Abstract
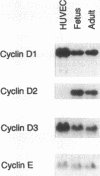
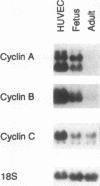
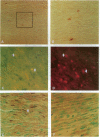
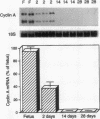
The regulated expression of cyclins controls the cell cycle. Because cardiomyocytes in adult mammals withdraw permanently from the cell cycle and thus cannot regenerate after injury, we examined cyclin expression during development by comparing cyclin A-E mRNA levels in fetal and adult human hearts. Cyclin B mRNA was detectable in adult hearts, although at a level markedly lower than that in fetal hearts. Levels of cyclin C, D1, D2, D3, and E mRNA were essentially identical in the two groups. In contrast, cyclin A mRNA was undetectable in adult hearts whereas cyclin A mRNA and protein were readily detectable in fetal hearts and cardiomyocytes, respectively. We then measured cyclin A mRNA and protein levels in rat hearts at four stages of development (fetal and 2, 14, and 28 d). Cyclin A mRNA and protein levels decreased quickly after birth (to 37% at day 2) and became undetectable within 14 d, an observation consistent with reports that cardiomyocytes stop replicating in rats by the second to third postnatal week. This disappearance of cyclin A gene expression in human and rat hearts at the time cardiomyocytes become terminally differentiated suggests that cyclin A downregulation is important in the permanent withdrawal of cardiomyocytes from the cell cycle.
Full text
PDF

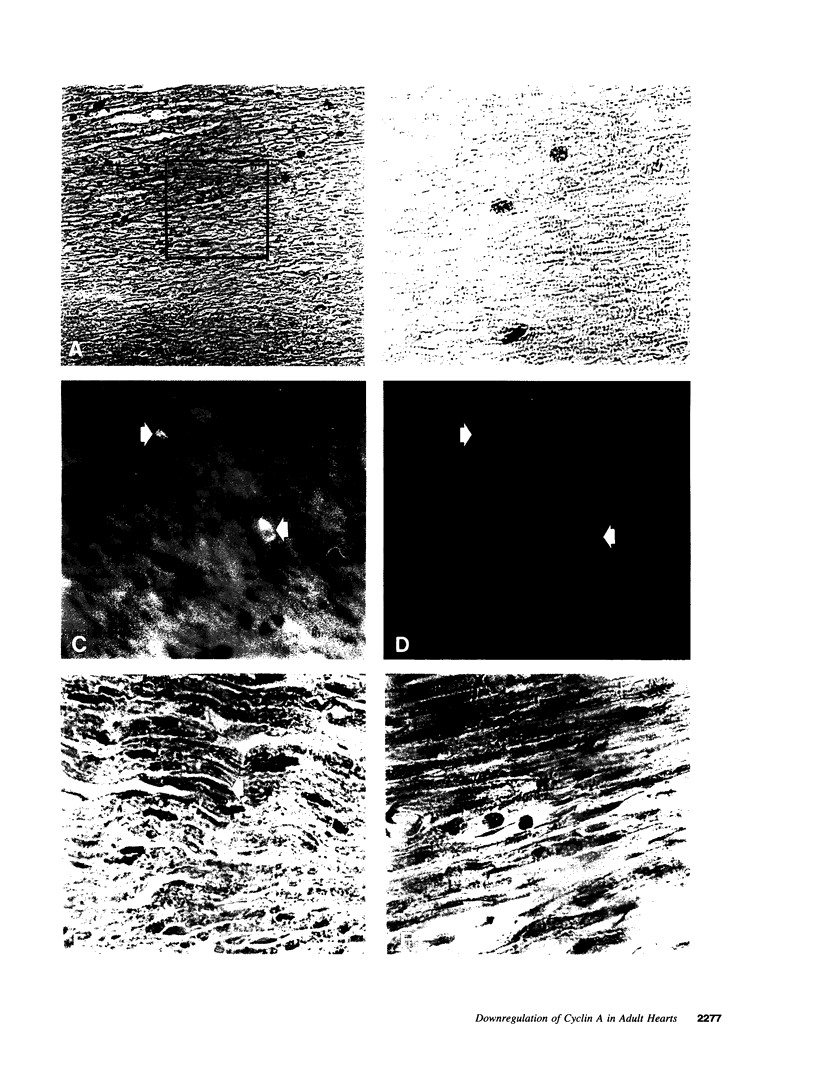


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbustini E., Diegoli M., Grasso M., Fasani R., D'Armini A., Martinelli L., Goggi C., Campana C., Gavazzi A., Viganò M. Expression of proliferating cell markers in normal and diseased human hearts. Am J Cardiol. 1993 Sep 1;72(7):608–614. doi: 10.1016/0002-9149(93)90360-o. [DOI] [PubMed] [Google Scholar]
- Beinlich C. J., Morgan H. E. Control of growth in neonatal pig hearts. Mol Cell Biochem. 1993 Feb 17;119(1-2):3–9. doi: 10.1007/BF00926846. [DOI] [PubMed] [Google Scholar]
- Cardoso M. C., Leonhardt H., Nadal-Ginard B. Reversal of terminal differentiation and control of DNA replication: cyclin A and Cdk2 specifically localize at subnuclear sites of DNA replication. Cell. 1993 Sep 24;74(6):979–992. doi: 10.1016/0092-8674(93)90721-2. [DOI] [PubMed] [Google Scholar]
- Casscells W., Speir E., Sasse J., Klagsbrun M., Allen P., Lee M., Calvo B., Chiba M., Haggroth L., Folkman J. Isolation, characterization, and localization of heparin-binding growth factors in the heart. J Clin Invest. 1990 Feb;85(2):433–441. doi: 10.1172/JCI114456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claycomb W. C. Biochemical aspects of cardiac muscle differentiation. Possible control of deoxyribonucleic acid synthesis and cell differentiation by adrenergic innervation and cyclic adenosine 3':5'-monophosphate. J Biol Chem. 1976 Oct 10;251(19):6082–6089. [PubMed] [Google Scholar]
