Abstract
We report an approach to the fabrication of freestanding and amine-reactive thin films that is based on the reactive layer-by-layer assembly and subsequent lift-off of azlactone-containing polymer multilayers. We demonstrate that covalently crosslinked multilayers fabricated using the azlactone-functionalized polymer poly(2-vinyl-4,4-dimethylazlactone) (PVDMA) and a primary amine-containing polymer [poly(ethyleneimine) (PEI)] can be delaminated from planar glass and silicon surfaces by immersion in mildly acidic aqueous environments to yield flexible freestanding membranes. These freestanding membranes are robust and can withstand exposure to strong acid, strong base, or incubation in high ionic strength solutions that typically lead to the disruption and erosion of polymer multilayers assembled by reversible weak interactions (e.g., ‘polyelectrolyte multilayers’ assembled by electrostatic interactions or hydrogen bonding). We demonstrate further that these PEI/PVDMA assemblies contain residual reactive azlactone functionality that can be exploited to chemically modify the films (either directly after fabrication or after they have been lifted off of the substrates on which they were fabricated) using a variety of amine-functionalized small molecules. These freestanding membranes can also be transferred readily onto other objects (for example, onto the surfaces of planar substrates containing holes or pores) to fabricate suspended polymer membranes and other film-functionalized interfaces. In addition to planar, two-dimensional freestanding films, this approach can be used to fabricate and isolate three-dimensional freestanding membranes (e.g., curved films or tubes) by layer-by-layer assembly on, and subsequent lift-off from, the surfaces of topologically complex substrates (e.g., the curved ends of glass tubing, etc.). The results of this investigation, when combined, suggest the basis of methods for the fabrication of stable, chemically-reactive, and flexible polymer thin films and membranes of potential utility in a variety of fundamental and applied contexts.
Introduction
Methods for the fabrication of freestanding and flexible polymer thin films are of interest in a broad range of fundamental and applied contexts. Freestanding polymer membranes are important, for example, for the design of miniaturized sensors and micromechanical devices,1–3 and as semi-permeable membranes for filtration or catalysis.3–5 Flexible freestanding polymer films have also been investigated broadly for the design of lightweight and flexible electronics6–8 and the development of new tissue engineering scaffolds and wound healing materials.9,10 Significant advances have been made toward the design of functional freestanding polymer films with defined physicochemical properties and architectures by exploiting a variety of materials and methods of fabrication.3,9,11 The continued development and application of these materials, however, could benefit substantially from the development of methods that permit the assembly of chemically and mechanically robust films with physical properties and functions that can be tuned more broadly (e.g., either during fabrication or after they have been assembled and isolated). The ability to tailor the surface chemistry or physical properties of freestanding films post-fabrication could enable a broad range of new applications for these materials and, more generally, (i) circumvent the current need to design and isolate new polymer structures prior to the assembly of new functional membranes, and (ii) permit the design of films bearing functional groups that might not otherwise survive the chemical treatments and other processing steps often associated with the fabrication and isolation of freestanding thin films. In this paper, we report an approach to the fabrication of freestanding and chemically reactive polymer films that takes a step toward addressing several of these issues by developing methods for the ‘reactive’ layer-by-layer assembly and subsequent lift-off of amine-reactive, azlactone-functionalized polymer multilayers.
Several approaches to the fabrication and isolation of freestanding or suspended polymer thin films have been reported, including methods such as Langmuir-Blodgett deposition,1,12 spin-coating or casting of polymer films,13 and layer-by-layer assembly.11,14 Films assembled using these methods can then be delaminated, lifted off, or peeled from the surfaces on which they are fabricated using a variety of techniques, and subsequently manipulated as a completely freestanding (i.e., unsupported) film or as a partially supported (i.e., suspended) membrane drawn over the opening of a porous substrate. Of these various approaches, the layer-by-layer assembly of polyelectrolyte multilayers15 on surfaces and interfaces has emerged as a particularly versatile method for the fabrication of freestanding films and membranes because it permits the facile assembly of polyelectrolyte-based films with a broad range of different physicochemical properties.16–20 This general layer-by-layer approach forms the basis of the approach to the fabrication of covalently crosslinked and reactive polymer multilayers investigated here.
Layer-by-layer methods of assembly generally involve the alternate and repetitive adsorption of oppositely charged, water-soluble polymers (or polyelectrolytes) on surfaces. While substrate-supported ‘polyelectrolyte multilayers’ (or PEMs) have been investigated extensively for a wide range of potential applications,16–20 there has also been considerable interest in approaches that permit the removal of PEMs from the substrates on which they are fabricated to design completely freestanding PEMs.2,11,14,21–36 Several past studies have demonstrated that PEMs assembled using weak interactions (e.g., electrostatic interactions or hydrogen bonding) can be released from surfaces using a variety of release or ‘lift-off’ mechanisms. For example, freestanding PEMs have been isolated by dissolving the substrates on which films are deposited (e.g., by using HF to dissolve silicon or glass substrates, etc.)23,24,26,27 or by exposure to environmental conditions that dissolve or remove sacrificial polymer layers assembled between substrates and films.21,22,25,29–31,36 Other reports have demonstrated that PEMs can be released from their substrates by changes in pH,27,33,35 the addition of metal ions,32 or by the physical peeling of hydrogen bonded films from low-energy surfaces.28 These and other past studies have demonstrated the versatility of layer-by-layer methods for the fabrication of freestanding and flexible membranes of potential utility in a broad range of applications such as sensing,2,11,25,30,37 the controlled administration of therapeutics,38 and the design of new filtration/separation media.29,39
As noted above, the majority of past work in this area has focused on the design of PEMs assembled through weak interactions (e.g., electrostatic interactions or hydrogen bonding) that can be disrupted upon exposure to changes in pH or ionic strength. In addition, methods based on the assembly of polyelectrolytes are generally restricted to water-based assembly procedures that preclude the incorporation of non-water soluble polymers or reactive polymers that may hydrolyze or react readily with water. In the work reported here, we demonstrate an approach to the fabrication of freestanding polymer multilayers that exploits methods for the ‘reactive’ layer-by-layer assembly40,41 of multilayers in organic solvents and that permit the incorporation of water-sensitive, amine-reactive polymers into freestanding membranes.
