenotypes. Importantly, these gene sets use standardized ontological nomenclature, which includes MeSH keywords for human disease annotation or mammalian phenotype codes for mouse phenotype designations. These gene sets have been recently presented (26). We have used these gene sets with D/P webPAGE in analyzing disease- or phenotype-related microarray-based gene expression datasets from the NCBI GEO (4) or EBI ArrayExpress (21) gene expression archival database. Information on these studies can be found in Table 1.
Human gene expression vs. human gene sets.
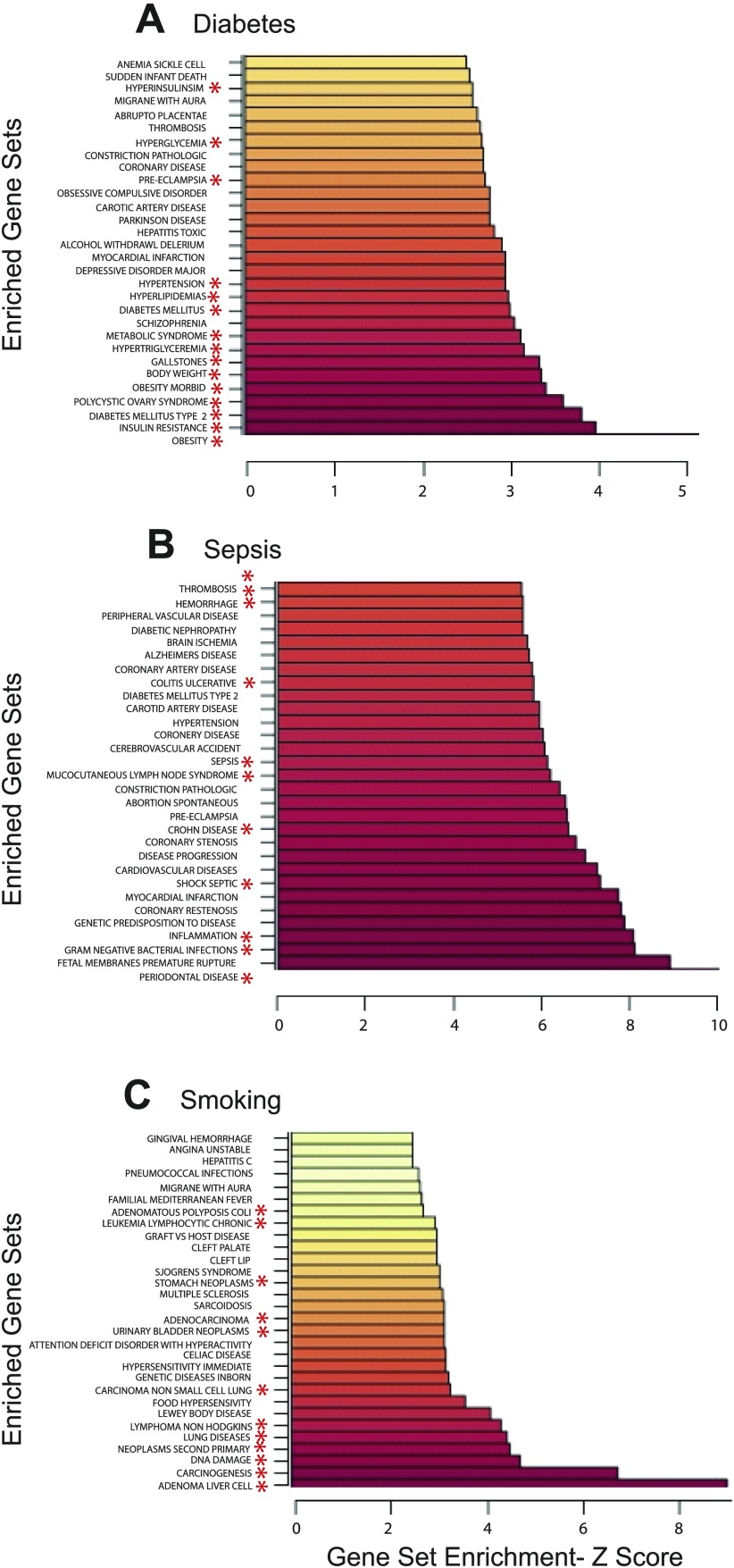
In these experiments we analyze human disease-related microarray studies with 1,317 GAD human disease gene sets. Figure 1 shows ribbon graphs of the top 30 Z-scores of the statistically significant (P < 0.05) gene sets of three independent microarray studies analyzed with the identical GAD human disease-based gene set file using the D/P webPAGE utility. These include human studies on Type 2 diabetes (18), sepsis (22), and smoking (23). The Z-score value is on the x-axis, while the most highly enriched gene sets are on the y-axis. In each case, statistically significantly altered gene sets (P < 0.05) show a clear relevance to the original disease topic of the gene expression study without significant overlap between studies (Supplemental Fig. S1). In Type 2 diabetes (Fig. 1A), the top altered gene sets include obesity, insulin resistance, diabetes mellitus Type 2, polycystic ovarian syndrome, and metabolic syndrome, among others.
Fig. 1.
Human gene expression vs. human gene sets. Ribbon graphs of the top 30 highest Z-scores of the statistically significant (P < 0.05) parametric analysis of gene expression (PAGE) results in human Type 2 diabetes -ArrayExpress #E-CBIL-30 (A), sepsis - Gene Expression Omnibus (GEO) #GSE8182 (B), and smoking - GEO #GSE994 (C), using the human Genetic Association Database (GAD) disease gene sets. *Gene sets with ontological relevance to the disease of reference.
