Abstract
Type I interferons (IFNα and β) are induced directly in response to viral infection, resulting in an antiviral state for the cell. In vitro studies have shown that IFNα is a potent inhibitor of viral replication; however, its role in HIV-1 infection is incompletely understood. In this study we describe the ability of IFNα to restrict HIV-1 infection in primary human macrophages in contrast to peripheral blood mononuclear cells and monocyte-derived dendritic cells. Inhibition to HIV-1 replication in cells pretreated with IFNα occurred at an early stage in the virus life cycle. Late viral events such as budding and subsequent rounds of infection were not affected by IFNα treatment. Analysis of early and late HIV-1 reverse transcripts and integrated proviral DNA confirmed an early post entry role for IFNα. First strand cDNA synthesis was slightly reduced but late and integrated products were severely depleted, suggesting that initiation or the nucleic acid intermediates of reverse transcription are targeted. The depletion of integrated provirus is disproportionally greater than that of viral cDNA synthesis suggesting the possibility of a least an additional later target. A role for either cellular protein APOBEC3G or tetherin in this IFNα mediated restriction has been excluded. Vpu, previously shown by others to rescue a viral budding restriction by tetherin, could not overcome this IFNα induced effect. Determining both the viral determinants and cellular proteins involved may lead to novel therapeutic approaches. Our results add to the understanding of HIV-1 restriction by IFNα.
Introduction
Type I interferon (IFN) α and β can be induced directly in response to viral infection and trigger the transcription of a diverse range of IFN-stimulated genes (ISGs) through activation of the Jak-STAT (signal transducer and activator transcription) pathway [1]. This establishes an antiviral state in target cells. However, most viruses can still replicate and cause disease in vivo, having evolved strategies to at least partially avoid or inhibit the IFN response. The efficiency by which the virus manages to circumvent the antiviral actions of IFN is important in the establishment of a productive infection.
Multiple clinical trials have tested the ability of IFNα to restrict HIV-1 replication or slow disease progression [2], [3], [4], [5], [6]. Overall, compared with the efficacy seen in treatment of viral infections such as Hepatitis C, the in vivo effects of IFNα in HIV-1 infected patients were modest at best, with conflicting results for negative outcomes such as toxicity, antiretroviral treatment failure and progression of HIV-1 disease. This was somewhat surprising as in vitro studies in both cell lines and primary human cells showed that IFNα is a potent inhibitor of HIV-1 infection, particularly in the early stages [7], [8], [9], [10], [11], [12], [13], [14]. The role of IFNα in HIV-1 infection is incompletely understood, however results from this and other studies suggest that IFNα may regulate the expression of a restriction factor/s able to specifically inhibit the replication of HIV-1.
More recent work has linked the ability of IFNα to inhibit HIV-1 infection in cell lines with a cellular membrane protein CD317 (tetherin) and known HIV-1 restriction factor APOBEC3G [15], [16], [17]. Tetherin is upregulated by IFNα and inhibits the release of newly assembled virions in cell lines. IFNα also upregulates the level of APOBEC3G in primary human macrophages. The enzymatic activity of APOBEC3G leads to the degradation of HIV-1 DNA [18], [19]. To mediate this effect however, APOBEC3G must be incorporated into the virion and thus is only able to restrict the establishment of new rounds of infection. Incorporation of APOBEC into virions is prevented by the viral accessory protein, Vif.
Here, we confirm previous studies in MDM and extend them to describe a dramatic reduction in HIV-1 infection by IFNα in primary human macrophages but interestingly not dendritic or T cells derived from the same donors. Our experiments support data indicating that IFNα acts at an early stage in the virus life cycle, upon establishment of infection. Our analysis of integrated proviral levels suggests the possibility of an additional target between the completion of reverse transcription (RT) and integration. The inhibition of replication is not due to IFNα-induced action of tetherin or APOBEC3G. Rather we suggest IFNα inhibits HIV-1 through an as yet unknown cellular pathway, possibly targeting the viral gag/pol, the reverse transcription process itself or RT intermediates. Identification of the viral and cellular factors involved in this response may provide novel therapeutic approaches without the historical negative outcomes of IFNα.
Results
IFNα dramatically inhibits HIV-1 replication in MDM but not PBMC/MDDC
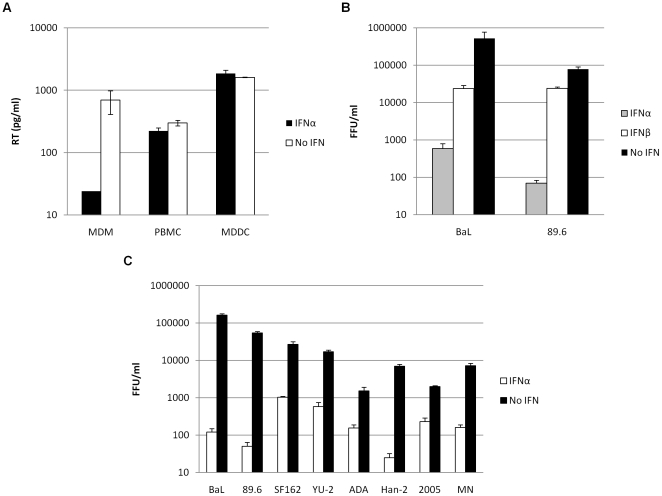
MDM, PBMC and MDDC were infected with HIV-1 89.6 in the presence or absence of IFNα. Viral replication was determined after 4–7 days following analysis of reverse transcriptase levels in the culture supernatants. IFNα was shown to inhibit HIV-1 replication in MDM. In contrast pretreatment of PBMC and MDDC with IFNα did not show any effect on the production of new virions (Fig. 1a). This suggests that IFNα can mediate a restriction to HIV-1 replication in a cell specific manner.
Figure 1. IFNα potently inhibits HIV-1 replication in MDM but not PBMC/MDDC.