We recently reported methods for the ‘reactive’ layer-by-layer fabrication of covalently crosslinked and reactive thin films that make use of reactive azlactone-functionalized polymers.42–45 Polymers containing azlactone functionality, such as poly(2-vinyl-4,4-dimethylazlactone) (PVDMA), undergo rapid, ‘click’-type ring-opening reactions in the presence of primary amines (Eq. 1).46 Our past studies demonstrate that this reactivity can be exploited to fabricate covalently crosslinked polymer multilayers using PVDMA and a primary amine-containing polymer [e.g., branched poly(ethylene imine) (PEI)].42–45 These methods permit the fabrication of thin polymer films on the surfaces of planar and topologically complex solid substrates,42,43,45 as well as the design of thin suspended membranes by assembly at interfaces created between two immiscible liquids.44 We have also demonstrated that residual, unreacted azlactone functionality present in these films can be used to chemically modify these materials post-fabrication (i.e., after film assembly) with a broad range of functional amines.42–45 This current investigation sought to determine whether this general approach could be used to assemble reactive multilayers that subsequently could be removed and isolated to fabricate reactive freestanding films and membranes. We note in this context that Seo et al. demonstrated recently that reactive films fabricated using polymers bearing activated ester groups can be removed from surfaces under mild conditions to yield chemically crosslinked freestanding films.47 We also note that several other groups have reported approaches to the covalent layer-by-layer assembly of polymer films on solid surfaces that could also be suitable for the fabrication of freestanding assemblies.40,41,48–52
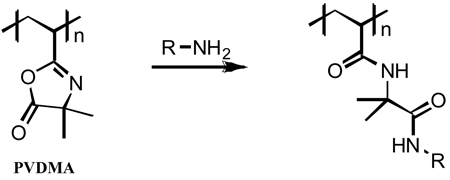 |
Eq. 1 |
We report here that polymer multilayers fabricated using PVDMA and PEI can be delaminated from planar glass and silicon surfaces to produce flexible and freestanding polymer membranes. These films readily detach and can be lifted off or transferred from substrates upon immersion in mildly acidic aqueous environments, and can be isolated as either hydrated films suspended in water or as dried, freestanding thin films in air. These materials retain their residual azlactone functionality under these conditions and can be chemically modified with primary amine-functionalized molecules either prior to or after isolation of the freestanding films. We demonstrate further that these reactive PEI/PVDMA films can be transferred onto other substrates or surfaces, suggesting opportunities for the use of these methods and materials to design functional thin films for incorporation into more complex devices or host geometries. These methods are also amenable to the fabrication of curved membranes and three-dimensional freestanding films by fabrication and subsequent lift-off of reactive films from the surfaces of non-planar substrates (e.g., tubes, etc.).
Materials and Methods
Materials
Test grade n-type silicon wafers were obtained from Si-Tech (Topsfield, MA). Glass microscope slides were purchased from Fisher Scientific (Pittsburgh, PA). Branched poly(ethyleneimine) (PEI, MW = 25,000), dansyl cadaverine, reagent grade acetone, and DMSO were purchased from Sigma Aldrich (Milwaukee, WI). Test grade n-type silicon wafers were purchased from Si-Tech, Inc. (Topsfield, MA). Glass microscope slides were purchased from Fischer Scientific (Pittsburgh, PA). The monomer 2-vinyl-4,4-dimethylazlactone (VDMA) was a kind gift from Dr. Steven M. Heilmann (3M Corporation, Minneapolis, MN). Glass substrates patterned with arrays of microholes (dia. = 10 µm) were kindly provided by Prof. Paul Steen (Cornell University). Propylamine was purchased from Alfa Aesar (Ward Hill, MA). Tetramethylrhodamine cadaverine was purchased from Invitrogen Corporation (Carlsbad, CA). All materials were used as received without further purification unless noted otherwise. Compressed air used to dry films and coated substrates was filtered through a 0.4 µm membrane syringe filter.
General Considerations
Silicon and glass substrates were cleaned with acetone, ethanol, methanol, and deionized water and dried under a stream of compressed air prior to the fabrication of multilayered films. Gel permeation chromatography (GPC) was performed using a GPCmax-VE2001 Solvent/Sample module (Viscotek Corp., Houston, TX) and two PlusPore Organic GPC Columns (Polymer Laboratories, Amherst, MA) equilibrated to 40 °C. THF was used as the eluent at a flow rate of 1.0 mL/min. Data were collected using the refractive index detector of a Viscotek TDA 302 triple detector array and processed using the OmniSEC 4.5 software package. Molecular weights and polydispersities are reported relative to monodisperse polystyrene standards. Attenuated total reflectance infrared spectroscopy data were acquired using a Bruker TENSOR 27 FTIR instrument (Billerica,MA) outfitted with an ATR transmission cell from PIKE Technologies (Madison, WI). Fluorescence microscopy images were acquired using an Olympus IX70 microscope and analyzed using the Metavue version 4.6 software package (Universal Imaging Corporation). Digital images were acquired using a Nikon Coolpix 4300 digital camera. Scanning electron micrographs were acquired using a LEO DSM 1530 scanning electron microscope at an accelerating voltage of 3 kV. Samples were coated with a thin layer of gold using a sputterer (30 s at 45 mA, 50 mTorr) prior to imaging. Stress/strain curves were generated using uniaxial tensile tests at ambient room temperature on a TA Instruments RSA 3 instrument.
Fabrication and Release (Lift-Off) of Polymer Multilayers
Solutions of PEI or PVDMA used for the fabrication of multilayered films were prepared in acetone (20 mM with respect to the molecular weight of the polymer repeat unit). Films were deposited manually layer-by-layer on silicon or glass substrates according to the following general protocol: 1) Substrates were submerged in a solution of PEI for 30 seconds, 2) substrates were removed and immersed in an initial acetone bath for 30 seconds followed by a second acetone bath for 30 seconds, 3) substrates were submerged in a solution of PVDMA for 30 seconds, and 4) substrates were rinsed in the manner described in step 2. This cycle was repeated until the desired number of PEI/PVDMA layers was reached (typically, 50–100 bilayers; the term ‘bilayer’, as used here, refers to a single PEI/PVDMA layer pair). Films were characterized or used in subsequent experiments immediately or were dried under a stream of filtered, compressed air and stored in a vacuum desiccator until use. All films were fabricated at ambient room temperature.
After fabrication, PEI/PVDMA films supported on glass or silicon substrates were cut into rectangular (approximately 3 cm × 1 cm) or square (approximately 2 cm × 2 cm) pieces by scoring film-coated silicon or glass substrates with a razor blade. Coated substrates were then immersed in deionized water adjusted to pH 2–3 with 1M HCl. After several minutes of immersion, the films detached from the surface and could be readily peeled from the substrate using forceps. The films were then removed from the aqueous solution using forceps and transferred to an acetone bath for approximately one minute. Dry freestanding films were obtained by transferring the film suspended in the acetone bath on a flat piece of poly(tetrafluoroethylene) (PTFE), removing the PTFE-supported film from the acetone bath, and then allowing the supported films to dry. Once dry, freestanding PEI/PVDMA films peeled readily away from the PTFE substrate and could be used for characterization and subsequent experiments. Freestanding films were transferred onto other substrates (e.g., TEM grids or glass substrates containing arrays of microholes) by placing a freestanding film isolated as described above on the surface of DI water and then lifting the film from the surface of the water with the substrate.
Post-Fabrication Functionalization of PEI/PVDMA Films
Experiments designed to investigate the reactivity of residual azlactone groups in freestanding PEI/PVDMA films were conducted by incubating films in solutions of dansyl cadaverine (2 mg/mL, in DMSO) for 24 hours. The films were removed from the fluorophore solution and rinsed in fresh DMSO by swirling gently for approximately 16 hours, exchanging the DMSO for fresh solvent two times during the rinse period. Freestanding films were then transferred into acetone and removed from the acetone bath as described above. Freestanding films that were incubated in a solution of propylamine (50 mM, in DMSO for 24 hours; to react with any remaining azlactone functionality) prior to treatment with dansyl cadaverine were used as controls. Dansyl cadaverine-treated films were characterized by fluorescence microscopy.