Significantly altered gene sets in human sepsis using D/P webPAGE (Fig. 1B) are related to bacterial infections, inflammation, septic shock, sepsis, and other inflammatory conditions, among others. Gene expression results from human lung tissue in a smoking study (Fig. 1C) were enriched for DNA damage and tumor-related gene sets, as well as inflammatory gene sets. All three studies were analyzed with the same primary GAD human disease gene set input file of 1,317 human disease gene sets using the same parameters and show minimal overlap in the top 30 significantly altered gene sets or in all gene sets (Supplemental Fig. 1A).
Comparison of D/P webPAGE and GSEA.
GSEA is a commonly used gene set computational tool for the analysis of microarray data (24). To test the comparative disease specificity of these two web tools and their underlying algorithms, we ran the same Type 2 diabetes gene expression data and GAD disease gene sets in parallel with both the D/P webPAGE utility and the GSEA software (24). Table 2 shows a comparative ranking of D/P webPAGE and GSEA results. Major clinical phenotypes and subphenotypes of Type 2 diabetes such as insulin resistance, polycystic ovarian syndrome, diabetes mellitus-Type 2, and morbid obesity are ranked in the top 20 statistically significant (P < 0.05) results in D/P webPAGE and are not found using GSEA. In comparison, the top 20 gene sets produced by GSEA are shown in Supplemental Table S1.
Table 2.
Comparative rank of gene sets
| Rank |
||
|---|---|---|
| GAD Disease Gene Set | D/P webPAGE | GSEA |
| Obesity | 1 | 4 |
| Insulin resistance | 2 | 31 |
| Diabetes mellitus, Type 2 | 3 | 40 |
| Polycystic ovary syndrome | 4 | 180 |
| West Nile fever | 5 | nss |
| Obesity, morbid | 6 | 51 |
| Body weight | 7 | 47 |
| Gallstones | 8 | 25 |
| Hypertriglyceridemia | 9 | 22 |
| Metabolic syndrome X | 10 | 10 |
| Bronchitis, chronic | 11 | 174 |
| Leukodystrophy, metachromatic | 12 | nss |
| Schizophrenia | 13 | 295 |
| Convalescence | 14 | nss |
| Diabetes mellitus | 15 | 141 |
| Hyperlipidemias | 16 | 7 |
| Hypertension | 17 | 88 |
| Depressive disorder, major | 18 | 228 |
| Myocardial infarction | 19 | 164 |
| Von Willebrand disease | 20 | nss |
GAD, Genetic Association Database; D/P webPAGE, disease/phenotype web-parametric analysis of gene expression; GSEA, gene set enrichment analysis; nss, not statistically significant.
