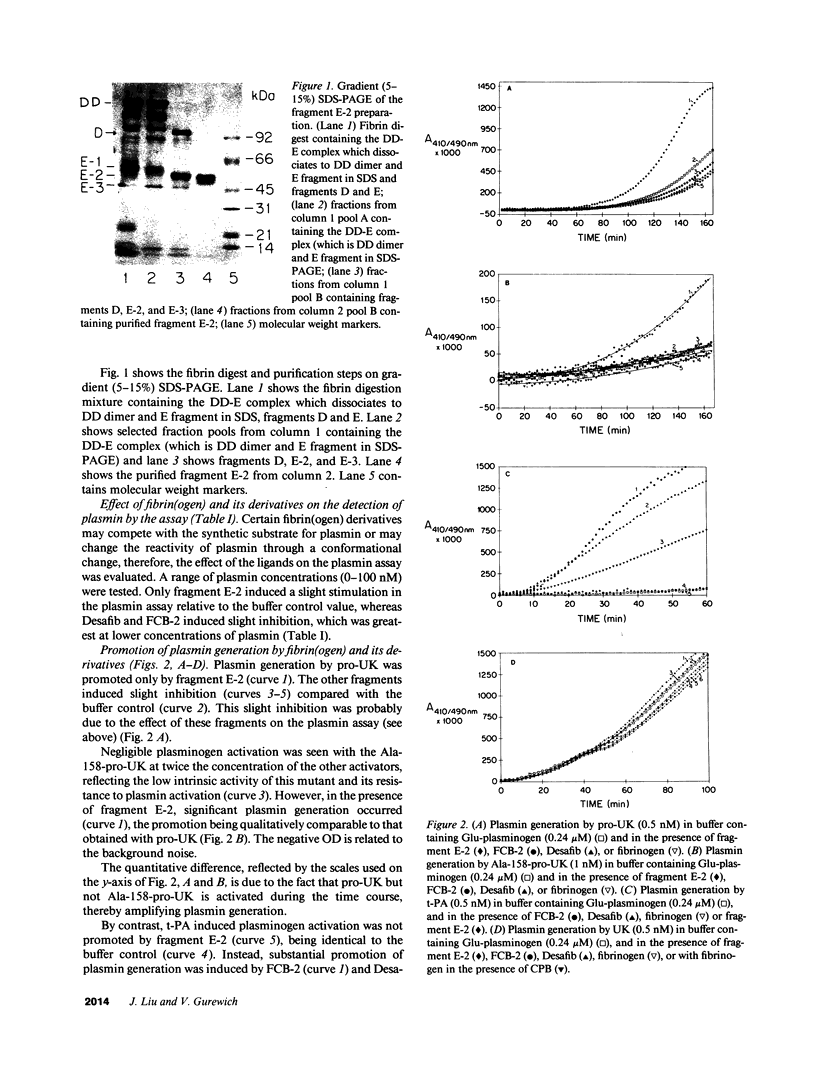
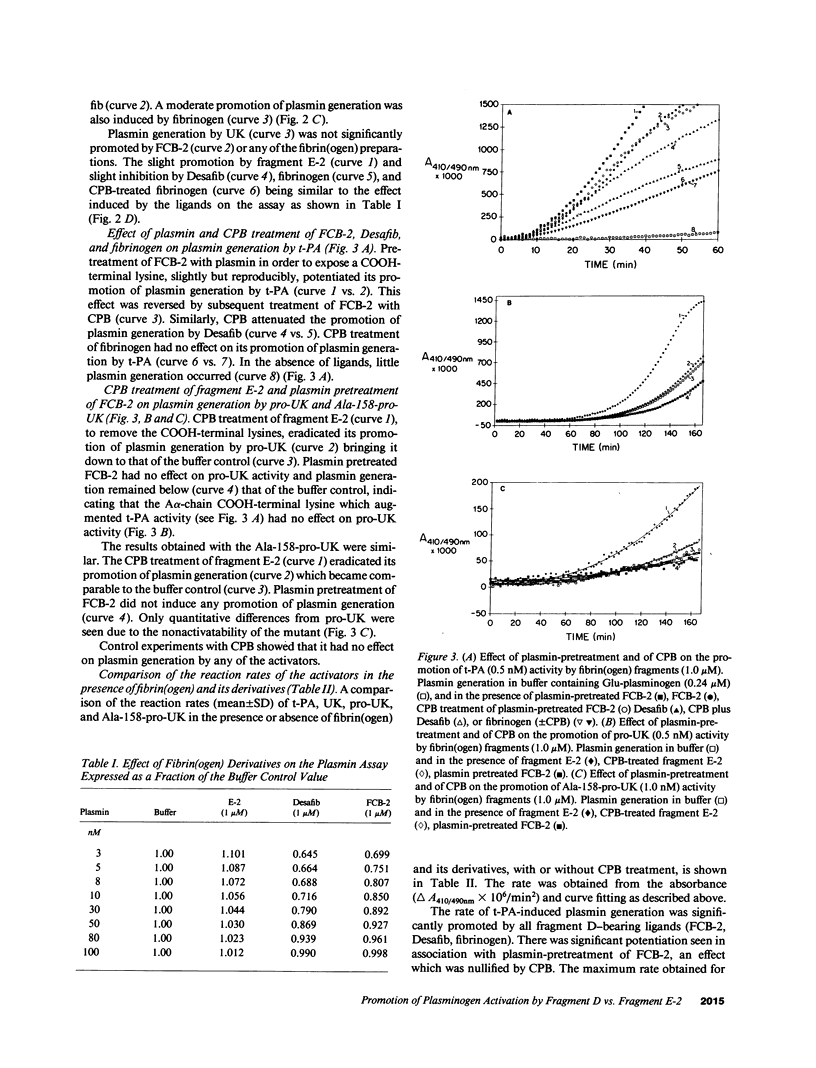
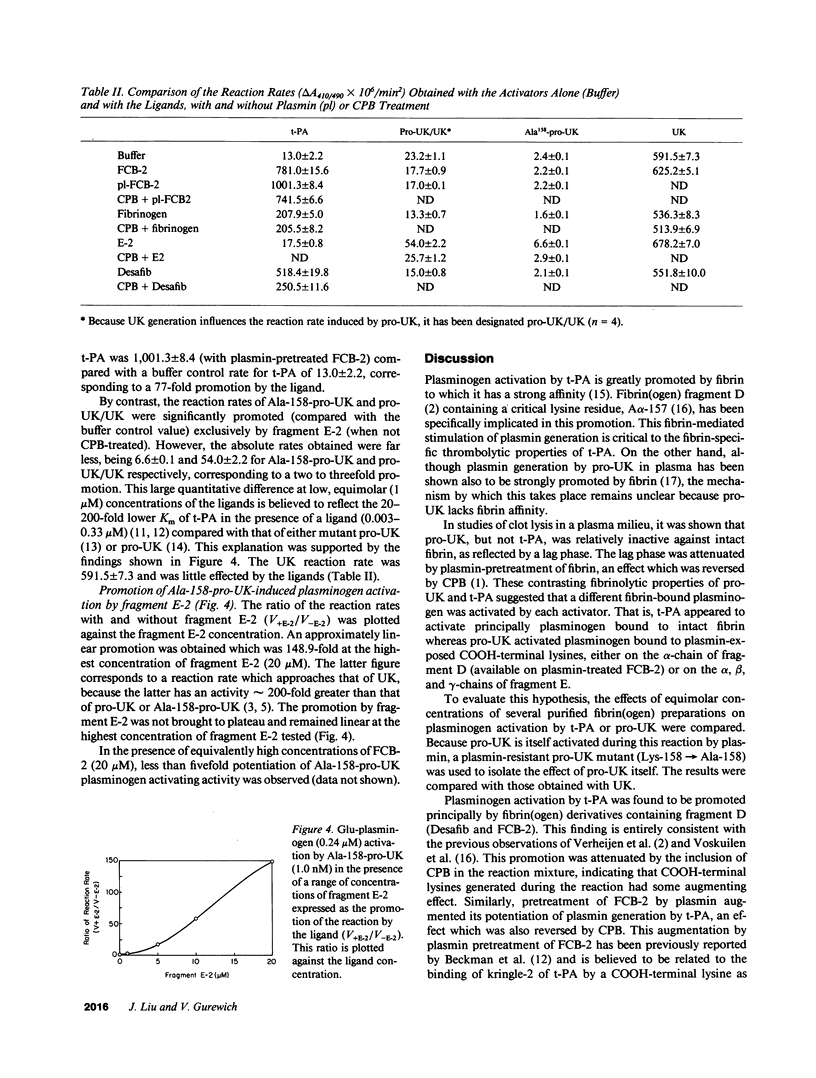
Abstract
Plasmin generation by equimolar concentrations of tissue plasminogen activator (t-PA), pro-urokinase (pro-UK), and urokinase (UK), and a twofold higher concentration of a plasmin-resistant mutant rpro-UK (Ala-158-pro-UK) was measured on a microtiter plate reader. The promoting effects on this reaction of equimolar concentrations of fibrinogen, soluble fibrin (Desafib), CNBr fragment FCB-2 (an analogue of fragment D), or purified fragment E-2 were compared. Plasmin generation by t-PA was moderately promoted by fibrinogen, substantially promoted by Desafib and FCB-2, but not at all promoted by fragment E-2. By contrast, plasmin generation by pro-UK or by Ala-158-pro-UK was not promoted either by fibrinogen, Desafib, or FCB-2, but was significantly promoted by fragment E-2. Plasmin generation by UK was not significantly promoted by any of the fibrin(ogen) preparations. Treatment of fragment E-2 by carboxypeptidase-B (CPB), eliminated its promotion of pro-UK and Ala-158-pro-UK-induced plasmin generation. Pretreatment of FCB-2 with plasmin slightly potentiated its promotion of t-PA activity. This effect of plasmin pretreatment of FCB-2 was reversed by CPB treatment. Plasmin pretreatment of FCB-2 did not induce any promotion of activity in pro-UK or Ala-158-pro-UK. The findings show that the intrinsic activity of pro-UK and the activity of t-PA are promoted by different regions of the fibrin(ogen) molecule. The latter is stimulated primarily by a determinant in the fragment D region, which is available in intact fibrin. By contrast, plasminogen activation by the intrinsic activity of pro-UK was stimulated