Results and Discussion
Fabrication of Freestanding PEI/PVDMA Thin Films
Our initial studies sought to determine whether PEI/PVDMA films fabricated on the surfaces of silicon or glass substrates could be detached and lifted off, and whether this could be achieved using conditions that would not disrupt the film or completely hydrolyze the residual reactive azlactone functionality within these materials. To this end, we fabricated films composed of 100 PEI/PVDMA layer pairs (or 100 ‘bilayers’ of PEI and PVDMA) by the alternate and repetitive dipping of glass or silicon substrates into solutions of PEI and PVDMA dissolved in acetone for 20 seconds each (see Materials and Methods for additional details). These films became visibly opaque and cloudy after the deposition of approximately 25 bilayers and, as a result, the thicknesses and growth profiles of films containing more than 25 bilayers could not be characterized using methods such as ellipsometry. We estimated the thickness of a 100-bilayer PEI/PVDMA film to be at least 5 µm by characterization of scratched films using atomic force microscopy (AFM). We note, however, that the exact thicknesses of these 100-bilayer films were difficult to measure accurately due to crumpling or flaking of these substrate-supported films. We return to further characterization and discussion of the thicknesses of freestanding films derived from these supported materials in the discussion below.
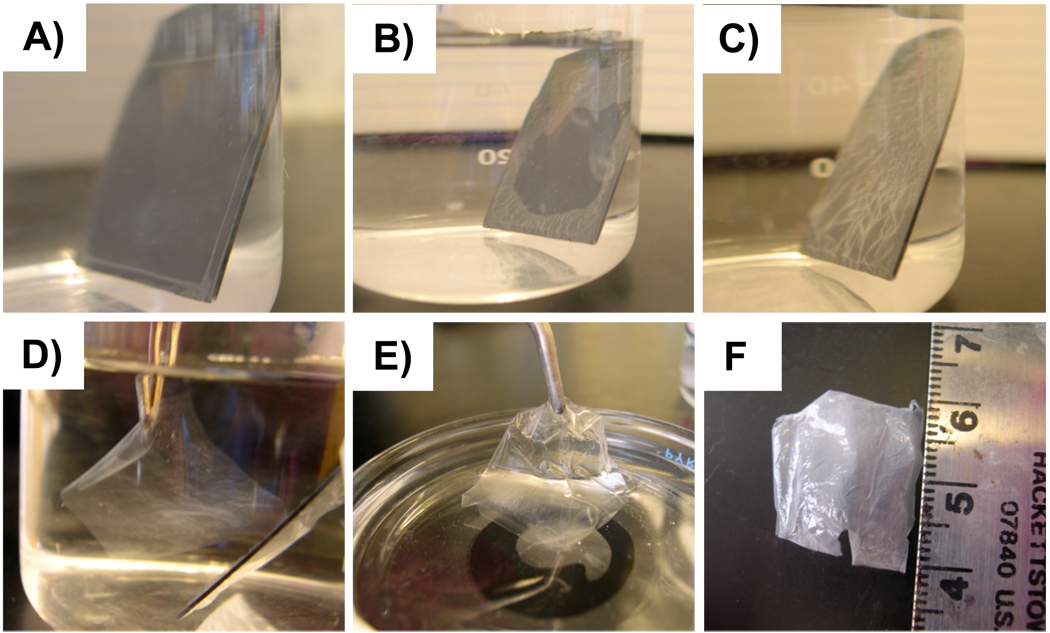
To isolate freestanding films with well-defined edges and shapes, film-coated silicon and glass substrates were scored near the edges of the substrate using a razor blade. These scored films were then submerged in DI water adjusted to pH 2–3 using 1M HCl. Figures 1A and 1B show digital images of a 100-bilayer film fabricated on a silicon substrate immersed in water at pH 3 for 10 and 23 minutes, respectively. After 23 minutes, these films began to wrinkle and gradually detach from the surface of the silicon (as shown in Figure 1B). After 38 minutes, the films were wrinkled uniformly across the surface of the substrate (Figure 1C) and could be peeled away from the substrate using forceps (Figure 1D). The films were then transferred to an acetone bath (Figure 1E) for approximately one minute, to facilitate removal of water in the films, and were then removed from the acetone bath using forceps and allowed to dry in ambient air. Films could also be removed from the acetone bath on a flat piece of PTFE to prevent wrinkling and curling of the film as the acetone evaporated. After drying, the films readily peeled from the PTFE to yield flat freestanding films (Figure 1F). Dried 100-bilayer freestanding films were sufficiently mechanically robust that they could be handled and manipulated using forceps without breaking or tearing. In addition to exposure to low pH environments, we explored several other solution conditions and substrate treatment protocols that could be used to remove and lift off these films from their substrates, including (i) the exposure of film-coated substrates to solutions of higher ionic strength (e.g., 0.1 M NaCl), (ii) sonication of film-coated substrates in organic solvents and in aqueous solutions at higher pH (e.g., pH ~4–5), and (iii) the fabrication of films on PTFE followed by incubation in water and/or peeling of the films from their substrates under air. These additional conditions either did not result in delamination of the films or resulted in disintegration of the films into small flakes (e.g., as observed during experiments using sonication). While the mildly acidic aqueous conditions described above likely do not represent a completely optimized protocol for the removal and lift-off of these films from their substrates, these conditions were sufficient for the isolation of all freestanding PEI/PVDMA films used in experiments described below.
Figure 1.
Digital photographs of a 100-bilayer PEI/PVDMA film immersed in water at pH 3. A–C) Images acquired of the film after A) 10 minutes, B) 23 minutes, and C) 38 minutes of immersion in the water bath. D) Image of a film detached from the silicon substrate and suspended in water. E) Image of a freestanding film partially submerged in an acetone bath. F) Image of a dry, freestanding film. Scale is in cm.
The acidic conditions used to promote the delamination of the PEI/PVDMA films described above did not result in visible tearing or disintegration of the films. In addition, these covalently crosslinked films remained visibly intact for at least two months under a range of aqueous conditions (e.g., when submerged at room temperature in 1 M HCl, 1 M NaOH, or in a saturated sodium chloride solution). Freestanding PEI/PVDMA films could also be cycled between exposure to strong acid and strong base without destruction or disintegration of the films. The results of these experiments demonstrate the stability of these freestanding films upon exposure to a range of conditions that have been demonstrated to physically disrupt and/or destroy PEMs fabricated using weak interactions (e.g., electrostatic interactions or hydrogen bonding).