(a) MDM, PBMC and MDDC were treated with 500 IU/ml IFNα 24 hr prior to challenge with HIV-1 89.6. Culture supernatants were assayed after 4 days for levels of RT by ELISA (pg/ml). (b & c) MDM were treated with 500 IU/ml IFNα or β 24 hr prior to infection with a panel of replication competent HIV-1. Infected foci were counted after 4 days. Fold reduction is the ratio of FFU/ml of no IFN compared to IFNα treated cells. Error bars represent SD of one representative experiment.
The general antiviral roles of the type I IFNs are well described, and to determine the relative ability of members of this IFN family to inhibit HIV-1, MDM were challenged with replication competent HIV-1 strains BaL and 89.6 in the presence or absence of IFNα or IFNβ. The level of HIV-1 infection was determined after 4 days by in situ intracellular p24 staining and enumeration of FFU/ml. Fold reduction is the ratio of FFU/ml of no IFN compared to IFNα treated cells. Replication in MDM by these HIV-1 isolates is dramatically inhibited (up to 1000-fold) when these cells are pretreated with IFNα (Fig. 1b). While it is known that IFNβ performs a unique role in viral infection and is essential for a fully effective general antiviral response, the potency of HIV-1 restriction was much less when MDM were pretreated with IFNβ. These data are consistent with previous reports that HIV-1 is susceptible to IFNα mediated inhibition [9], [10]. Results for IFNβ have not been previously reported. In this study we specifically investigate IFNα, and its ability to mediate restriction of HIV-1 infection.
Further analysis was then performed on an extended panel of full length HIV-1 isolates. The well characterised macrophage- (BaL, YU-2) and dual-tropic (89.6) HIV-1 strains along with several primary isolates were used. HIV-1 isolates were dramatically inhibited (up to 1000-fold) when these cells were pretreated with IFNα (Fig. 1c). The extent of inhibition across the panel of viruses tested was variable (10-1000-fold), indicating that some isolates are less sensitive to the antiviral action of IFNα and suggesting the evolution of viral escape mechanisms.
IFNα inhibits the establishment of infection and acts via unknown cellular factor/s
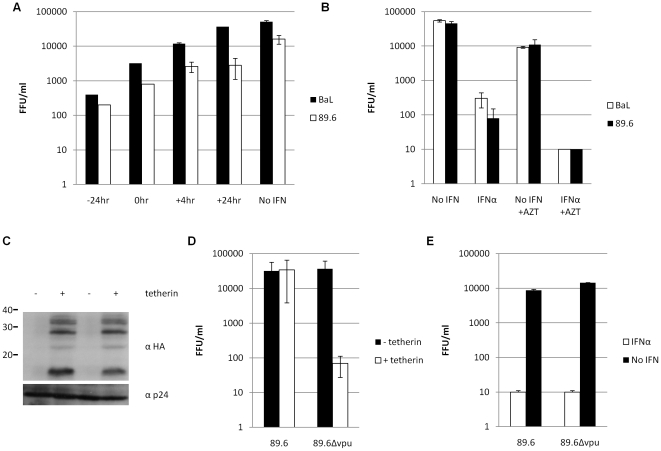
MDM cultures were treated with IFNα at various times prior to, at and post infection with HIV-1 strains 89.6 and BaL, previously shown to be highly sensitive to its inhibitory effects. The most potent restriction was observed when IFNα was present before the cells were infected (Fig. 2a). IFNα was still seen to have a modest effect on replication when added at the same time as the virus, but this soon decreased to low/negligible levels if IFNα was introduced after first round infection was complete (+24 hr). These results show that suitable expression levels of antiviral proteins or non-translated RNA can take at least 4 hr following induction by IFNα (up to 24 hr for maximum effect) and suggest that the resultant antiviral state is most effective at inhibiting the initial establishment of infection.
Figure 2. IFNα inhibits the establishment of infection and acts via unknown cellular protein/s.
MDM were treated with 500 IU/ml IFNα 24 hr prior to infection with replication competent HIV-1 89.6 and BaL. Infected foci were counted after 4 days. (a) IFNα was added at various time points prior to, at or after infection. (b) AZT (final concentration 100 nM) was added to cultures 24 hr post infection to block second round infection. (c) HEK 293T cells were co-transfected with pcDNA3.1-HA-tetherin and either WT or Δvpu 89.6 molecular clones. Cells were lysed and the presence or absence of tetherin was confirmed by Western blot using an anti-HA Ab. Tetherin is a 30–36 kDa protein that migrates as several species by SDS-PAGE, as a result of post-translational modifications [15]. The levels of p24 protein were monitored as a loading control. (d) Supernatants from transfected HEK 293T cells were serially diluted and used to infect NP2-CD4-CXCR4 cells. Virus titres were determined after 48 hr. (e) MDM were challenged with tetherin resistant (WT) and tetherin sensitive (Δvpu) HIV-1 molecular clones ± IFNα. Data is presented as mean ± SD.
APOBEC3G is a well known IFNα responsive gene that inhibits HIV-1 replication at an early post entry stage (reviewed in [20]). The action of APOBEC3G on nascent DNA requires its presence in virions made by the producer cell. HIV-1 Vif counteracts the action of APOBEC3G by preventing its incorporation into virions. Here it is unlikely that APOBEC3G restriction is responsible for the IFNα mediated inhibition because the HEK 293T producer cells are APOBEC3G- [21]. Furthermore virion incorporation of APOBEC3G is unlikely because all viruses are Vif+. It could be argued however that the higher cellular levels of APOBEC3G following IFNα induction overcome Vif mediated exclusion. This could result in APOBEC3G being packaged into virions after first round infection and contributing to the total inhibition. To exclude this possibility, experiments were performed in which the RT inhibitor AZT was introduced 24 hr post infection, to block late stages and second round infection (Fig. 2b).
