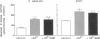Abstract
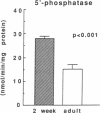
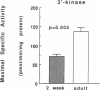
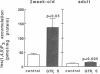
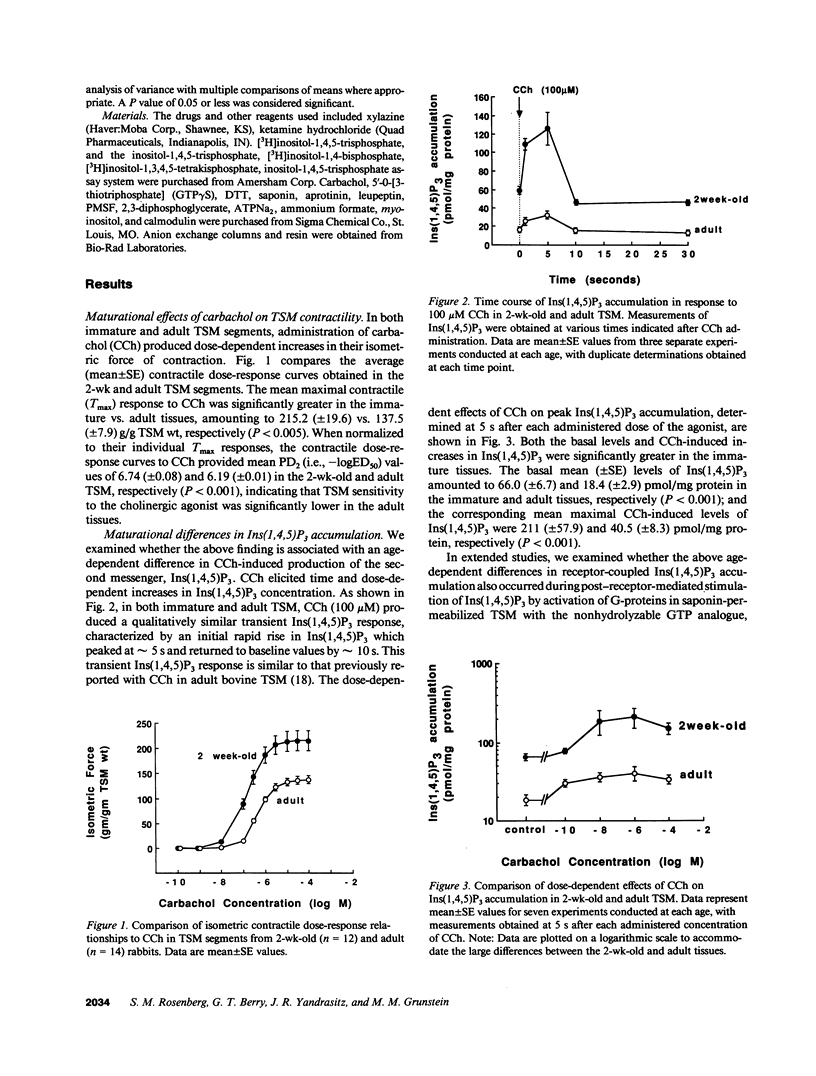
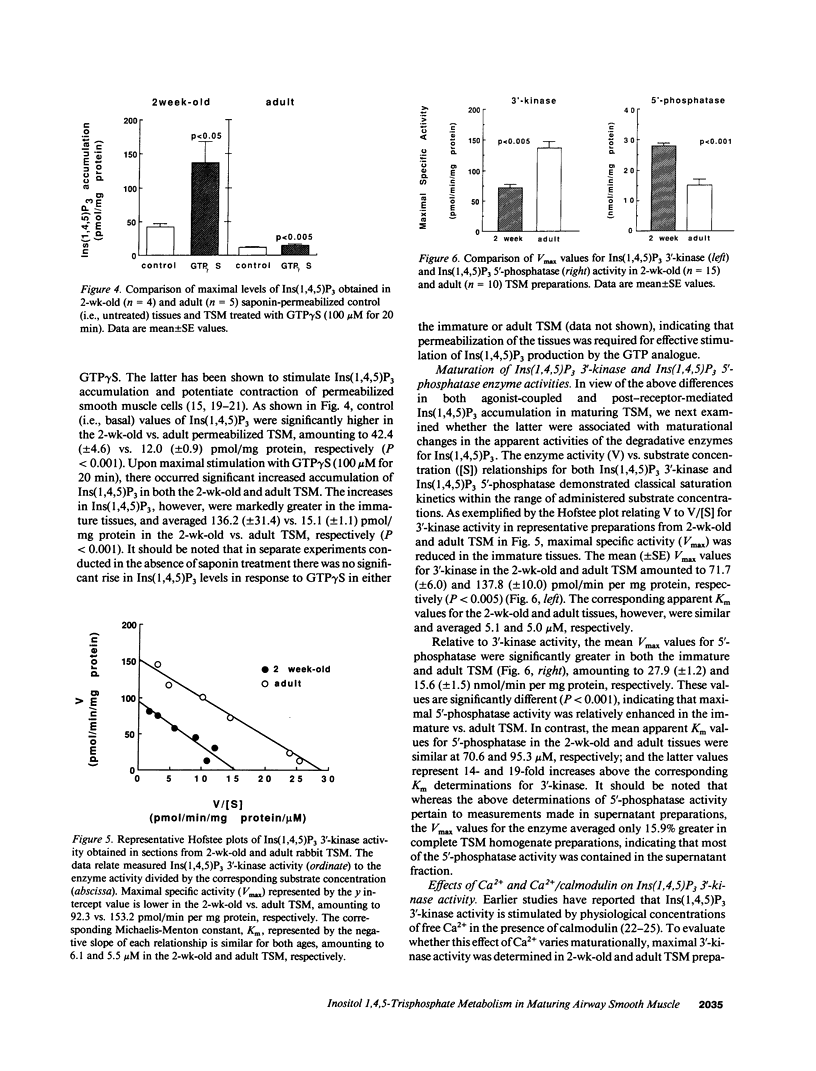
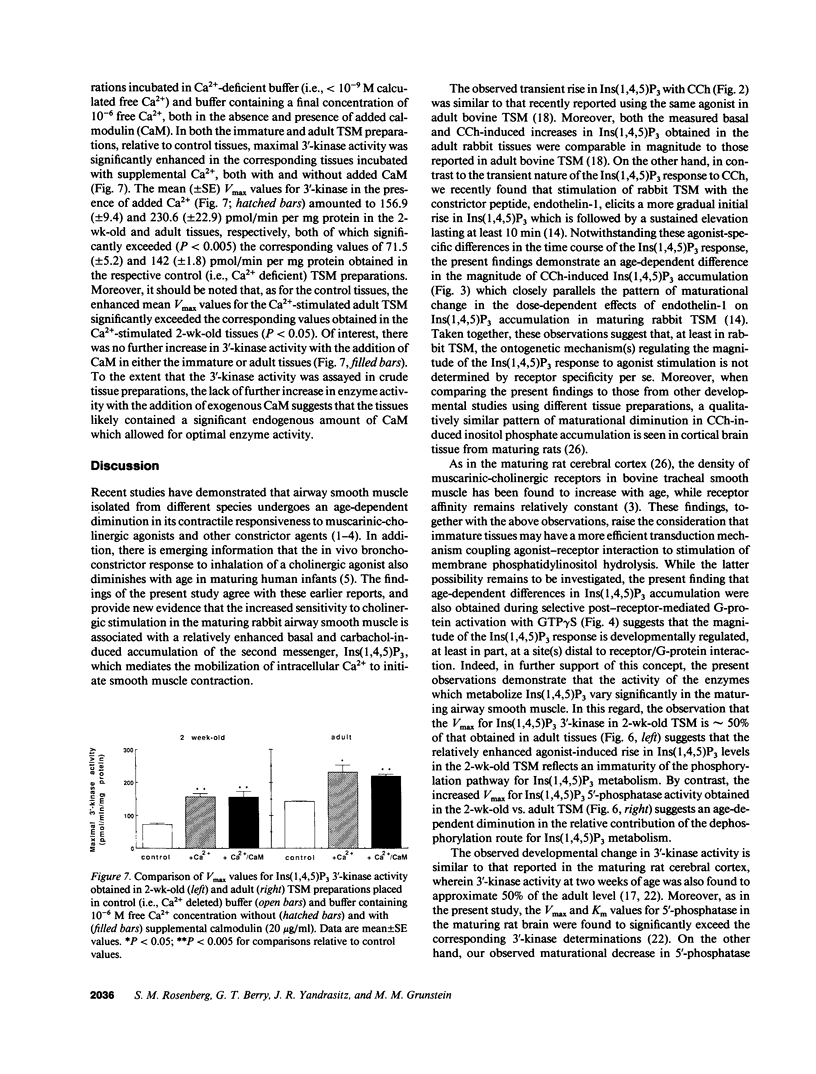
Airway reactivity has been shown to vary with age; however, the mechanism(s) underlying this process remain unidentified. To elucidate the role of ontogenetic changes in phosphoinositide-linked signal transduction, we examined whether age-related differences in tracheal smooth muscle (TSM) contractility to carbachol (CCh) are associated with developmental changes in the production and metabolism of the second messenger, inositol 1,4,5-trisphosphate (Ins (1,4,5)P3). In TSM segments isolated from 2-wk-old and adult rabbits, both the maximal isometric contractile force and sensitivity (i.e., -logED50) to CCh (10(-10)-10(-4) M) were significantly greater in the immature vs. adult tissues (P less than 0.001). Similarly, Ins(1,4,5)P3 accumulation elicited by either receptor-coupled stimulation with CCh (10(-10)-10(-4) M) or post-receptor-mediated guanine nucleotide binding protein activation of permeabilized TSM with GTP gamma S (100 microM) was also significantly enhanced in 2-wk-old vs. adult TSM. Measurement of the activities of the degradative enzymes for Ins(1,4,5)P3 demonstrated that: (a) mean +/- SE maximal Ins(1,4,5)P3 3'-kinase activity was significantly reduced in the immature vs. adult TSM (i.e., approximately 71.7 +/- 6.0 vs. 137.8 +/- 10.0 pmol/min per mg protein, respectively; P less than 0.005); (b) by contrast, maximal Ins(1,4,5)P3 5'-phosphatase activity was significantly increased in the immature vs. adult TSM (i.e., 27.9 +/- 1.2 vs. 15.6 +/- 1.5 nmol/min per mg protein, respectively; P less than 0.001); and (c) the Km values for Ins(1,4,5)P3 5'-phosphatase were 14- and 19-fold greater than those for Ins(1,4,5)P3 3'-kinase in the 2-wk-old and adult TSM, respectively. Collectively, the findings suggest that the age-related decrease in agonist-induced rabbit TSM contractility is associated with a diminution in Ins(1,4,5)P3 accumulation which is attributed, at least in part, to ontogenetic changes in the relative activities of the degradative enzymes for Ins(1,4,5)P3.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A. Calcium-mobilizing receptors, polyphosphoinositides, and the generation of second messengers. Pharmacol Rev. 1986 Sep;38(3):227–272. [PubMed] [Google Scholar]
- Balduini W., Murphy S. D., Costa L. G. Developmental changes in muscarinic receptor-stimulated phosphoinositide metabolism in rat brain. J Pharmacol Exp Ther. 1987 May;241(2):421–427. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Biden T. J., Comte M., Cox J. A., Wollheim C. B. Calcium-calmodulin stimulates inositol 1,4,5-trisphosphate kinase activity from insulin-secreting RINm5F cells. J Biol Chem. 1987 Jul 15;262(20):9437–9440. [PubMed] [Google Scholar]
- Brink C., Duncan P. G., Midzenski M., Douglas J. S. Response and sensitivity of female guinea-pig respiratory tissues to agonists during ontogenesis. J Pharmacol Exp Ther. 1980 Nov;215(2):426–433. [PubMed] [Google Scholar]
- Chilvers E. R., Challiss R. A., Barnes P. J., Nahorski S. R. Mass changes of inositol(1,4,5)trisphosphate in trachealis muscle following agonist stimulation. Eur J Pharmacol. 1989 May 30;164(3):587–590. doi: 10.1016/0014-2999(89)90269-0. [DOI] [PubMed] [Google Scholar]
- Connolly T. M., Lawing W. J., Jr, Majerus P. W. Protein kinase C phosphorylates human platelet inositol trisphosphate 5'-phosphomonoesterase, increasing the phosphatase activity. Cell. 1986 Sep 12;46(6):951–958. doi: 10.1016/0092-8674(86)90077-2. [DOI] [PubMed] [Google Scholar]