Using the general procedure described above for the isolation of 100-bilayer freestanding films, we were also able to isolate and manually manipulate uniform, defect-free PEI/PVDMA films as thin as 20 bilayers thick (approximately 200 nm thick as measured using ellipsometry prior to film delamination). Freestanding films composed of as few as 10 bilayers (~100 nm, as measured by ellipsometry prior to film removal) could be isolated by immersion of film-coated substrates in acidic aqueous solutions, however these thin films typically required longer immersion times to induce delamination and were generally not robust enough to be isolated as unsupported, dry films without breaking. These thinner films could, however, be suspended in aqueous media and then transferred onto other solid supports (as discussed in further detail below). Films containing fewer than 10 bilayers of PEI and PVDMA either did not detach from the substrate surface or fractured into small pieces of film during delamination from the surface under the conditions used here.
The detachment of PEI/PVDMA multilayered films under the mildly acidic conditions described above is likely facilitated by the disruption of hydrogen bonding between the bottommost layer of PEI (i.e., the first layer of polymer deposited on the substrate during fabrication) and the hydroxyl groups of the silicon and glass substrates. In addition, it is also possible that the hydroxyl groups of silicon oxide surfaces are partially positively charged at low pH and that electrostatic repulsion between these surfaces and this PEI layer (PEI is protonated and positively charged at low pH) could account for the release of these films.33,53 These potential mechanisms are supported by the results of additional experiments demonstrating that films delaminated more rapidly at lower values of pH. For example, whereas films immersed in water adjusted to pH 3 required ~40 minutes to fully detach, films submerged in water at pH 2 exfoliated from glass substrates within 5–10 minutes. Finally, we note that similar mechanisms for delamination have been suggested in studies of the lift-off and isolation of other covalently crosslinked polymer multilayers.47
Characterization of the Physical and Mechanical Properties of PEI/PVDMA Freestanding Films
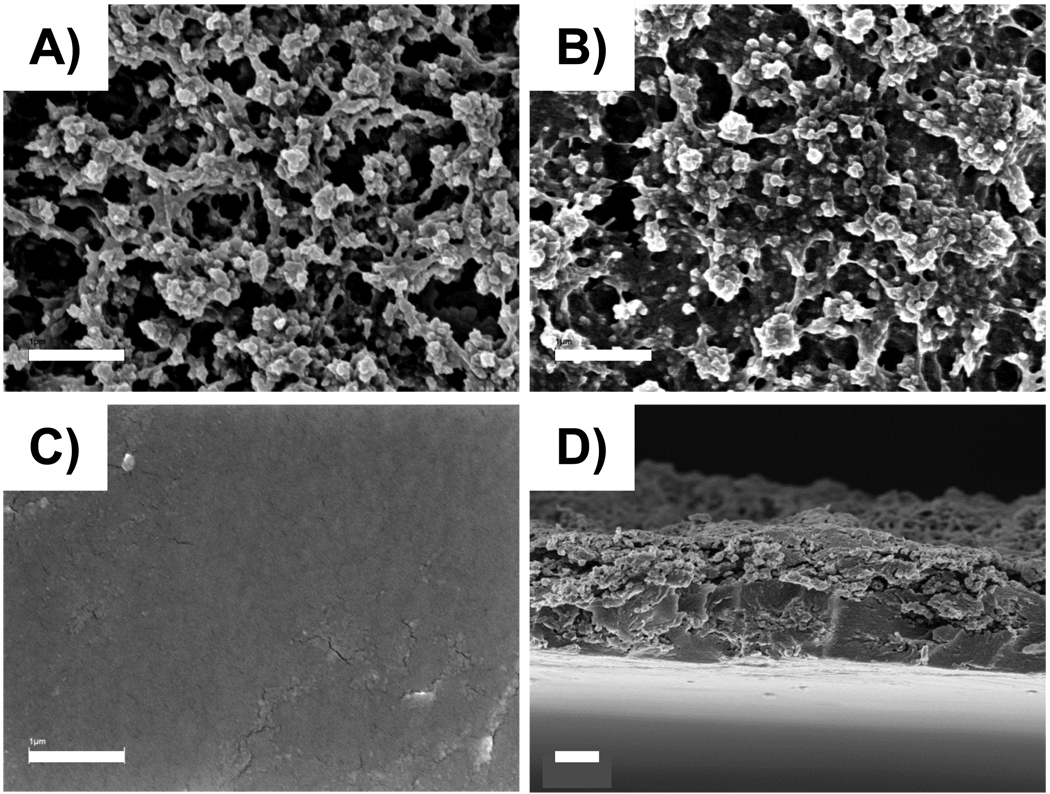
Characterization of freestanding films using scanning electron microscopy (SEM) revealed significant differences in the surface morphologies of the top and bottom faces of the films (the term ‘bottom face’ refers to the side of the film in contact with the substrate during assembly; the term ‘top face’ refers to the side of the film in contact with the polymer solutions during assembly). Figures 2A and 2B show representative SEM images of the top faces of a 50-bilayer and a 100-bilayer freestanding film, respectively. Inspection of these images reveals that the surfaces of these films are rough and textured with nanometer and micrometer-scale features. These topographical features contrast with the smooth and uniform surfaces of the bottom faces of these films (e.g., Figure 2C). The roughness of the top faces of these films likely accounts for the opaque appearances of these thicker freestanding films (e.g., as shown in Figure 1E), and give rise to different and side-dependent physical properties that could influence the behaviors or potential applications of these freestanding films. Additional studies focused on investigating the origins of this surface roughness suggest that it arises, at least in part, from the presence of cyclic azlactone-functionalized oligomers present during the polymerization of PVDMA. These additional investigations and a report on the impact of these surface morphologies on the physical properties of these films (e.g., wettability, etc.) will be reported in a separate publication.54
Figure 2.
SEM images of A) the top face (i.e., side of the film exposed to polymer solutions) of a 50-bilayer freestanding PEI/PVDMA film, B) the top face of a 100-bilayer freestanding PEI/PVDMA film, C) the bottom face (i.e., side of the film attached to the glass substrate) of a 100-bilayer freestanding PEI/PVDMA film, and D) a cross-section of a freestanding film (top face is pointing up, bottom face is pointing down). Scale bars = A–C) 1 µm and D) 2 µm.
Figure 2D shows a cross-sectional image of a 100-bilayer film that was torn prior to imaging and reveals an average film thickness of ~4 micrometers (as determined by measuring the average point-to-point distance between the top and bottom faces of the film). This value is, in general, on the order of the thicknesses measured by AFM for 100-bilayer films prior to delamination (as discussed above; measured to be, on average, at least 5 µm thick). Finally, initial characterization of the tensile mechanical properties of freestanding PEI/PVDMA films 100-bilayer thick measured by subjecting freestanding films to uniaxial tensile tests revealed ultimate tensile strengths of ~150 MPa and elastic moduli of ~55 MPa (a representative stress vs. strain curve for a 100-bilayer PEI/PVDMA thin film is shown in Figure S1 of the Supporting Information). We note that the nature of the layer-by-layer assembly process used here creates opportunities for the incorporation of a range of different polyamines or other materials (polymers, nanoparticles, etc.) that could be used to tune further the mechanical properties of these materials. In addition, the copolymerization of azlactone-containing monomers with other functional monomers46 can be used to generate reactive copolymers that could be used to tune the crosslinking density within these films and/or introduce additional functionality to manipulate further the physical properties of these materials.