If late events or APOBEC3G contributed to the inhibition induced by IFNα, we would expect a relative reduction of IFNα mediated inhibition in cells treated with AZT. In MDM infected with HIV-1 BaL and 89.6, no difference in the ability of IFNα to restrict infection was observed in the presence or absence of AZT (Fig. 2b), confirming the notion that IFNα-induced restrictions act at an early stage of viral replication.
A role has recently been attributed to IFNα in the regulation of tetherin (CD317, BST-2), a cell surface molecule capable of inhibiting viral production. The inhibition is overcome by HIV-1 Vpu [15], [16].
We determined whether the IFNα mediated restriction described here could be due to the action of tetherin. We engineered an HIV-1 molecular clone 89.6 with a premature stop codon in vpu (89.6Δvpu). We first confirmed that the 89.6Δvpu construct was susceptible to tetherin: HEK 293T cells were cotransfected with HA-tagged tetherin and either wild type (WT) or Δvpu HIV-1 89.6 molecular clones. Tetherin and viral protein expression was confirmed by Western blot (Fig. 2c). The titre of supernatants collected from these cultures was determined confirming that tetherin was capable of inhibiting virus release in these assays (Fig. 2d). The presence of tetherin had no effect on WT 89.6 virus titre however, as expected, it was able to reduce the titre of 89.6Δvpu at least 100-fold.
WT and Δvpu HIV-1 strains were then used to challenge MDM in the presence or absence of IFNα. The results shown in Fig. 2e demonstrate that Vpu is not required for the IFNα restriction, and thus neither tetherin nor Vpu are involved in the IFNα induced restriction in MDM. Supporting this, a reduction was also observed in the levels of RT detected in the supernatants of MDM, PBMC and MDDC cultures when challenged with 89.6Δvpu compared with WT 89.6 but no difference in the ability of IFNα to restrict HIV-1 replication was observed (data not shown). The IFNα mediated restriction described in this study therefore, is distinct from the effects of tetherin and Vpu.
IFNα inhibits HIV-1 at an early post entry stage in the virus life cycle
To probe the mechanism behind the inhibition of HIV in MDM, we sought to determine the stage of the virus life cycle at which IFNα was effective.
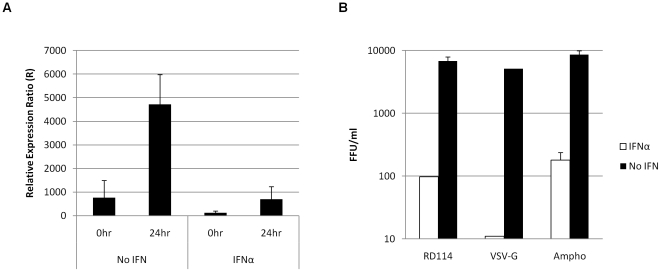
The lack of p24 protein in the MDM detected by staining for FFU indicated that the IFNα mediated block of viral replication was in the early part of the life cycle and at least prior to protein translation. Furthermore to support this we isolated mRNA from MDM infected with HIV-1 BaL and performed qRT-PCR to determine whether the quantity of HIV-1 transcripts produced in the presence of IFNα was affected. HIV-1 mRNA levels were negligible in the presence of IFNα (Fig. 3a). These results support the observations in Fig. 1c that IFNα acts at an earlier stage in the viral life cycle.
Figure 3. IFNα inhibits HIV-1 at an early post entry stage in the virus life cycle.
(a) MDM were treated with 500 IU/ml IFNα 24 hr prior to challenge with HIV-1 BaL. Cells were collected 0 and 24 hr post infection and total RNA was extracted. RT-qPCR was used to detect late HIV-1 products and results were normalised to GAPDH cDNA and compared with uninfected controls. Error bars represent SD of two independent experiments. (b) HIV-1 89.6Δenv was pseudotyped with VSV-G, RD114 and Ampho MLV envs and used to challenge MDM. Infected foci were counted after 4 days and are presented as mean ± SD.
To expand on these results and characterise the viral determinants of IFNα restriction, MDM were infected with HIV-1 pseudotypes carrying various retroviral and other envelopes. Significant restriction was seen in all cases, most notably for HIV-1 cores pseudotyped with VSV-G, for which 4-5-log reductions in replication were observed (Fig. 3b).
Viruses pseudotyped with retroviral envelopes from feline endogenous virus (RD114) and amphotrophic murine leukaemia virus (Ampho) enter the cell following binding to the receptors RDR and PiT2 respectively, while VSV-G triggers entry by clathrin-mediated endocytosis through a ubiquitously expressed glycolipid. Therefore these results show that IFNα mediated restriction of HIV-1 is not dependent on the Env-host cell interaction. IFNα may mediate a restriction that targets the viral gag-pol.
Post entry restriction affects the kinetics of late HIV-1 RT products in MDM but not PBMC/MDDC
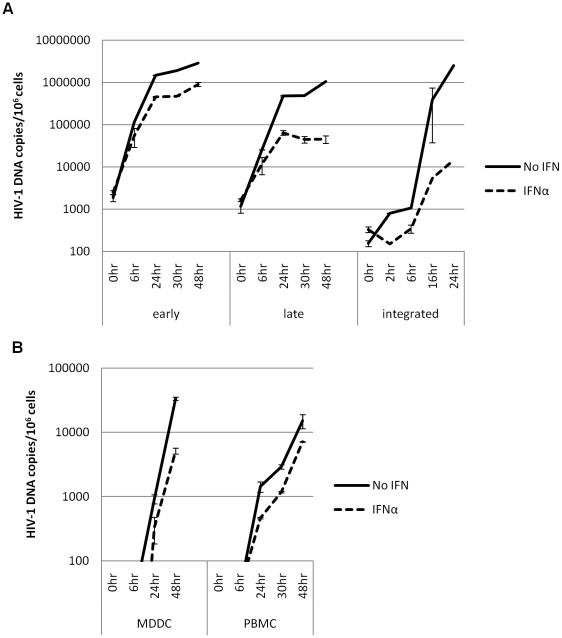
To identify more precisely the post entry point of the restriction induced by IFNα, qPCR analysis was performed on DNA extracted from HIV-1 89.6 infected MDM at various time points post infection. Early (negative strand strong stop, -sss), late (gag-LTR) and integrated (Alu-gag) HIV-1 products were quantified. The results in Fig. 4a confirm that inhibition occurs post entry, as HIV-1 DNA products are detected in the presence and absence of IFNα. No HIV-1 DNA products are detected in the presence of a CCR5 inhibitor (data not shown). Up to 6 hr after virus challenge the levels of both early and late HIV-1 DNA RT products are the same in the presence or absence of IFNα, indicating the same efficiency of viral entry. After 6 hr IFNα treated samples showed a delay in both RT products. This was more pronounced for late RT transcripts, and more so for integrated proviruses, of which a 100-fold decrease was observed following treatment with IFNα. Thus this IFNα-induced restriction acts after the initiation of RT, preventing the completion and/or integration of full length products.