exclusively by fragment E-2, which is unavailable in intact fibrin. The findings are believed relevant to fibrinolysis and support the concept that t-PA and pro-UK are complementary, sequential, and synergistic in their actions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckmann R., Geiger M., Binder B. R. Plasminogen activation by tissue plasminogen activator in the presence of stimulating CNBr fragment FCB-2 of fibrinogen is a two-phase reaction. Kinetic analysis of the initial phase of slow plasmin formation. J Biol Chem. 1988 May 25;263(15):7176–7180. [PubMed] [Google Scholar]
- Castellino F. J., Powell J. R. Human plasminogen. Methods Enzymol. 1981;80(Pt 100):365–378. doi: 10.1016/s0076-6879(81)80031-6. [DOI] [PubMed] [Google Scholar]
- Chibber B. A., Morris J. P., Castellino F. J. Effects of human fibrinogen and its cleavage products on activation of human plasminogen by streptokinase. Biochemistry. 1985 Jul 2;24(14):3429–3434. doi: 10.1021/bi00335a006. [DOI] [PubMed] [Google Scholar]
- Gurewich V., Pannell R., Broeze R. J., Mao J. Characterization of the intrinsic fibrinolytic properties of pro-urokinase through a study of plasmin-resistant mutant forms produced by site-specific mutagenesis of lysine(158). J Clin Invest. 1988 Dec;82(6):1956–1962. doi: 10.1172/JCI113815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurewich V., Pannell R., Louie S., Kelley P., Suddith R. L., Greenlee R. Effective and fibrin-specific clot lysis by a zymogen precursor form of urokinase (pro-urokinase). A study in vitro and in two animal species. J Clin Invest. 1984 Jun;73(6):1731–1739. doi: 10.1172/JCI111381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoylaerts M., Rijken D. C., Lijnen H. R., Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982 Mar 25;257(6):2912–2919. [PubMed] [Google Scholar]