Reactivity of Freestanding PEI/PVDMA Films: Post-Fabrication Functionalization
We have demonstrated in past reports that the reactivity of residual azlactone functionality within PEI/PVDMA multilayers fabricated on solid surfaces can be exploited to modify these materials using a range of different amine-functionalized nucleophiles.42–45 In the context of this current work, however, we note that azlactone functionality can react (i.e., hydrolyze) upon contact with water,46 and that it is possible that the unreacted azlactone groups present in these films after fabrication could be consumed by hydrolysis upon exposure to the weakly acidic aqueous media described above used to isolate these materials as freestanding films.
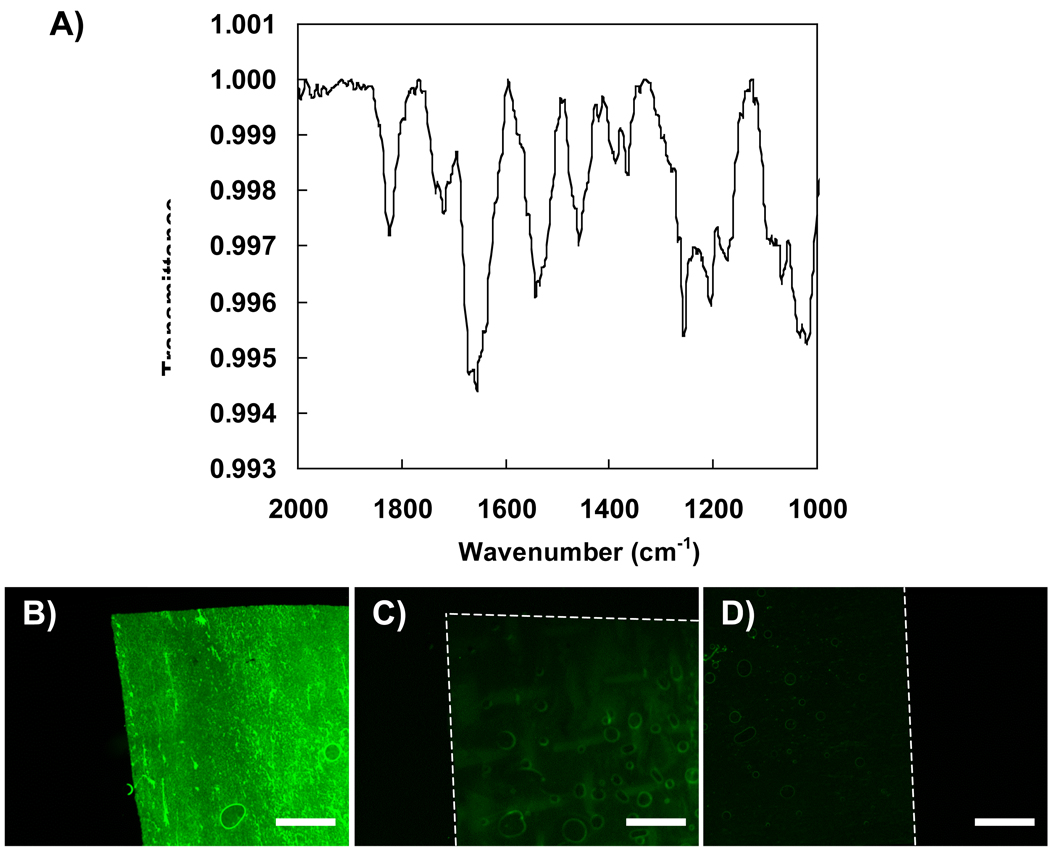
Our next experiments sought to characterize the presence (or absence) of residual azlactone functionality in our freestanding materials and determine whether it was possible to use these groups to chemically modify the films using amine-functionalized nucleophiles. Characterization of freestanding films using infrared spectroscopy (IR) revealed peaks characteristic of unreacted azlactone functionality. Figure 3A shows the carbonyl region of an IR spectrum acquired using a 100-bilayer freestanding film prepared as described above. Inspection of these data reveals a peak at 1821 cm−1, which is characteristic of the C=O stretch of unreacted azlactone functionality. The peak centered at approximately 1725 cm−1 corresponds with what has been reported previously to be the C=O stretch of carboxylic acid functionality formed upon hydrolysis of the azlactone ring.46 The strong peak centered at approximately 1655 cm−1 corresponds to both the C=N bond in unreacted azlactone groups as well as the carbonyl peaks of amide bonds formed during film fabrication (e.g., see Equation 1) and the hydrolysis of azlactone groups. These results demonstrate that while some of the azlactone groups present in the films after film fabrication (in organic solvents) are likely hydrolyzed during the incubation of the films in mildly acidic aqueous environments, a significant amount of azlactone functionality remains intact and unhydrolyzed.
Figure 3.
A) Infrared spectrum acquired for a freestanding PEI/PVDMA film. Fluorescence micrographs of B) a freestanding film treated with dansyl cadaverine, C) an untreated free-standing film, and D) a freestanding film treated first with propylamine followed by dansyl cadaverine. Dotted lines show the edges of the films. Scale bars = 500 µm.
To determine whether the residual azlactone functionality in these isolated, freestanding films could be used for further functionalization, a 100-bilayer freestanding film was submerged in a solution of the amine-functionalized fluorophore dansyl cadaverine (2 mg/mL in DMSO) for ~12 hours followed by rinsing in fresh DMSO overnight. Figure 3B shows a fluorescence microscopy image of the edge of a dansyl-functionalized freestanding film. Inspection of this image reveals uniform levels of fluorescence that are significantly greater than those observed for an otherwise identical, untreated freestanding film (shown in Figure 3C). Control experiments in which a freestanding film was treated with propylamine (to react with and consume all reactive azlactone groups) prior to treatment with dansyl cadaverine (Figure 3D) revealed levels of fluorescence similar to those observed for untreated freestanding films. These results demonstrate that the residual azlactone functionality in these freestanding PEI/PVDMA films can be used to chemically modify these assemblies post-fabrication. We note here that our past studies establish that it is also possible to functionalize these films (and thereby influence the physical properties (e.g., wettability) of the surfaces of these materials) prior to removing these films from their substrates.42–45 However, the ability to modify these multilayers after the delamination and manipulation of these freestanding films creates opportunities to functionalize freestanding films and membranes with chemical or biological functionality that may otherwise be degraded or altered by exposure to mild acidic conditions.