Figure 4. Post entry restriction affects the kinetics of late HIV-1 RT products and integration in MDM but not PBMC/MDDC.
(a) MDM and (b) PBMC and MDDC were treated with 500 IU/ml IFNα 24 hr prior to challenge with HIV-1 BaL. Cells were collected at various time points after infection and DNA was extracted. Early (MDM), late (MDM, PBMC & MDDC) and integrated (MDM) HIV-1 DNA was normalised to genomic GAPDH and copy number is presented per 106 cells. Data is presented as mean ± SD and is from one representative experiment.
We next compared the effect of IFNα on RT in PBMC and MDDC. As expected from the infectivity data in Fig. 1a the potency of any IFNα induced restriction(s) was much weaker in PBMC and MDDC compared to MDM following analysis of the kinetics of HIV-1 DNA production (Fig. 4b).
Discussion
Here we show a potent (3-log) IFNα induced antiviral response against HIV-1 infection which is specific to MDM. The inhibition of replication occurs after the virus delivers its capsid into the cytoplasm, acting after initiation, but before completion of RT. We have excluded the involvement of previously described IFNα responsive genes tetherin or APOBEC3G.
The overall modest responses of HIV-1 infected patients to IFN therapy in past years [2], [3], [4], [5], [6] have forced researchers to investigate alternative strategies for the development of pharmaceuticals to control disease progression. Subsequently, while long observed, the inhibitory effects of IFNα on HIV-1 replication in primary cells are relatively poorly understood. Here we have taken further steps in identifying and characterising its specific action on HIV-1 infection of primary human macrophages.
It has previously been reported that IFNα preferentially inhibits HIV-1 replication in MDM compared with CD4+ T cells [10]. Here we confirm this observation using RT ELISA and real-time qPCR analysis (Fig. 1a and 4b). We build upon previous studies of this nature with analysis of MDDC, in which we show a profile of HIV-1 replication more similar to PBMC than MDM. Thus we confirm the strong antiviral response to IFNα to be cell type specific, which may contribute to the more refractory nature of macrophages to HIV-1 infection than T-cells in vivo.
qPCR analysis of DNA from various time points following HIV-1 infection of MDMs shows that, on top of an overall decrease in the total amount of detectable reverse transcribed HIV-1 DNA, IFNα acts at a point after the initiation of reverse transcription which specifically delays the completion of full-length preintegration genomes and significantly reduces the levels of integrated provirus. This is supported by our observation of the redundancy of HIV-1 Env in determining susceptibility to IFNα, which indicates that this effect is not dependent on the Env-host cell interaction. The restriction acts after the viral core is uncoated and released into the cytoplasm, suggesting that the HIV-1 gag/pol, the reverse transcription process and events leading to integration may be targeted.
Analysis of the levels of integrated viral DNA following treatment with IFNα shows a 100-fold decrease compared with untreated samples 24 hr post infection. Yet there is only a 10-fold decrease in late RT products which could point to the existence of additional blocks after reverse transcription but before completion of integration due to the upregulation and action of multiple ISGs.
As the experiments in this study were performed using replication competent viruses, it was important to determine the extent to which the overall inhibition described could be accounted for by the action of known HIV-1 specific IFNα-inducible proteins tetherin and APOBEC3G. We have excluded a role for either protein in this IFNα mediated antiviral effect. Tetherin was excluded following the comparison of WT (tetherin resistant) and Δvpu (tetherin sensitive) HIV-1 viruses wherein no difference in the ability of IFNα to restrict was observed. Interestingly, in a recent report of potential IFNα-induced antiviral genes, tetherin was upregulated less than 2-fold in MDM [22].
Evidence for IFNα regulation of APOBEC3G as an important intracellular defence against HIV-1 infection is compelling. It is unlikely that the IFNα mediated restriction described in this study is due to APOBEC3G. All viruses were Vif+ and produced in the absence of APOBEC3G. However it could be argued that IFNα overcomes APOBEC3G sequestration by HIV-1 Vif, allowing enhanced APOBEC3G packaging into budding virions. Our data using AZT to inhibit subsequent rounds of infection demonstrates that this scenario is unlikely as there was no relative difference in the potency of IFNα mediated restriction in the presence of AZT.
Generally, the IFNs are known to have wide ranging protective immunomodulatory, antiproliferative and antiviral effects, and exert their activity through multiple pathways. Effector proteins and pathways induced by IFN include protein kinase regulated by RNA (PKR)/eukaryotic initiation factor 2α (eIF-2α) for mRNA translation inhibition, RNase L for RNA degradation, adenosine deaminase acting on RNA (ADAR1) for RNA editing and others such as protein GTPase Mx and nitric oxide synthetase (NOS) (reviewed in [23]). In the case of HIV-1 infection, IFNα can itself be regulated by cytokines such as IL-27 [24], and has been implicated in the antiviral actions of activator proteins such as tetherin [15] and APOBEC3G [17], [25]. In addition to these mechanisms, we describe a role for IFNα in the early stages of establishment of initial infection that is peculiar to primary MDM.
