Abstract
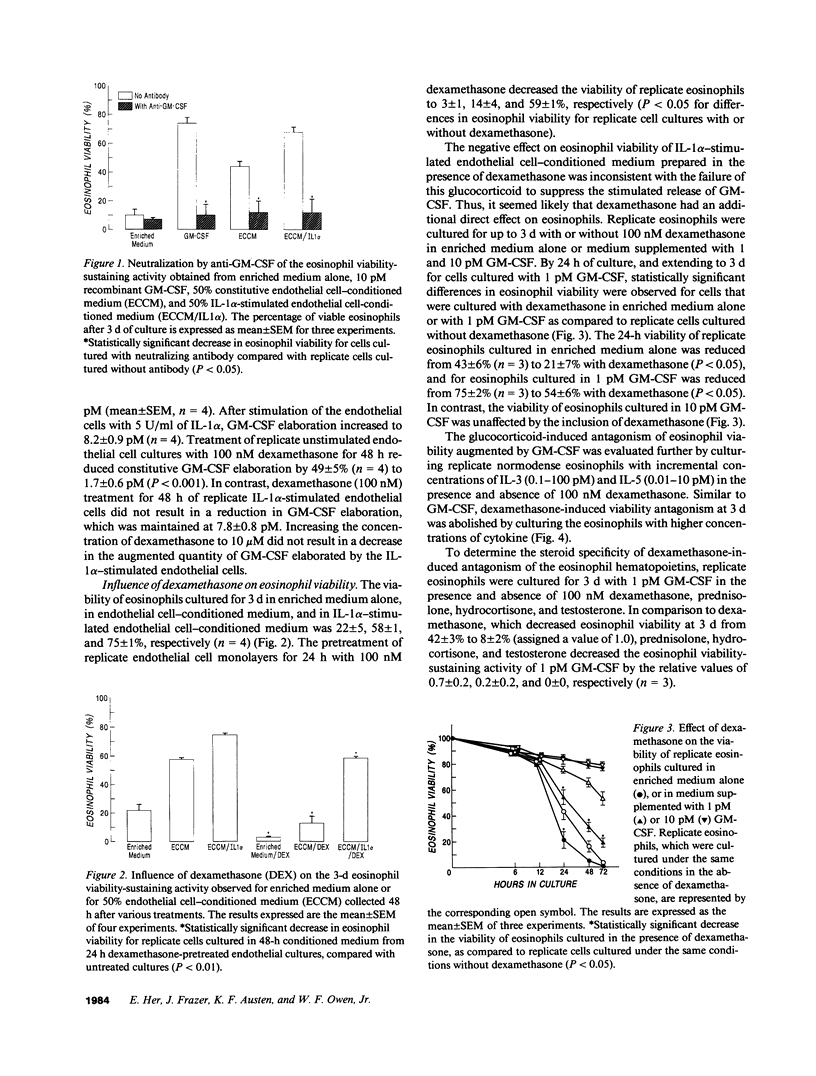
Granulocyte-macrophage colony-stimulating factor (GM-CSF) was established as the constitutive and elicited human umbilical vein endothelial cell-derived eosinophil viability-sustaining factor. Stimulation of endothelium cell monolayers with IL-1 alpha (5 U/ml) increased the 48-h elaboration of GM-CSF from a mean of 3.2 to a mean of 8.2 pM (P less than 0.05). Dexamethasone (100 nM) decreased the constitutive GM-CSF elaboration by 49% (P less than 0.001) but did not diminish production by IL-1 alpha-stimulated endothelium. However, eosinophil viability decreased by 21% in dexamethasone-pretreated IL-1 alpha-stimulated endothelial cell-conditioned medium (P less than 0.05), which suggested viability antagonism by glucocorticoids. After 24 h of culture, eosinophil viability for replicate cells in enriched medium alone or with 1 pM GM-CSF decreased from means of 43 and 75% to means of 21 and 54%, respectively, when dexamethasone was included (P less than 0.05). However, 10 pM GM-CSF, IL-3, or IL-5 protected the cells against dexamethasone and against endonuclease-specific DNA fragmentation. In this model system of eosinophil-tissue interactions, dexamethasone prevents the endothelial cells from inducing a pathobiologic phenotypic change in the eosinophil by suppression of GM-CSF elaboration to concentrations that are not cytoprotective. Cytokine priming by GM-CSF, IL-3, or IL-5 may account for the differential responsiveness of select eosinophilic disorders to glucocorticoids.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broudy V. C., Kaushansky K., Harlan J. M., Adamson J. W. Interleukin 1 stimulates human endothelial cells to produce granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor. J Immunol. 1987 Jul 15;139(2):464–468. [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Compton M. M., Cidlowski J. A. Rapid in vivo effects of glucocorticoids on the integrity of rat lymphocyte genomic deoxyribonucleic acid. Endocrinology. 1986 Jan;118(1):38–45. doi: 10.1210/endo-118-1-38. [DOI] [PubMed] [Google Scholar]
- Cox G., Ohtoshi T., Vancheri C., Denburg J. A., Dolovich J., Gauldie J., Jordana M. Promotion of eosinophil survival by human bronchial epithelial cells and its modulation by steroids. Am J Respir Cell Mol Biol. 1991 Jun;4(6):525–531. doi: 10.1165/ajrcmb/4.6.525. [DOI] [PubMed] [Google Scholar]
- Enokihara H., Furusawa S., Nakakubo H., Kajitani H., Nagashima S., Saito K., Shishido H., Hitoshi Y., Takatsu K., Noma T. T cells from eosinophilic patients produce interleukin-5 with interleukin-2 stimulation. Blood. 1989 May 15;73(7):1809–1813. [PubMed] [Google Scholar]
- Enokihara H., Kajitani H., Nagashima S., Tsunogake S., Takano N., Saito K., Furusawa S., Shishido H., Hitoshi Y., Takatsu K. Interleukin 5 activity in sera from patients with eosinophilia. Br J Haematol. 1990 Aug;75(4):458–462. doi: 10.1111/j.1365-2141.1990.tb07782.x. [DOI] [PubMed] [Google Scholar]