Transfer of Freestanding Films onto Other Substrates and the Fabrication of Three-Dimensional Membranes
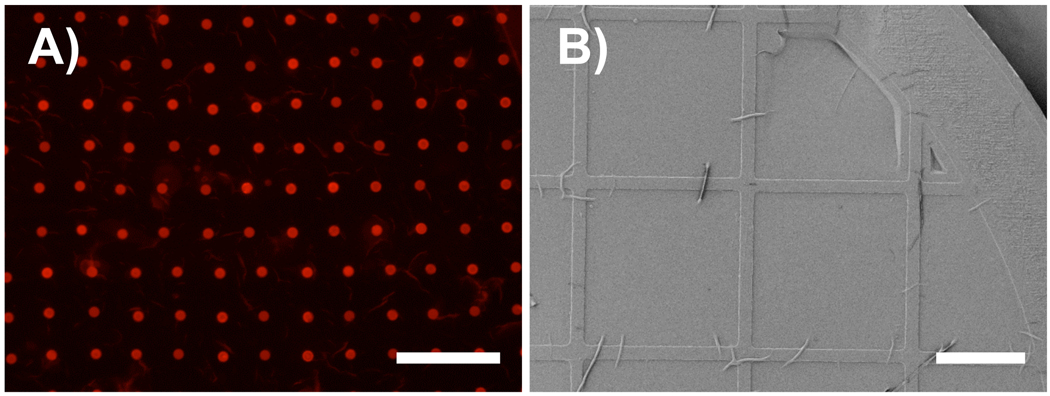
Semipermeable and pliable polyelectrolyte multilayers suspended over pores or cavities have been investigated for the development of micromechanical devices, sensors, and separation/filtration membranes.2,11,25,29,30,37 Several past studies have demonstrated that suspended PEMs can be created by transferring freestanding PEMs onto substrates containing holes, cavities, or pores.2,25,29,30,37 To determine whether our freestanding PEI/PVDMA multilayers could be transferred onto other substrates and suspended across holes or pores, we fabricated 100-bilayer freestanding films and treated the films with the amine-functionalized fluorophore tetramethylrhodamine cadaverine (TMR). These fluorescently labeled freestanding films were then placed on the surface of a water bath and lifted from the air/water interface using a glass substrate patterned with an array of small holes (dia. = 10 µm). Figure 4A shows a fluorescence micrograph of a TMR-labeled freestanding film transferred in this manner and reveals uniform red fluorescence across the glass substrate and across each of the holes of the array, demonstrating that the film is both adhered to the surface of the glass and suspended across the holes in the substrate (the red circles correspond to areas of the film suspended across the pores and appear brighter than the rest of the film due to optical effects). We note here that the ability to transfer these films to glass and silicon substrate was strongly side-dependent. In general, freestanding films situated such that the bottom face of the film (defined as described above and shown in Figure 2C) resulted in films that transferred smoothly and adhered strongly to the surfaces of the new substrates to which they were transferred. Films that were situated such that the rougher top faces of these films were placed in contact with a new substrate did not adhere strongly and were generally more difficult to transfer. These differences in behavior are likely the result of the large differences in the roughness and surface morphologies of the top and bottom faces of these films (as shown in Figure 2). Freestanding PEI/PVDMA films could also be transferred and overlaid onto other substrates having larger pore sizes and smaller supporting regions. Figure 4B shows an SEM image of a film lifted and transferred onto a gold TEM grid (grid openings are 283 µm on a side and the bar width is 55 µm). When combined, these results demonstrate the ability to transfer PEI/PVDMA films onto a variety of substrates with different geometries and pore sizes and suggest a general approach for the fabrication of suspended thin films of potential use in filtration/separation applications3,5 or the design of micromechanical devices3,11 and microactuators.55
Figure 4.
A) Fluorescence micrograph of a TMR-functionalized freestanding film ransferred onto a glass substrate patterned with microholes. B) SEM image of a freestanding film transferred onto a gold TEM grid. Scale bars = A) 100 µm and B) 200 µm.
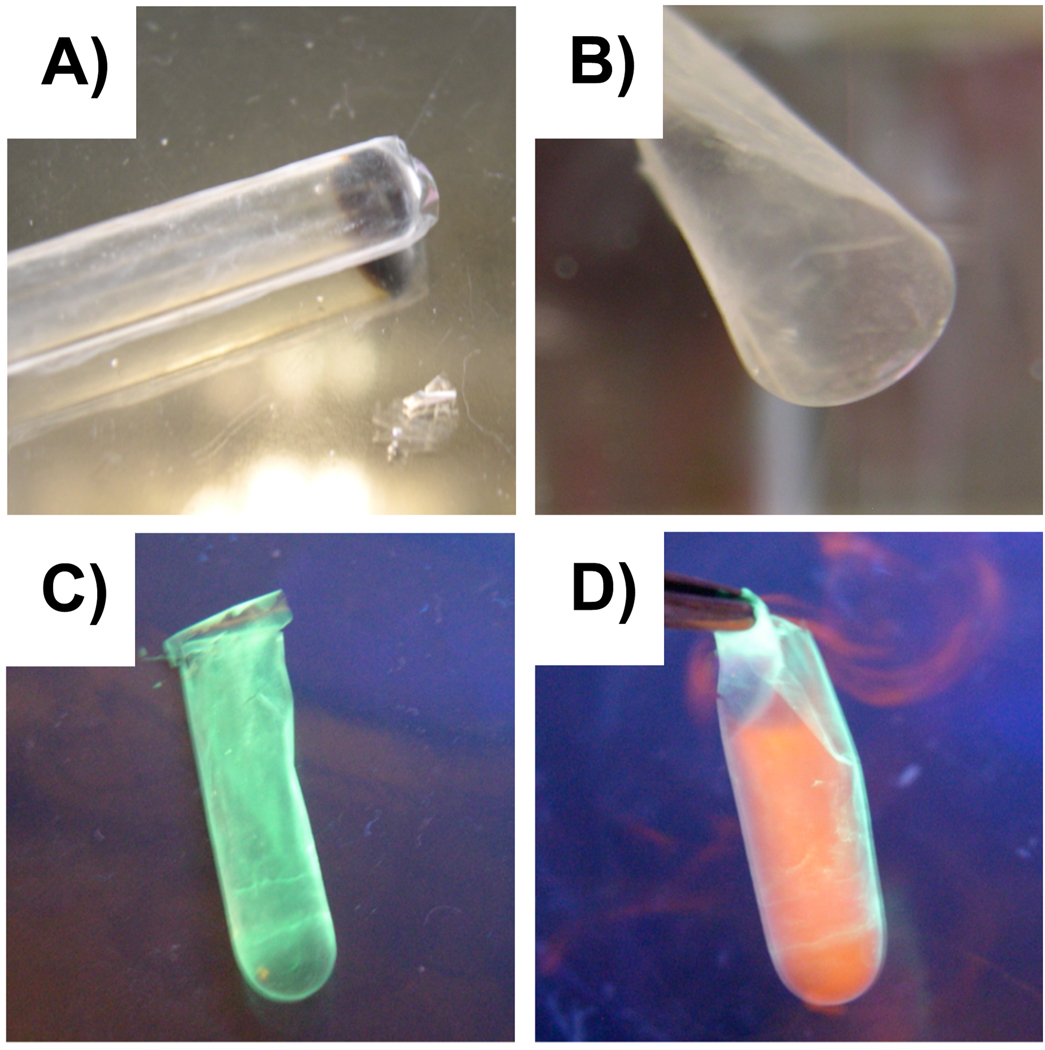
Finally, one key advantage of layer-by-layer methods for the fabrication of thin films on surfaces is the ability to assemble thin films directly on the surfaces of topologically-complex substrates.17–20,34 We performed a final series of experiments to demonstrate that the general approach described above for the fabrication and isolation of two-dimensional (i.e., planar) freestanding films can be used to fabricate and isolate three-dimensional freestanding membranes (e.g., curved films or thin film tubes) by layer-by-layer assembly on, and subsequent lift-off from, the surfaces of topologically complex substrates. To demonstrate the feasibility of this approach, we fabricated 75-bilayer PEI/PVDMA films on the curved ends of a standard glass nuclear magnetic resonance (NMR) tube (outer dia. = ~6 mm). These film-coated tubes were then immersed in water adjusted to pH ~2.