In the last few years, several groups have characterised the ability of the retroviral protein Vpx to rescue HIV-1 infection in MDM by overcoming a restriction imposed by an unknown antiviral factor in these cells [26], [27], [28]. More recently, one study has suggested that the cellular factor overcome by Vpx is IFNα-inducible, as introduction of Vpx into IFNα treated MDM prior to HIV-1 infection rescues the level of inhibition approximately 100-fold [29]. The phenotype of the Vpx-counteractable restriction bears many similarities to the IFNα mediated restriction described here, as the antiviral action is at an early stage in the viral life cycle and the accumulation of full length reverse transcripts is prevented.
It seems unlikely that the cellular restriction factors responsible for the Vpx-counteractable phenomenon and the inhibition described here are one and the same. A recent study demonstrates that primate and nonprimate lentiviruses containing Vpx are also susceptible to restriction by IFNα [14]. Together with the observation that Vpx is not able to completely rescue HIV-1 from IFNα mediated inhibition, and that it has been shown to have the same enhancement of viral infection in MDDC [30] does not completely explain the total impact of IFNα on HIV-1 replication and thus it is likely that other unidentified IFNα-inducible antiviral factors exist.
Macrophages have a key role in the success of the innate immune response. A recent paper shows that HIV-1 infected MDM fail to produce type I IFNα suggesting that HIV-1 can specifically interfere with the IFNα mediated response to establish infection in vivo [31]. If the cellular proteins involved in this interference can be determined and targeted, without the toxicities and negative disease outcomes of previous IFNα therapies, further rounds of macrophage infection may be eliminated. Additionally, cell-cell transmission and/or establishment of cellular reservoirs with the potential to generate drug resistant strains may be reduced, enhancing the effectiveness of current long-term ART regimens [32].
Materials and Methods
Cells
Buffy coats from seronegative donors were obtained from the National Blood Service (Brentwood, UK). Donors were anonymous and thus patient consent was not required. Peripheral blood mononuclear cells (PBMC) were prepared by density-gradient centrifugation (Lymphoprep, Axis-Shield). Monocyte-derived macrophages (MDM) were prepared by adherence as described previously [33]. Cells were plated at 2×106 cells/ml and left to differentiate for 7–14 days in RPMI 1640/20% autologous human serum and 20 ng/ml macrophage colony stimulating factor (M-CSF; R&D Systems).
PBMC were cultured from the same donor at 2×106 cells/ml in RPMI 1640, supplemented with 10% foetal calf serum (FCS; Biosera), 1% penicillin/streptomycin (PAA) and phytohemagglutinin (PHA-P, 0.5 µg/ml; Sigma). After 3–5 days, PHA-P was replaced by recombinant human interleukin-2 (rhIL-2, 20 U/ml; Invitrogen).
Monocyte-derived dendritic cells (MDDC) were isolated from PBMC using CD14+ MACS Microbeads (Miltenyi Biotech) and were cultured for 7 days in RPMI 1640/10% FCS, 1% penicillin/streptomycin, recombinant human interleukin-4 (rhIL-4, 50 U/ml; R&D Systems) and recombinant human granulocyte-macrophage colony stimulating factor (GM-CSF, 0.1 µg/ml; Sigma).
HEK 293T [34] cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) with 10% FCS and 1% penicillin/streptomycin. DMEM for NP2-CD4-CXCR4 [35] cells additionally contained geneticin (G418, 1 mg/ml; Melford Laboratories) and puromycin (1 µg/ml; PAA).
Plasmid constructs
HIV-1 89.6Δvpu and 89.6Δenv were generated from the 89.6 molecular clone using overlap extension PCR. For 89.6Δvpu, the fragments obtained with the primers 89.6-ERI-F/Vpu stop-XbaI-R (89.6-ERI-F 5′-GGAGTGGAAGCCTTAATAAGAATTCTGCA-3′, Vpu stop-XbaI-R 5′-GCTAATATTTGTCTAGAAAGTTATACATGTAC-3′) and Vpu stop-XbaI-F/89.6-StuI-R (Vpu stop XbaI-F 5′-GTACATGTATAACTTTCTAGACAAATATTAGC-3′, 89.6-StuI-R 5′- GATACCTTTGGACAGGCCTGTGTA-3′) were used as a template for another PCR using the primers 89.6-ERI-F/89.6-StuI-R. The resulting fragment was cloned at EcoRI and StuI sites in the 89.6 molecular clone. For 89.6Δenv, the fragments obtained with the primers 89.6-ERI-F/Env stop-HIII-R (Env stop-HIII-R 5′-TCCTGATCTCCTTCAAGCTTTATGCCACTGTC-3′) and Env stop-HIII-F/89.6-StuI-R (Env stop HIII-F 5′-GACAGTGGCATAAAGCTTGAAGGAGATCAGGA-3′) were used as a template for another PCR using the primers 89.6-ERI-F/89.6-StuI-R. The resulting fragment was cloned at EcoRI and StuI sites in the 89.6 molecular clone.
To generate the HA-tagged pcDNA3.1-tetherin construct, tetherin coding sequence was amplified using forward (5′-CACACACACACAGCGGCCGCATGTACCCATACGATGTTCCAGATTACGCTATGGCATCTACTTCGTATGACTA-3′) and reverse (5′-CACACACACACAGGATCCTCACTGCAGCAGAGCGCTGA-3′) primers from 293T cDNA and cloned in-frame at NotI and BamHI sites in the pcDNA3.1(-) expression vector (Invitrogen).
Primary HIV-1 stocks
2005 and HAN-2 were isolated as previously described [36]. All primary strains were minimally passaged in PBMC to expand virus stocks.