- Lijnen H. R., Van Hoef B., Nelles L., Collen D. Plasminogen activation with single-chain urokinase-type plasminogen activator (scu-PA). Studies with active site mutagenized plasminogen (Ser740----Ala) and plasmin-resistant scu-PA (Lys158----Glu). J Biol Chem. 1990 Mar 25;265(9):5232–5236. [PubMed] [Google Scholar]
- Nieuwenhuizen W., Voskuilen M., Vermond A., Hoegee-de Nobel B., Traas D. W. The influence of fibrin(ogen) fragments on the kinetic parameters of the tissue-type plasminogen-activator-mediated activation of different forms of plasminogen. Eur J Biochem. 1988 May 16;174(1):163–169. doi: 10.1111/j.1432-1033.1988.tb14077.x. [DOI] [PubMed] [Google Scholar]
- Olexa S. A., Budzynski A. Z., Doolittle R. F., Cottrell B. A., Greene T. C. Structure of fragment E species from human cross-linked fibrin. Biochemistry. 1981 Oct 13;20(21):6139–6145. doi: 10.1021/bi00524a035. [DOI] [PubMed] [Google Scholar]
- Pannell R., Black J., Gurewich V. Complementary modes of action of tissue-type plasminogen activator and pro-urokinase by which their synergistic effect on clot lysis may be explained. J Clin Invest. 1988 Mar;81(3):853–859. doi: 10.1172/JCI113394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannell R., Gurewich V. Activation of plasminogen by single-chain urokinase or by two-chain urokinase--a demonstration that single-chain urokinase has a low catalytic activity (pro-urokinase). Blood. 1987 Jan;69(1):22–26. [PubMed] [Google Scholar]
- Pannell R., Gurewich V. Pro-urokinase: a study of its stability in plasma and of a mechanism for its selective fibrinolytic effect. Blood. 1986 May;67(5):1215–1223. [PubMed] [Google Scholar]