Mouse gene expression vs. mouse phenotype gene sets.
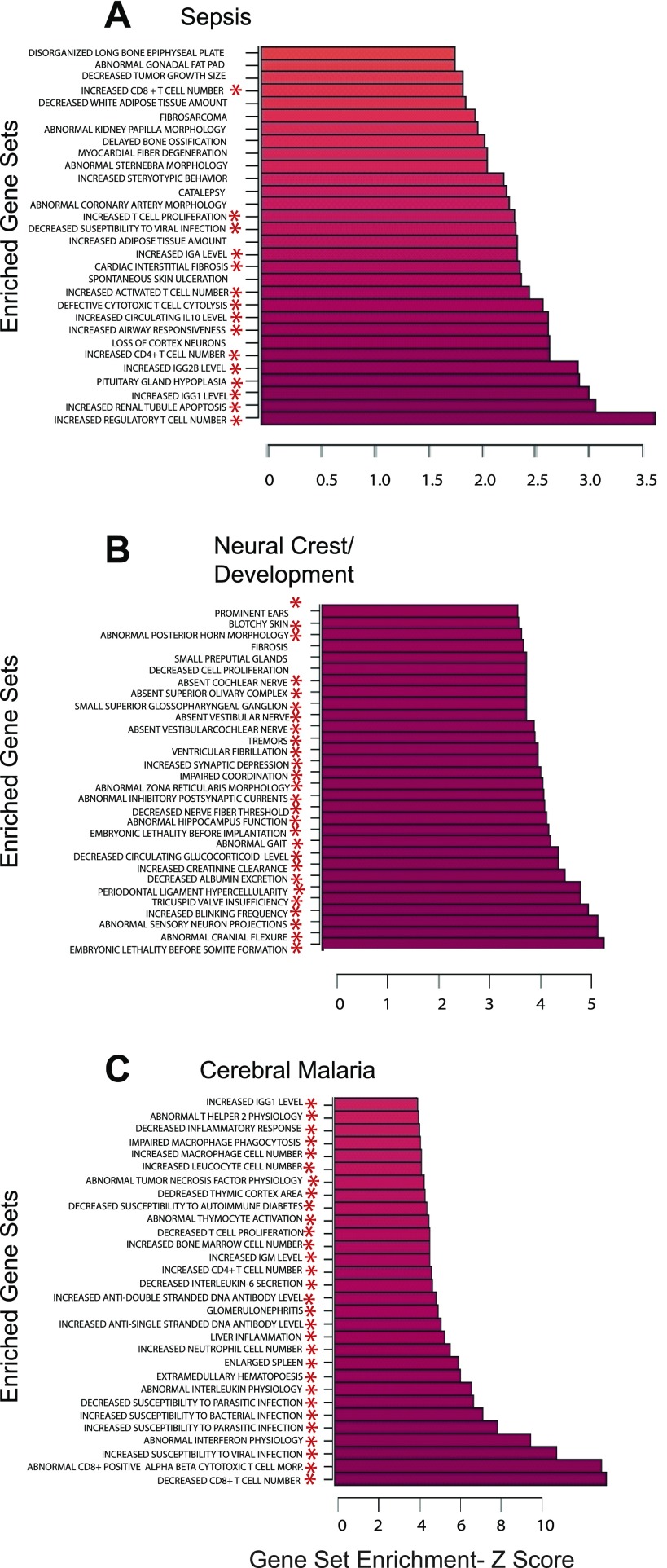
In these examples, mouse gene expression studies were analyzed with 5,142 mouse phenotype gene sets (MP gene sets) that were derived from mouse genetic models of disease (26) from the mouse MGI phenotypes database. Figure 2A shows three independent analyses of gene expression in mouse sepsis (17), cerebral malaria (16), and neural crest development (Albino D, 2008, unpublished) analyzed with the D/P webPAGE utility. Mouse sepsis (Fig. 2A) shows significant alteration in mouse immune gene sets highly relevant to sepsis including increased regulatory T cells, alterations in apoptosis, and alterations in immunoglobulin genes, among others. Gene expression in neural crest development is shown (Fig. 2B) enriched for mouse gene sets involved in developmental processes or neural function, such as abnormal sensory neuron projections, embryonic lethality before somite formation, and abnormal posterior horn morphology. There are no immune or inflammatory gene sets in the top 30 results for neural crest in marked contrast to sepsis (Fig. 2A). Results for cerebral malaria are shown in Fig. 2C. Although the top 30 gene sets shown here are all immune or inflammatory related, there is little overlap to the immune and inflammatory gene sets of sepsis (Supplemental Fig. S1b).