Virus stocks
Virus stocks were prepared from infectious full-length and chimeric HIV-1 clones by polyethylenimine (PEI; Polysciences) transfection of HEK 293T cells. Infectivity assays. NP2-CD4-CXCR4 cells were seeded in 48-well trays at 1.5×104 cells/well in 200 µl antibiotic-free DMEM. The following day viruses were added to the cells and incubated for 2 hr before the media was changed. Cells were cultured for 48 hr before infection was assessed following intracellular p24 staining.
MDM in 48-well trays (4×105 cells/well) were infected with 500–5000 FFU viral stock (titred on NP2 cells) in 200 µl RPMI 1640/20% autologous serum. Media was replaced after 24 hr and virus production was detected after 4 days following intracellular p24 staining.
Time courses of virus infectivity of MDM, PBMC and MDDC were performed in 12- and 24-well trays. Cells were infected 3–5 days (PBMC) or 7 days (MDM and MDDC) post isolation. 500 IU/ml IFNα was added 24 hr prior to infection where appropriate. CCR5 coreceptor inhibitor SCH-D (10 nM) was added 1 hr before infection. MDM were challenged with 6000 focus forming units (FFU) virus/well in a total of 1 ml RPMI 1640/20% autologous serum while all PBMC or MDDC were initially pooled, pelleted and challenged with 250000 FFU virus. 2 hrs after infection these cells were divided into 24-well plates and made up to 1 ml in RPMI 1640/10% FCS supplemented with appropriate cytokines. At the collection of each time point, cells were washed twice with PBS.
Determination of RT activity
Virus supernatants from infections were assessed for RT activity by RT-ELISA (Cavidi Tech Inc.).
In situ immunostaining for p24 antigen
Infected cells were fixed with cold (−20°C) methanol:acetone (1∶1), washed with PBS then immunostained for p24 using mouse anti-HIV-1 p24 monoclonal antibodies EVA365 and 366 (NIBSC, UK) (diluted 1∶50), as previously described [37]. Infected cells were blue (regarded as foci of infection (FFU/ml)) and counted by light microscopy.
Western blot
Western blots carrying lysed HEK 293T extracts were first incubated with primary antibody (rat anti-HA or human anti-p24) followed by the appropriate HRP-conjugated antibodies. Proteins were visualised using a chemiluminescence kit (ECL, Amersham Biosciences).
First round Alu-gag PCR
DNA was extracted at various time points after infection with a QIAamp DNA Blood Mini Kit (QIAGEN). Integrated HIV-1 DNA was measured using a nested PCR protocol, as previously described [38]. Briefly, a standard curve was prepared using a 1∶1∶1 ratio of genomic DNA from chronically HIV-1 infected cell lines ACH-2 [39], 8E5/LAV [40] and OM-10.1 [41] in a background of HeLa genomic DNA (data not shown). First round products were amplified using 300 nM each primer (Alu-F 5′-GCCTCCCAAAGTGCTGGGATTACAG-3′; gag-R 5′-GTTCCTGCTATGTCACTTCC-3′) and approximately 50 ng total DNA in a 25 µl reaction. PCR conditions consisted of one cycle of denaturation (94° for 2 min), 39 cycles of amplification (94° for 15 sec, 50° for 30 sec, 68° for 8 min), and one cycle of 68° for 7 min. 5 µl of resulting PCR product was used as the template for measurement of integrated HIV-1 DNA in a nested real-time quantitative PCR.
Real-time quantitative PCR for HIV-1 DNA
The isolated DNA was subjected to real-time quantitative PCR (qPCR) to determine the number of early (negative strand strong stop, -sss) and late (gag) transcripts present, normalised for cell number by genomic GAPDH. Integrated HIV-1 DNA copies were determined in a nested qPCR using 5 µl of first round product as template. Each 25 µl reaction contained the following components: 1× MegaMix-Gold PCR buffer (Microzone), 300 nM each forward primer (early 5′-GCTAACTAGGGAACCCACTGCTT-3′; late 5′-tgggcaagcagggagcta-3′; genomic GAPDH 5′-GAAGGTGAAGGTCGGAGT-3′; integrated 5′- TTAAGCCTCAATAAAGCTTGCC-3′), 300 nM each reverse primer (early 5′-CAACAGACGGGCACACACTACT-3′; late 5′-tcctgtctgaagggatggttgt-3′; genomic GAPDH 5′-CATGGGTGGAATCATATTGGAA-3′; integrated 5′- GTTCGGGCGCCACTGCTAGA-3′), 150 nM each probe (early 5′-CY3-AGCCTCAATAAAGCTTGCCTTGAGTGCTTC-BHQ2-3′; late 5′-6-FAM-aacgattcgcagttaatcctggcctgtt-BHQ1-3′; genomic GAPDH 5′-CY5-CAACGGATTTGGTCGTATTGGGCGC-BHQ2-3′; integrated 5′- CY3-CCAGAGTCACACAACAGACGGGCACA-BHQ2-3′) and approximately 500 ng total DNA (early, late, GAPDH) or 5 µl of first round Alu-gag PCR product (integrated). A standard curve was prepared with the NL4.3 molecular clone (early and late HIV-1 DNA) and HEK 293T genomic DNA (GAPDH) in a background of 200 ng salmon sperm carrier DNA (data not shown). PCR conditions consisted of one cycle of denaturation (95°C for 5 min) followed by 40 cycles of amplification (95°C for 15 sec, 60°C for 1 min). Data acquisition and analysis was performed using the ABI PRISM 7500 SDS software.
cDNA synthesis
Total RNA was extracted from MDM using an RNeasy Plant Mini Kit (QIAGEN) and cDNA was synthesised with Superscript III First Strand Synthesis System (Invitrogen), according to manufacturer's instructions. The cDNA produced was subjected to real-time qPCR as above, using the primer/probe set designated ‘late’, and was normalised for input cDNA by primers and probe targeting GAPDH transcripts (300 nM forward primer GAPDH 5′- CCACATCGCTCAGACACCAT-3′,300 nM reverse primer GAPDH 5′-CCAGGCGCCCAATACG-3′, 150 nM probe GAPDH 5′-6-FAM-AGGTGAAGGTCGGAGTCAACGGATTTG-BHQ1-3′).