Figure 5A shows a digital photograph of the coated end of a tube submerged in water and shows the film wrinkling and beginning to detach from the end of the tube. Once the film had completely detached from the surface of the glass, the tube was removed to yield a curved and three-dimensional hollow tube with one closed end (as shown in Figure 5B). Figure 5C shows a digital photograph (imaged under UV illumination) of a freestanding tube that was chemically functionalized with dansyl cadaverine after the removal of the film from the glass tube substrate. These three-dimensional freestanding films were mechanically robust and could be removed from the water to yield thin film ‘sacks’ that could also be filled with solutions of other agents (for example, an aqueous solution of TMR, as shown in Figure 5D). The ability to fabricate intact and three-dimensional membranes using this approach could offer new opportunities to characterize the permeabilities of these materials and develop new thin film platforms for separations-based applications. More generally, methods that are amenable to the fabrication and subsequent transfer of two- or three-dimensional freestanding films suggest broader opportunities to design functional thin films that can be incorporated into, or used to functionalize the surfaces of, more complex devices or host geometries.
Figure 5.
A) Digital photograph of a 75-bilayer PEI/PVDMA film coated on the end of a standard NMR tube and immersed in water at pH 3. B) Photograph of the curved end of a freestanding film removed from an NMR tube and suspended in water. C) Digital image of a freestanding film reacted with dansyl cadaverine and illuminated under a UV lamp. D) Digital photograph of a dansyl cadaverine-functionalized freestanding “pouch” filled with a solution of TMR in water illuminated under a UV lamp.
Summary and Conclusions
We have reported a layer-by-layer approach to the fabrication of covalently crosslinked and amine reactive freestanding thin films. We demonstrated that multilayered films fabricated from PEI and PVDMA could be delaminated from and lifted off of silicon or glass surfaces when immersed in a mildly acidic water bath (pH 2–3). These assemblies were stable and could withstand exposure to a range of aqueous conditions (e.g., high and low pH values and high ionic strength) that typically lead to the disruption of polyelectrolyte-based multilayers assembled through reversible physical interactions. Additional experiments demonstrated that freestanding PEI/PVDMA films contained residual azlactone functionality after film fabrication and lift-off, and that these residual reactive groups could be used to functionalize PEI/PVDMA freestanding films post-fabrication by treatment with amine-functionalized nucleophiles. These flexible membranes could be readily transferred onto other objects, for example onto pore- or cavity-containing substrates, and, thus, offer opportunities for the fabrication of suspended polymer membranes. Finally, in addition to planar, two-dimensional freestanding films, the approach reported here permitted the fabrication of more complex, three-dimensional freestanding films by assembly and subsequent lift-off from topologically complex substrates. The methods and materials described here offer a modular approach to the design and rapid fabrication of stable, chemically crosslinked, and reactive polymer thin films of potential utility in a range of applications such as catalysis, sensing, separation/filtration, or drug delivery.
Supplementary Material
Acknowledgments
Financial support was provided by the NSF (DMR-0520527) through a grant to the Materials Research Science and Engineering Center (MRSEC) at the University of Wisconsin, and the Alfred P. Sloan Foundation. We thank Dr. Steven M. Heilmann and Dr. Gerald K. Rasmussen (3M Corporation) for providing samples of 2-vinyl-4,4-dimethylazlactone. We thank the Steen Group at Cornell University for providing plates for testing; these plates were fabricated under support by DARPA grant (ARO W911NF0610236). We thank Eric M. Saurer for assistance with scanning electron microscopy experiments and the Soft Materials Laboratory at the University of Wisconsin-Madison for assistance with gel permeation chromatography and dynamic mechanical analysis experiments. M.E.B. was funded in part by an NIH Chemistry Biology Interface Training Grant (NIGMS T32 GM008505).
References
- 1.Mallwitz F, Goedel WA. Angew. Chem. Int. Ed. 2001;40:2645–2647. [PubMed] [Google Scholar]
- 2.Jiang CY, Markutsya S, Pikus Y, Tsukruk VV. Nat. Mater. 2004;3:721–728. doi: 10.1038/nmat1212. [DOI] [PubMed] [Google Scholar]
- 3.Cheng WL, Campolongo MJ, Tan SJ, Luo D. Nano Today. 2009;4:482–493. [Google Scholar]
- 4.Pandey P, Chauhan RS. Prog. Polym. Sci. 2001;26:853–893. [Google Scholar]
- 5.Ulbricht M. Polymer. 2006;47:2217–2262. [Google Scholar]
- 6.Forrest SR. Nature. 2004;428:911–918. doi: 10.1038/nature02498. [DOI] [PubMed] [Google Scholar]
- 7.Kim DH, Ahn JH, Choi WM, Kim HS, Kim TH, Song JZ, Huang YGY, Liu ZJ, Lu C, Rogers JA. Science. 2008;320:507–511. doi: 10.1126/science.1154367. [DOI] [PubMed] [Google Scholar]
- 8.LeMieux MC, Bao ZN. Nat. Nanotechnol. 2008;3:585–586. doi: 10.1038/nnano.2008.296. [DOI] [PubMed] [Google Scholar]
- 9.Stamatialis DF, Papenburg BJ, Gironés M, Saiful S, Bettahalli SNM, Schmitmeier S, Wessling M. J. Membr. Sci. 2008;308:1–34. [Google Scholar]