- Duncan P. G., Brink C., Douglas J. S. Beta-receptors during aging in respiratory tissues. Eur J Pharmacol. 1982 Feb 19;78(1):45–52. doi: 10.1016/0014-2999(82)90370-3. [DOI] [PubMed] [Google Scholar]
- Grunstein M. M., Rosenberg S. M., Schramm C. M., Pawlowski N. A. Mechanisms of action of endothelin 1 in maturing rabbit airway smooth muscle. Am J Physiol. 1991 Jun;260(6 Pt 1):L434–L443. doi: 10.1152/ajplung.1991.260.6.L434. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Toda N. Age-related alterations in the response of rabbit tracheal smooth muscle to agents. J Pharmacol Exp Ther. 1980 Sep;214(3):675–681. [PubMed] [Google Scholar]
- Heacock A. M., Seguin E. B., Agranoff B. W. Developmental and regional studies of the metabolism of inositol 1,4,5-trisphosphate in rat brain. J Neurochem. 1990 Apr;54(4):1405–1411. doi: 10.1111/j.1471-4159.1990.tb01976.x. [DOI] [PubMed] [Google Scholar]
- Huang C. L., Ives H. E. Guanosine 5'-O-(3-thiotrisphosphate) potentiates both thrombin- and platelet-derived growth factor-induced inositol phosphate release in permeabilized vascular smooth muscle cells. Signaling mechanisms distinguished by sensitivity to pertussis toxin and phorbol esters. J Biol Chem. 1989 Mar 15;264(8):4391–4397. [PubMed] [Google Scholar]
- Kitazawa T., Kobayashi S., Horiuti K., Somlyo A. V., Somlyo A. P. Receptor-coupled, permeabilized smooth muscle. Role of the phosphatidylinositol cascade, G-proteins, and modulation of the contractile response to Ca2+. J Biol Chem. 1989 Apr 5;264(10):5339–5342. [PubMed] [Google Scholar]
- Kobayashi S., Kitazawa T., Somlyo A. V., Somlyo A. P. Cytosolic heparin inhibits muscarinic and alpha-adrenergic Ca2+ release in smooth muscle. Physiological role of inositol 1,4,5-trisphosphate in pharmacomechanical coupling. J Biol Chem. 1989 Oct 25;264(30):17997–18004. [PubMed] [Google Scholar]