Acknowledgments
The authors thank Dr. Matthias Dittmar for helpful discussions and critical reading of the manuscript. The monoclonal antibodies to p24 (EVA365 and 366) were provided by the EU Programme EVA Centre for AIDS Reagents, NIBSC, UK (AVIP Contract Number LSHP-CT-2004-503487). The following cell lines were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: ACH-2 and 8E5/LAV from Dr. Thomas Folks, OM-10.1 from Dr. Salvatore Butera.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by an MRC Senior Non-Clinical Fellowship awarded to AM (G117/547) and a Wellcome Trust grant (WT075853MA). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 2.Katabira ET, Sewankambo NK, Mugerwa RD, Belsey EM, Mubiru FX, et al. Lack of efficacy of low dose oral interferon alfa in symptomatic HIV-1 infection: a randomised, double blind, placebo controlled trial. Sex Transm Infect. 1998;74:265–270. doi: 10.1136/sti.74.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krown SE, Lee JY, Lin L, Fischl MA, Ambinder R, et al. Interferon-alpha2b with protease inhibitor-based antiretroviral therapy in patients with AIDS-associated Kaposi sarcoma: an AIDS malignancy consortium phase I trial. J Acquir Immune Defic Syndr. 2006;41:149–153. doi: 10.1097/01.qai.0000194237.15831.23. [DOI] [PubMed] [Google Scholar]
- 4.Krown SE, Li P, Von Roenn JH, Paredes J, Huang J, et al. Efficacy of low-dose interferon with antiretroviral therapy in Kaposi's sarcoma: a randomized phase II AIDS clinical trials group study. J Interferon Cytokine Res. 2002;22:295–303. doi: 10.1089/107999002753675712. [DOI] [PubMed] [Google Scholar]
- 5.Lane HC, Davey V, Kovacs JA, Feinberg J, Metcalf JA, et al. Interferon-alpha in patients with asymptomatic human immunodeficiency virus (HIV) infection. A randomized, placebo-controlled trial. Ann Intern Med. 1990;112:805–811. doi: 10.7326/0003-4819-112-11-805. [DOI] [PubMed] [Google Scholar]
- 6.Stylianou E, Aukrust P, Bendtzen K, Muller F, Froland SS. Interferons and interferon (IFN)-inducible protein 10 during highly active anti-retroviral therapy (HAART)-possible immunosuppressive role of IFN-alpha in HIV infection. Clin Exp Immunol. 2000;119:479–485. doi: 10.1046/j.1365-2249.2000.01144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitha PM. Multiple effects of interferon on the replication of human immunodeficiency virus type 1. Antiviral Res. 1994;24:205–219. doi: 10.1016/0166-3542(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto JK, Barre-Sinoussi F, Bolton V, Pedersen NC, Gardner MB. Human alpha- and beta-interferon but not gamma- suppress the in vitro replication of LAV, HTLV-III, and ARV-2. J Interferon Res. 1986;6:143–152. doi: 10.1089/jir.1986.6.143. [DOI] [PubMed] [Google Scholar]
- 9.Baca-Regen L, Heinzinger N, Stevenson M, Gendelman HE. Alpha interferon-induced antiretroviral activities: restriction of viral nucleic acid synthesis and progeny virion production in human immunodeficiency virus type 1-infected monocytes. J Virol. 1994;68:7559–7565. doi: 10.1128/jvi.68.11.7559-7565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gendelman HE, Baca L, Turpin JA, Kalter DC, Hansen BD, et al. Restriction of HIV replication in infected T cells and monocytes by interferon-alpha. AIDS Res Hum Retroviruses. 1990;6:1045–1049. doi: 10.1089/aid.1990.6.1045. [DOI] [PubMed] [Google Scholar]
- 11.Gendelman HE, Baca LM, Turpin J, Kalter DC, Hansen B, et al. Regulation of HIV replication in infected monocytes by IFN-alpha. Mechanisms for viral restriction. J Immunol. 1990;145:2669–2676. [PubMed] [Google Scholar]
- 12.Shirazi Y, Pitha PM. Interferon alpha-mediated inhibition of human immunodeficiency virus type 1 provirus synthesis in T-cells. Virology. 1993;193:303–312. doi: 10.1006/viro.1993.1126. [DOI] [PubMed] [Google Scholar]
- 13.Meylan PR, Guatelli JC, Munis JR, Richman DD, Kornbluth RS. Mechanisms for the inhibition of HIV replication by interferons-alpha, -beta, and -gamma in primary human macrophages. Virology. 1993;193:138–148. doi: 10.1006/viro.1993.1110. [DOI] [PubMed] [Google Scholar]
- 14.Goujon C, Malim MH. Characterization of the alpha interferon-induced postentry block to HIV-1 infection in primary human macrophages and T cells. J Virol. 2010;84:9254–9266. doi: 10.1128/JVI.00854-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 16.Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, et al. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008;3:245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng G, Lei KJ, Jin W, Greenwell-Wild T, Wahl SM. Induction of APOBEC3 family proteins, a defensive maneuver underlying interferon-induced anti-HIV-1 activity. J Exp Med. 2006;203:41–46. doi: 10.1084/jem.20051512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Yang B, Pomerantz RJ, Zhang C, Arunachalam SC, et al. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature. 2003;424:94–98. doi: 10.1038/nature01707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris RS, Bishop KN, Sheehy AM, Craig HM, Petersen-Mahrt SK, et al. DNA deamination mediates innate immunity to retroviral infection. Cell. 2003;113:803–809. doi: 10.1016/s0092-8674(03)00423-9. [DOI] [PubMed] [Google Scholar]