Fig. 2.
Mouse gene expression vs. mouse gene sets. Ribbon graphs of the top 30 highest Z-scores of the statistically significant (P < 0.05) PAGE results in mouse sepsis - GEO #GSE4479 (A), neural crest/development - GEO #GSE11356 (B), and cerebral malaria - GEO #GSE7814 (C) using the mouse mammalian phenotype (MP) gene sets. *Gene sets with ontological relevance to the disease of reference.
Figure 3 shows a more dynamic view of brain gene expression in cerebral malaria progression as a heat map generated by D/P webPAGE. Highlighted in day 1 of infection are altered gene sets involved in brain function, while infection at day 6 is predominated by brain inflammation. In addition, gene expression from mouse brain in an α-synuclein gene knockout (Conrad CG, 2006, unpublished) was analyzed with the same mouse gene set file as a comparison. The enriched gene sets were markedly different compared with cerebral malaria demonstrating no brain tissue bias (Supplemental Fig. S2).
Fig. 3.
Mouse gene expression vs. mouse gene sets. Heat map of mouse B6 cerebral malaria GEO #GSE7814. B6 day 1 vs. B6 day 0; B6 day 3 vs. B6 day 0; B6 day 6 vs. B6 day 0.
Human gene expression vs. mouse phenotype gene sets.
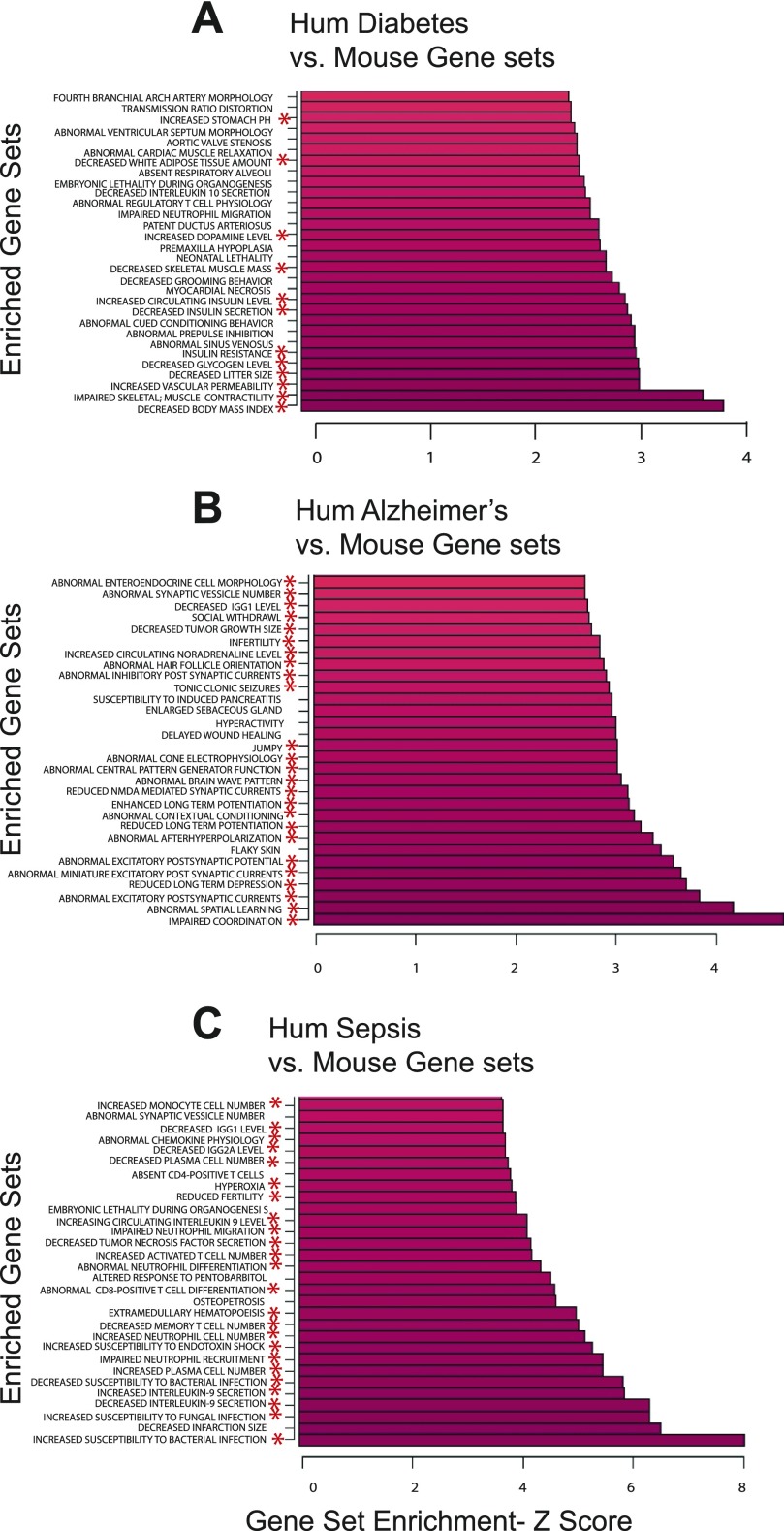
A primary rationale for the use of animals in biomedical research is the ability of animal models of disease to inform on the human condition. In Fig. 4 we show the use of the mouse phenotype gene sets to analyze human disease-related gene expression studies. Using the mouse gene