- Majerus P. W., Connolly T. M., Bansal V. S., Inhorn R. C., Ross T. S., Lips D. L. Inositol phosphates: synthesis and degradation. J Biol Chem. 1988 Mar 5;263(7):3051–3054. [PubMed] [Google Scholar]
- Majerus P. W., Connolly T. M., Deckmyn H., Ross T. S., Bross T. E., Ishii H., Bansal V. S., Wilson D. B. The metabolism of phosphoinositide-derived messenger molecules. Science. 1986 Dec 19;234(4783):1519–1526. doi: 10.1126/science.3024320. [DOI] [PubMed] [Google Scholar]
- Miller-Hance W. C., Miller J. R., Wells J. N., Stull J. T., Kamm K. E. Biochemical events associated with activation of smooth muscle contraction. J Biol Chem. 1988 Oct 5;263(28):13979–13982. [PubMed] [Google Scholar]
- Molina y Vedia L. M., Lapetina E. G. Phorbol 12,13-dibutyrate and 1-oleyl-2-acetyldiacylglycerol stimulate inositol trisphosphate dephosphorylation in human platelets. J Biol Chem. 1986 Aug 15;261(23):10493–10495. [PubMed] [Google Scholar]
- Montgomery G. L., Tepper R. S. Changes in airway reactivity with age in normal infants and young children. Am Rev Respir Dis. 1990 Dec;142(6 Pt 1):1372–1376. doi: 10.1164/ajrccm/142.6_Pt_1.1372. [DOI] [PubMed] [Google Scholar]
- Moon K. H., Lee S. Y., Rhee S. G. Developmental changes in the activities of phospholipase c, 3-kinase, and 5-phosphatase in rat brain. Biochem Biophys Res Commun. 1989 Oct 16;164(1):370–374. doi: 10.1016/0006-291x(89)91728-2. [DOI] [PubMed] [Google Scholar]
- Murphy T. M., Mitchell R. W., Blake J. S., Mack M. M., Kelly E. A., Munoz N. M., Leff A. R. Expression of airway contractile properties and acetylcholinesterase activity in swine. J Appl Physiol (1985) 1989 Jul;67(1):174–180. doi: 10.1152/jappl.1989.67.1.174. [DOI] [PubMed] [Google Scholar]