- 10.Wan LS, Liu ZM, Xu ZK. Soft Matter. 2009;5:1775–1785. [Google Scholar]
- 11.Jiang CY, Tsukruk VV. Adv. Mater. 2006;18:829–840. [Google Scholar]
- 12.Goedel WA, Heger R. Langmuir. 1998;14:3470–3474. [Google Scholar]
- 13.Lim MH, Ast DG. Adv. Mater. 2001;13:718–721. [Google Scholar]
- 14.Nolte M, Fery A. IEE Proc.-Nanobiotechnol. 2006;153:112–120. doi: 10.1049/ip-nbt:20050017. [DOI] [PubMed] [Google Scholar]
- 15.Decher G. Science. 1997;277:1232–1237. [Google Scholar]
- 16.Bertrand P, Jonas A, Laschewsky A, Legras R. Macromol. Rapid Comm. 2000;21:319–348. [Google Scholar]
- 17.Hammond PT. Adv. Mater. 2004;16:1271–1293. [Google Scholar]
- 18.Peyratout CS, Dahne L. Angew. Chem. Int. Ed. 2004;43:3762–3783. doi: 10.1002/anie.200300568. [DOI] [PubMed] [Google Scholar]
- 19.Tang ZY, Wang Y, Podsiadlo P, Kotov NA. Adv. Mater. 2006;18:3203–3224. [Google Scholar]
- 20.Boudou T, Crouzier T, Ren K, Blin G, Picart C. Adv. Mater. 2010;22:441–467. doi: 10.1002/adma.200901327. [DOI] [PubMed] [Google Scholar]
- 21.Mamedov AA, Kotov NA. Langmuir. 2000;16:5530–5533. [Google Scholar]
- 22.Dubas ST, Farhat TR, Schlenoff JB. J. Am. Chem. Soc. 2001;123:5368–5369. doi: 10.1021/ja015774+. [DOI] [PubMed] [Google Scholar]
- 23.Mamedov AA, Kotov NA, Prato M, Guldi DM, Wicksted JP, Hirsch A. Nat. Mater. 2002;1:190–194. doi: 10.1038/nmat747. [DOI] [PubMed] [Google Scholar]
- 24.Tang ZY, Kotov NA, Magonov S, Ozturk B. Nat. Mater. 2003;2 doi: 10.1038/nmat906. 413-U8. [DOI] [PubMed] [Google Scholar]
- 25.Jiang CY, Markutsya S, Tsukruk VV. Adv. Mater. 2004;16:157–161. [Google Scholar]
- 26.Volodkin DV, Larionova NI, Sukhorukov GB. Biomacromolecules. 2004;5:1962–1972. doi: 10.1021/bm049669e. [DOI] [PubMed] [Google Scholar]
- 27.Lavalle P, Boulmedais F, Ball V, Mutterer J, Schaaf P, Voegel JC. J. Membr. Sci. 2005;253:49–56. [Google Scholar]
- 28.Lutkenhaus JL, Hrabak KD, McEnnis K, Hammond PT. J. Am. Chem. Soc. 2005;127:17228–17234. doi: 10.1021/ja053472s. [DOI] [PubMed] [Google Scholar]
- 29.Nolte M, Schoeler B, Peyratout CS, Kurth DG, Fery A. Adv. Mater. 2005;17:1665–1669. [Google Scholar]
- 30.Jiang CY, Kommireddy DS, Tsukruk VV. Adv. Funct. Mater. 2006;16:27–32. [Google Scholar]
- 31.Ono SS, Decher G. Nano Lett. 2006;6:592–598. doi: 10.1021/nl0515504. [DOI] [PubMed] [Google Scholar]
- 32.Ma Y, Sun J, Shen J. Chem. Mater. 2007;19:5058–5062. [Google Scholar]
- 33.Bernsmann F, Richert L, Senger B, Lavalle P, Voegel JC, Schaaf P, Ball V. Soft Matter. 2008;4:1621–1624. doi: 10.1039/b806649c. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Angelatos AS, Caruso F. Chem. Mater. 2008;20:848–858. [Google Scholar]
- 35.Bernsmann F, Ponche A, Ringwald C, Hemmerle J, Raya J, Bechinger B, Voegel JC, Schaaf P, Ball V. J. Phys. Chem. C. 2009;113:8234–8242. [Google Scholar]
- 36.Gui ZL, Qian JW, An QF, Zhao Q, Jin HT, Du BY. J. Mater. Chem. 2010;20:1467–1474. [Google Scholar]
- 37.Nolte M, Donch I, Fery A. ChemPhysChem. 2006;7:1985–1989. doi: 10.1002/cphc.200600300. [DOI] [PubMed] [Google Scholar]
- 38.Mansouri S, Winnik FM, Tabrizian M. Expert Opin. Drug Del. 2009;6:585–597. doi: 10.1517/17425240902967599. [DOI] [PubMed] [Google Scholar]
- 39.Zimnitsky D, Shevchenko VV, Tsukruk VV. Langmuir. 2008;24:5996–6006. doi: 10.1021/la7038575. [DOI] [PubMed] [Google Scholar]
- 40.Quinn JF, Johnston APR, Such GK, Zelikin AN, Caruso F. Chem. Soc. Rev. 2007;36:707–718. doi: 10.1039/b610778h. [DOI] [PubMed] [Google Scholar]
- 41.Bergbreiter DE, Liao K-S. Soft Matter. 2009;5:23–28. [Google Scholar]
- 42.Buck ME, Zhang J, Lynn DM. Adv. Mater. 2007;19:3951–3955. [Google Scholar]
- 43.Buck ME, Breitbach AS, Belgrade SK, Blackwell HE, Lynn DM. Biomacromolecules. 2009;10:1564–1574. doi: 10.1021/bm9001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buck ME, Lynn DM. Adv. Mater. 2010;22:994–998. doi: 10.1002/adma.200903054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buck ME, Lynn DM. ACS Appl. Mater. Interfaces. 2010;2:1421–1429. doi: 10.1021/am1000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heilmann SM, Rasmussen JK, Krepski LR. J. Polym. Sci. Part A. 2001;39:3655–3677. [Google Scholar]
- 47.Seo J, Schattling P, Lang T, Jochum F, Nilles K, Theato P, Char K. Langmuir. 2010;26:1830–1836. doi: 10.1021/la902574z. [DOI] [PubMed] [Google Scholar]
- 48.Liang ZQ, Wang Q. Langmuir. 2004;20:9600–9606. doi: 10.1021/la048828c. [DOI] [PubMed] [Google Scholar]
- 49.Such GK, Quinn JF, Quinn A, Tjipto E, Caruso F. J. Am. Chem. Soc. 2006;128:9318–9319. doi: 10.1021/ja063043+. [DOI] [PubMed] [Google Scholar]
- 50.Vestberg R, Malkoch M, Kade M, Wu P, Fokin VV, Sharpless KB, Drockenmuller E, Hawker CJ. J. Polym. Sci. Part A. 2007;45:2835–2846. [Google Scholar]
- 51.Liao KS, Wan A, Batteas JD, Bergbreiter DE. Langmuir. 2008;24:4245–4253. doi: 10.1021/la703730b. [DOI] [PubMed] [Google Scholar]
- 52.Rydzek G, Thomann JS, Ben Ameur N, Jierry L, Mesini P, Ponche A, Contal C, El Haitami AE, Voegel JC, Senger B, Schaaf P, Frisch B, Boulmedais F. Langmuir. 2010;26:2816–2824. doi: 10.1021/la902874k. [DOI] [PubMed] [Google Scholar]
- 53.Kosmulski M. Chemical Properties of Material Surfaces. New York: Marcel Dekker; 2001. [Google Scholar]
- 54.Buck ME, Schwartz SC, Lynn DM. 2010 Manuscript Submitted. [Google Scholar]
- 55.Vogel MJ, Steen PH. Proc. Nat. Acad. Sci. 2010;107:3377–3381. doi: 10.1073/pnas.0914720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.