sets, we find that human Type 2 diabetes is greatly enriched for gene sets highly relevant to diabetes including alterations in body mass, insulin resistance, insulin secretion, and skeletal muscle gene set alterations, among others. Human Alzheimer's (6) (Fig. 4B) is shown enriched for gene sets in brain function including spatial learning, long-term potentiation, and synaptic alterations. Gene expression patterns in human sepsis (Fig. 4C) are altered in mouse gene sets for immune function and inflammation. In these cases, the identical mouse gene set input file displays striking disease or experimental relevance with little overlap of significantly altered gene sets between the studies shown (Supplemental Fig. S1c).
Fig. 4.
Human gene expression vs. mouse gene sets. Ribbon graphs of the top 30 highest Z-scores of the statistically significant (P < 0.05) PAGE results in human Type 2 diabetes ArrayExpress #E-CBIL-30 (A), Alzheimer's disease GEO #GSE 1297 (B), and human sepsis GEO #GSE 8121 (C) using the mouse MP gene sets. *Gene sets with clear ontological relevance to the disease of reference.
DISCUSSION
The molecular and genetic understanding of common human disease and experimental animal models of disease is complex. Broad based pleiotropy, multifactorial and combinatorial gene-gene interactions, small effect size of individual genes, and complex gene-environment interactions, often occurring over many decades, make interpretation of genetic and molecular studies of disease quite challenging (2, 3, 11–13).