- Petersen L. C., Lund L. R., Nielsen L. S., Danø K., Skriver L. One-chain urokinase-type plasminogen activator from human sarcoma cells is a proenzyme with little or no intrinsic activity. J Biol Chem. 1988 Aug 15;263(23):11189–11195. [PubMed] [Google Scholar]
- Stump D. C., Lijnen H. R., Collen D. Purification and characterization of single-chain urokinase-type plasminogen activator from human cell cultures. J Biol Chem. 1986 Jan 25;261(3):1274–1278. [PubMed] [Google Scholar]
- Verheijen J. H., Nieuwenhuizen W., Wijngaards G. Activation of plasminogen by tissue activator is increased specifically in the presence of certain soluble fibrin(ogen) fragments. Thromb Res. 1982 Aug 15;27(4):377–385. doi: 10.1016/0049-3848(82)90055-x. [DOI] [PubMed] [Google Scholar]
- Voskuilen M., Vermond A., Veeneman G. H., van Boom J. H., Klasen E. A., Zegers N. D., Nieuwenhuizen W. Fibrinogen lysine residue A alpha 157 plays a crucial role in the fibrin-induced acceleration of plasminogen activation, catalyzed by tissue-type plasminogen activator. J Biol Chem. 1987 May 5;262(13):5944–5946. [PubMed] [Google Scholar]
- Váradi A., Patthy L. Beta(Leu121-Lys122) segment of fibrinogen is in a region essential for plasminogen binding by fibrin fragment E. Biochemistry. 1984 Apr 24;23(9):2108–2112. doi: 10.1021/bi00304a036. [DOI] [PubMed] [Google Scholar]
- Váradi A., Patthy L. Location of plasminogen-binding sites in human fibrin(ogen). Biochemistry. 1983 May 10;22(10):2440–2446. doi: 10.1021/bi00279a021. [DOI] [PubMed] [Google Scholar]
- de Vries C., Veerman H., Pannekoek H. Identification of the domains of tissue-type plasminogen activator involved in the augmented binding to fibrin after limited digestion with plasmin. J Biol Chem. 1989 Jul 25;264(21):12604–12610. [PubMed] [Google Scholar]