- Murray R. K., Bennett C. F., Fluharty S. J., Kotlikoff M. I. Mechanism of phorbol ester inhibition of histamine-induced IP3 formation in cultured airway smooth muscle. Am J Physiol. 1989 Oct;257(4 Pt 1):L209–L216. doi: 10.1152/ajplung.1989.257.4.L209. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Formation and actions of calcium-mobilizing messenger, inositol 1,4,5-trisphosphate. Am J Physiol. 1987 Feb;252(2 Pt 1):G149–G157. doi: 10.1152/ajpgi.1987.252.2.G149. [DOI] [PubMed] [Google Scholar]
- Rana R. S., Hokin L. E. Role of phosphoinositides in transmembrane signaling. Physiol Rev. 1990 Jan;70(1):115–164. doi: 10.1152/physrev.1990.70.1.115. [DOI] [PubMed] [Google Scholar]
- Shears S. B. Metabolism of the inositol phosphates produced upon receptor activation. Biochem J. 1989 Jun 1;260(2):313–324. doi: 10.1042/bj2600313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takazawa K., Lemos M., Delvaux A., Lejeune C., Dumont J. E., Erneux C. Rat brain inositol 1,4,5-trisphosphate 3-kinase. Ca2(+)-sensitivity, purification and antibody production. Biochem J. 1990 May 15;268(1):213–217. doi: 10.1042/bj2680213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsett J. A., Machulskis A., Noguchi A., Burdsall J. A. Ontogeny of alpha 1- and beta-adrenergic receptors in rat lung. Life Sci. 1982 Jan 11;30(2):139–145. doi: 10.1016/0024-3205(82)90645-2. [DOI] [PubMed] [Google Scholar]
- Wills M., Douglas J. S. Aging and cholinergic responses in bovine trachealis muscle. Br J Pharmacol. 1988 Apr;93(4):918–924. doi: 10.1111/j.1476-5381.1988.tb11480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., Hirata M., Kuriyama H. Calmodulin activates inositol 1,4,5-trisphosphate 3-kinase activity in pig aortic smooth muscle. Biochem J. 1987 Jun 15;244(3):787–791. doi: 10.1042/bj2440787. [DOI] [PMC free article] [PubMed] [Google Scholar]