Here, we use thousands of gene combinations representing over 1,300 human disease phenotypes and greater than 5,000 experimentally determined mouse phenotypes to systematically analyze molecular studies of human and mouse disease. Unlike other collections of gene sets (24) each gene assignment in a gene set was originally identified in either population-based studies of human disease or mouse experimental genetic models of disease. We integrate this information with independent disease-related microarray-based gene expression studies from different groups, demonstrating disease relevance and specificity. Moreover, unlike other gene sets commonly used (24), both the human and mouse gene sets described here were developed using systematic ontological terminology, MeSH disease headings, or mammalian phenotype codes. Therefore, these gene sets have an internal consistency and comparability that can be placed in the hierarchy of disease and phenotype ontology.
In addition, we cross-species barriers in using gene set analysis. In this way we automatically and systematically leverage over 9,000 independent mouse genetic experimental models, and over 8,000 mouse genes with defined mouse phenotypes to study human disease in an unbiased manner. We have also used mouse gene sets on rat gene expression data (data not shown). It is likely that useful biological insights can be extracted from cross-species analysis of gene expression data from other species with proper formatting of files. Moreover, as we gain experience in the use of these gene sets, we believe the human gene sets tend to represent broader end-stage clinical disease classifications, while the mouse gene sets tend to represent more narrow subphenotypes, providing useful complementary information. Preliminary analysis also suggests these disease gene sets are useful in analysis of both GWAS-based single nucleotide polymorphism (8–10) and high-throughput proteomic data (not shown).
The interpretation of enriched gene sets described here should be used with caution due to any number of factors, including the uncertainty of genetic association assignments, influences of genetic background in mouse models, peculiarities in disease and phenotype ontologies, and especially in cross-species biological inferences. In addition, variation and peculiarities in microarray experiments will influence the biological and disease relevance of gene set analysis results.
However, this approach can be a useful tool in the analysis of complex disease providing a rapid, systematic approach to help validate and interpret disease-associated gene assignments, experimental genetic models, and gene expression studies, all with a focus on disease models and human disease. This should aid in the translation of ongoing and archival human gene expression studies as well as mouse experimental models of disease into a clinical context.
GRANTS
This research was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Institute on Aging, and the NIH Center for Information Technology.
Supplementary Material
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G; The Gene Ontology Consortium. Gene ontology: tool for the unification of biology. Nat Genet 25: 25–29, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attia J, Ioannidis JP, Thakkinstian A, McEvoy M, Scott RJ, Minelli C, Thompson J, Infante-Rivard C, Guyatt G. How to use an article about genetic association. A: Background concepts. JAMA 301: 74–81, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Barnes KC. Gene-environment and gene-gene interaction studies in the molecular genetic analysis of asthma and atopy. Clin Exp Allergy 29, Suppl 4: 47–51, 1999. [PubMed] [Google Scholar]
- 4.Barrett T, Troup DB, Wilhite SE, Ledoux P, Rudnev D, Evangelista C, Kim IF, Soboleva A, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Muertter RN, Edgar R. NCBI GEO: archive for high-throughput functional genomic data. Nucleic Acids Res 37: D885–D890, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker KG, Barnes KC, Bright TJ, Wang SA. The genetic association database. Nat Genet 36: 431–432, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Blalock EM, Geddes JW, Chen KC, Porter NM, Markesbery WR, Landfield PW. Incipient Alzheimer's disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc Natl Acad Sci USA 101: 2173–2178, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bult