- Kaushansky K. Control of granulocyte-macrophage colony-stimulating factor production in normal endothelial cells by positive and negative regulatory elements. J Immunol. 1989 Oct 15;143(8):2525–2529. [PubMed] [Google Scholar]
- Lamas A. M., Leon O. G., Schleimer R. P. Glucocorticoids inhibit eosinophil responses to granulocyte-macrophage colony-stimulating factor. J Immunol. 1991 Jul 1;147(1):254–259. [PubMed] [Google Scholar]
- Lamas A. M., Marcotte G. V., Schleimer R. P. Human endothelial cells prolong eosinophil survival. Regulation by cytokines and glucocorticoids. J Immunol. 1989 Jun 1;142(11):3978–3984. [PubMed] [Google Scholar]
- Le Beau M. M., Lemons R. S., Espinosa R., 3rd, Larson R. A., Arai N., Rowley J. D. Interleukin-4 and interleukin-5 map to human chromosome 5 in a region encoding growth factors and receptors and are deleted in myeloid leukemias with a del(5q). Blood. 1989 Feb 15;73(3):647–650. [PubMed] [Google Scholar]
- Limaye A. P., Abrams J. S., Silver J. E., Ottesen E. A., Nutman T. B. Regulation of parasite-induced eosinophilia: selectively increased interleukin 5 production in helminth-infected patients. J Exp Med. 1990 Jul 1;172(1):399–402. doi: 10.1084/jem.172.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan D. F., Welch G. R., Wahl S. M. Lipopolysaccharide, tumor necrosis factor-alpha, and IL-1 beta prevent programmed cell death (apoptosis) in human peripheral blood monocytes. J Immunol. 1991 Mar 1;146(5):1541–1546. [PubMed] [Google Scholar]
- Owen W. F., Jr, Petersen J., Sheff D. M., Folkerth R. D., Anderson R. J., Corson J. M., Sheffer A. L., Austen K. F. Hypodense eosinophils and interleukin 5 activity in the blood of patients with the eosinophilia-myalgia syndrome. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8647–8651. doi: 10.1073/pnas.87.21.8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen W. F., Jr, Rothenberg M. E., Silberstein D. S., Gasson J. C., Stevens R. L., Austen K. F., Soberman R. J. Regulation of human eosinophil viability, density, and function by granulocyte/macrophage colony-stimulating factor in the presence of 3T3 fibroblasts. J Exp Med. 1987 Jul 1;166(1):129–141. doi: 10.1084/jem.166.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen W. F., Rothenberg M. E., Petersen J., Weller P. F., Silberstein D., Sheffer A. L., Stevens R. L., Soberman R. J., Austen K. F. Interleukin 5 and phenotypically altered eosinophils in the blood of patients with the idiopathic hypereosinophilic syndrome. J Exp Med. 1989 Jul 1;170(1):343–348. doi: 10.1084/jem.170.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachar A., Fleischer S., Frickhofen N., Heimpel H., Fleischer B. T lymphocyte control of human eosinophilic granulopoiesis. Clonal analysis in an idiopathic hypereosinophilic syndrome. J Immunol. 1987 Dec 1;139(11):3753–3758. [PubMed] [Google Scholar]
- Rothenberg M. E., Owen W. F., Jr, Silberstein D. S., Soberman R. J., Austen K. F., Stevens R. L. Eosinophils cocultured with endothelial cells have increased survival and functional properties. Science. 1987 Aug 7;237(4815):645–647. doi: 10.1126/science.3110954. [DOI] [PubMed] [Google Scholar]