- 20.Malim MH. APOBEC proteins and intrinsic resistance to HIV-1 infection. Philos Trans R Soc Lond B Biol Sci. 2009;364:675–687. doi: 10.1098/rstb.2008.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- 22.Greenwell-Wild T, Vazquez N, Jin W, Rangel Z, Munson PJ, et al. Interleukin-27 inhibition of HIV-1 involves an intermediate induction of type I interferon. Blood. 2009;114:1864–1874. doi: 10.1182/blood-2009-03-211540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809, table of contents. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imamichi T, Yang J, Huang DW, Brann TW, Fullmer BA, et al. IL-27, a novel anti-HIV cytokine, activates multiple interferon-inducible genes in macrophages. AIDS. 2008;22:39–45. doi: 10.1097/QAD.0b013e3282f3356c. [DOI] [PubMed] [Google Scholar]
- 25.Chen K, Huang J, Zhang C, Huang S, Nunnari G, et al. Alpha interferon potently enhances the anti-human immunodeficiency virus type 1 activity of APOBEC3G in resting primary CD4 T cells. J Virol. 2006;80:7645–7657. doi: 10.1128/JVI.00206-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharova N, Wu Y, Zhu X, Stranska R, Kaushik R, et al. Primate lentiviral Vpx commandeers DDB1 to counteract a macrophage restriction. PLoS Pathog. 2008;4:e1000057. doi: 10.1371/journal.ppat.1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergamaschi A, Ayinde D, David A, Le Rouzic E, Morel M, et al. The human immunodeficiency virus type 2 Vpx protein usurps the CUL4A-DDB1 DCAF1 ubiquitin ligase to overcome a postentry block in macrophage infection. J Virol. 2009;83:4854–4860. doi: 10.1128/JVI.00187-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaushik R, Zhu X, Stranska R, Wu Y, Stevenson M. A cellular restriction dictates the permissivity of nondividing monocytes/macrophages to lentivirus and gammaretrovirus infection. Cell Host Microbe. 2009;6:68–80. doi: 10.1016/j.chom.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gramberg T, Sunseri N, Landau NR. Evidence for an activation domain at the amino terminus of simian immunodeficiency virus Vpx. J Virol. 2010;84:1387–1396. doi: 10.1128/JVI.01437-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goujon C, Jarrosson-Wuilleme L, Bernaud J, Rigal D, Darlix JL, et al. With a little help from a friend: increasing HIV transduction of monocyte-derived dendritic cells with virion-like particles of SIV(MAC). Gene Ther. 2006;13:991–994. doi: 10.1038/sj.gt.3302753. [DOI] [PubMed] [Google Scholar]
- 31.Tsang J, Chain BM, Miller RF, Webb BL, Barclay W, et al. HIV-1 infection of macrophages is dependent on evasion of innate immune cellular activation. AIDS. 2009. [DOI] [PMC free article] [PubMed]
- 32.Neumann A, Polis M, Rozenberg L, Jackson J, Reitano K, et al. Differential antiviral effect of PEG-interferon-alpha-2b on HIV and HCV in the treatment of HIV/HCV co-infected patients. AIDS. 2007;21:1855–1865. doi: 10.1097/QAD.0b013e32825eaba7. [DOI] [PubMed] [Google Scholar]
- 33.Marchant D, Neil SJ, McKnight A. Human immunodeficiency virus types 1 and 2 have different replication kinetics in human primary macrophage culture. J Gen Virol. 2006;87:411–418. doi: 10.1099/vir.0.81391-0. [DOI] [PubMed] [Google Scholar]
- 34.DuBridge RB, Tang P, Hsia HC, Leong PM, Miller JH, et al. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol Cell Biol. 1987;7:379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soda Y, Shimizu N, Jinno A, Liu HY, Kanbe K, et al. Establishment of a new system for determination of coreceptor usages of HIV based on the human glioma NP-2 cell line. Biochem Biophys Res Commun. 1999;258:313–321. doi: 10.1006/bbrc.1999.0633. [DOI] [PubMed] [Google Scholar]
- 36.Reeves JD, Hibbitts S, Simmons G, McKnight A, Azevedo-Pereira JM, et al. Primary human immunodeficiency virus type 2 (HIV-2) isolates infect CD4-negative cells via CCR5 and CXCR4: comparison with HIV-1 and simian immunodeficiency virus and relevance to cell tropism in vivo. J Virol. 1999;73:7795–7804. doi: 10.1128/jvi.73.9.7795-7804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKnight A, Clapham PR, Weiss RA. HIV-2 and SIV infection of nonprimate cell lines expressing human CD4: restrictions to replication at distinct stages. Virology. 1994;201:8–18. doi: 10.1006/viro.1994.1260. [DOI] [PubMed] [Google Scholar]
- 38.Liszewski MK, Yu JJ, O'Doherty U. Detecting HIV-1 integration by repetitive-sampling Alu-gag PCR. Methods. 2009;47:254–260. doi: 10.1016/j.ymeth.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clouse KA, Powell D, Washington I, Poli G, Strebel K, et al. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J Immunol. 1989;142:431–438. [PubMed] [Google Scholar]
- 40.Folks TM, Powell D, Lightfoote M, Koenig S, Fauci AS, et al. Biological and biochemical characterization of a cloned Leu-3- cell surviving infection with the acquired immune deficiency syndrome retrovirus. J Exp Med. 1986;164:280–290. doi: 10.1084/jem.164.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butera ST, Roberts BD, Lam L, Hodge T, Folks TM. Human immunodeficiency virus type 1 RNA expression by four chronically infected cell lines indicates multiple mechanisms of latency. J Virol. 1994;68:2726–2730. doi: 10.1128/jvi.68.4.2726-2730.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]