CJ, Eppig JT, Kadin JA, Richardson JE, Blake JA. The Mouse Genome Database (MGD): mouse biology and model systems. Nucleic Acids Res 36: D724–D728, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chai HS, Sicotte H, Bailey KR, Turner ST, Asmann YW, Kocher JP. GLOSSI: a method to assess the association of genetic loci-sets with complex diseases. BMC Bioinformatics 10: 102, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chasman DI. On the utility of gene set methods in genomewide association studies of quantitative traits. Genet Epidemiol 32: 658–668, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Holden M, Deng S, Wojnowski L, Kulle B. GSEA-SNP: applying gene set enrichment analysis to SNP data from genome-wide association studies. Bioinformatics 24: 2784–2785, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Iles MM. What can genome-wide association studies tell us about the genetics of common disease? PLoS Genet 4: e33, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khoury MJ, Little J, Gwinn M, Ioannidis JP. On the synthesis and interpretation of consistent but weak gene-disease associations in the era of genome-wide association studies. Int J Epidemiol 36: 439–445, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Khoury MJ, Little J, Higgins J, Ioannidis JP, Gwinn M. Reporting of systematic reviews: the challenge of genetic association studies. PLoS Med 4: e211, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SB, Yang S, Kim SK, Kim SC, Woo HG, Volsky DJ, Kim SY, Chu IS. GAzer: gene set analyzer. Bioinformatics 23: 1697–1699, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Kim SY, Volsky DJ. PAGE: parametric analysis of gene set enrichment. BMC Bioinformatics 6: 144, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovegrove FE, Gharib SA, Patel SN, Hawkes CA, Kain KC, Liles WC. Expression microarray analysis implicates apoptosis and interferon-responsive mechanisms in susceptibility to experimental cerebral malaria. Am J Pathol 171: 1894–1903, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDunn JE, Turnbull IR, Polpitiya AD, Tong A, MacMillan SK, Osborne DF, Hotchkiss RS, Colonna M, Cobb JP. Splenic CD4+ T cells have a distinct transcriptional response six hours after the onset of sepsis. J Am Coll Surg 203: 365–375, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34: 267–273, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Nam D, Kim SY. Gene-set approach for expression pattern analysis. Brief Bioinform 9: 189–197, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Okuda S, Yamada T, Hamajima M, Itoh M, Katayama T, Bork P, Goto S, Kanehisa M. KEGG Atlas mapping for global analysis of metabolic pathways. Nucleic Acids Res 36: W423–W426, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parkinson H, Kapushesky M, Kolesnikov N, Rustici G, Shojatalab M, Abeygunawardena N, Berube H, Dylag M, Emam I, Farne A, Holloway E, Lukk M, Malone J, Mani R, Pilicheva E, Rayner TF, Rezwan F, Sharma A, Williams E, Bradley XZ, Adamusiak T, Brandizi M, Burdett T, Coulson R, Krestyaninova M, Kurnosov P, Maguire E, Neogi SG, Rocca-Serra P, Sansone SA, Sklyar N, Zhao M, Sarkans U, Brazma A. ArrayExpress update–from an archive of functional genomics experiments to the atlas of gene expression. Nucleic Acids Res 37: D868–D872, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shanley TP, Cvijanovich N, Lin R, Allen GL, Thomas NJ, Doctor A, Kalyanaraman M, Tofil NM, Penfil S, Monaco M, Odoms K, Barnes M, Sakthivel B, Aronow BJ, Wong HR. Genome-level longitudinal expression of signaling pathways and gene networks in pediatric septic shock. Mol Med 13: 495–508, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spira A, Beane J, Shah V, Liu G, Schembri F, Yang X, Palma J, Brody JS. Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proc Natl Acad Sci USA 101: 10143–10148, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102: 15545–15550, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Belle G, Fisher LD, Heagerty PJ, Lumley TS. Biostatistics: A Methodology for Health Science. Hoboken, NJ: John Wiley and Sons, 2004. [Google Scholar]
- 26.Zhang Y, De S, Garner JR, Smith K, Wang SA, Becker KG. Systematic analysis, comparison, and integration of disease based human genetic association data and mouse genetic phenotypic information. BMC Med Genomics 3: 1, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.