- Rothenberg M. E., Owen W. F., Jr, Silberstein D. S., Woods J., Soberman R. J., Austen K. F., Stevens R. L. Human eosinophils have prolonged survival, enhanced functional properties, and become hypodense when exposed to human interleukin 3. J Clin Invest. 1988 Jun;81(6):1986–1992. doi: 10.1172/JCI113547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg M. E., Petersen J., Stevens R. L., Silberstein D. S., McKenzie D. T., Austen K. F., Owen W. F., Jr IL-5-dependent conversion of normodense human eosinophils to the hypodense phenotype uses 3T3 fibroblasts for enhanced viability, accelerated hypodensity, and sustained antibody-dependent cytotoxicity. J Immunol. 1989 Oct 1;143(7):2311–2316. [PubMed] [Google Scholar]
- Rothenberg M. E., Pomerantz J. L., Owen W. F., Jr, Avraham S., Soberman R. J., Austen K. F., Stevens R. L. Characterization of a human eosinophil proteoglycan, and augmentation of its biosynthesis and size by interleukin 3, interleukin 5, and granulocyte/macrophage colony stimulating factor. J Biol Chem. 1988 Sep 25;263(27):13901–13908. [PubMed] [Google Scholar]
- Shi Y. F., Szalay M. G., Paskar L., Sahai B. M., Boyer M., Singh B., Green D. R. Activation-induced cell death in T cell hybridomas is due to apoptosis. Morphologic aspects and DNA fragmentation. J Immunol. 1990 May 1;144(9):3326–3333. [PubMed] [Google Scholar]
- Sieff C. A., Tsai S., Faller D. V. Interleukin 1 induces cultured human endothelial cell production of granulocyte-macrophage colony-stimulating factor. J Clin Invest. 1987 Jan;79(1):48–51. doi: 10.1172/JCI112806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein D. S., Austen K. F., Owen W. F., Jr Hemopoietins for eosinophils. Glycoprotein hormones that regulate the development of inflammation in eosinophilia-associated disease. Hematol Oncol Clin North Am. 1989 Sep;3(3):511–533. [PubMed] [Google Scholar]
- Silberstein D. S., Owen W. F., Gasson J. C., DiPersio J. F., Golde D. W., Bina J. C., Soberman R., Austen K. F., David J. R. Enhancement of human eosinophil cytotoxicity and leukotriene synthesis by biosynthetic (recombinant) granulocyte-macrophage colony-stimulating factor. J Immunol. 1986 Nov 15;137(10):3290–3294. [PubMed] [Google Scholar]
- Smith C. A., Williams G. T., Kingston R., Jenkinson E. J., Owen J. J. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989 Jan 12;337(6203):181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- Williams G. T., Smith C. A., Spooncer E., Dexter T. M., Taylor D. R. Haemopoietic colony stimulating factors promote cell survival by suppressing apoptosis. Nature. 1990 Jan 4;343(6253):76–79. doi: 10.1038/343076a0. [DOI] [PubMed] [Google Scholar]
- Zucali J. R., Dinarello C. A., Oblon D. J., Gross M. A., Anderson L., Weiner R. S. Interleukin 1 stimulates fibroblasts to produce granulocyte-macrophage colony-stimulating activity and prostaglandin E2. J Clin Invest. 1986 Jun;77(6):1857–1863. doi: 10.1172/JCI112512. [DOI] [PMC free article] [PubMed] [Google Scholar]