- Dulić V., Drullinger L. F., Lees E., Reed S. I., Stein G. H. Altered regulation of G1 cyclins in senescent human diploid fibroblasts: accumulation of inactive cyclin E-Cdk2 and cyclin D1-Cdk2 complexes. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11034–11038. doi: 10.1073/pnas.90.23.11034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard F., Strausfeld U., Fernandez A., Lamb N. J. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 1991 Dec 20;67(6):1169–1179. doi: 10.1016/0092-8674(91)90293-8. [DOI] [PubMed] [Google Scholar]
- Guadagno T. M., Ohtsubo M., Roberts J. M., Assoian R. K. A link between cyclin A expression and adhesion-dependent cell cycle progression. Science. 1993 Dec 3;262(5139):1572–1575. doi: 10.1126/science.8248807. [DOI] [PubMed] [Google Scholar]
- Hall P. A., Levison D. A., Woods A. L., Yu C. C., Kellock D. B., Watkins J. A., Barnes D. M., Gillett C. E., Camplejohn R., Dover R. Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol. 1990 Dec;162(4):285–294. doi: 10.1002/path.1711620403. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Heichman K. A., Roberts J. M. Rules to replicate by. Cell. 1994 Nov 18;79(4):557–562. doi: 10.1016/0092-8674(94)90541-x. [DOI] [PubMed] [Google Scholar]
- Henglein B., Chenivesse X., Wang J., Eick D., Bréchot C. Structure and cell cycle-regulated transcription of the human cyclin A gene. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5490–5494. doi: 10.1073/pnas.91.12.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. Braking the cycle. Cell. 1993 Dec 3;75(5):839–841. doi: 10.1016/0092-8674(93)90528-x. [DOI] [PubMed] [Google Scholar]
- Hunter T., Pines J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell. 1994 Nov 18;79(4):573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- Hunter T., Pines J. Cyclins and cancer. Cell. 1991 Sep 20;66(6):1071–1074. doi: 10.1016/0092-8674(91)90028-w. [DOI] [PubMed] [Google Scholar]
- Jahn L., Sadoshima J., Izumo S. Cyclins and cyclin-dependent kinases are differentially regulated during terminal differentiation of C2C12 muscle cells. Exp Cell Res. 1994 Jun;212(2):297–307. doi: 10.1006/excr.1994.1147. [DOI] [PubMed] [Google Scholar]
- Kanai Y., Stelzner M. G., Lee W. S., Wells R. G., Brown D., Hediger M. A. Expression of mRNA (D2) encoding a protein involved in amino acid transport in S3 proximal tubule. Am J Physiol. 1992 Dec;263(6 Pt 2):F1087–F1092. doi: 10.1152/ajprenal.1992.263.6.F1087. [DOI] [PubMed] [Google Scholar]
- Knowlton K. U., Michel M. C., Itani M., Shubeita H. E., Ishihara K., Brown J. H., Chien K. R. The alpha 1A-adrenergic receptor subtype mediates biochemical, molecular, and morphologic features of cultured myocardial cell hypertrophy. J Biol Chem. 1993 Jul 25;268(21):15374–15380. [PubMed] [Google Scholar]
- Koff A., Cross F., Fisher A., Schumacher J., Leguellec K., Philippe M., Roberts J. M. Human cyclin E, a new cyclin that interacts with two members of the CDC2 gene family. Cell. 1991 Sep 20;66(6):1217–1228. doi: 10.1016/0092-8674(91)90044-y. [DOI] [PubMed] [Google Scholar]
- Krek W., Nigg E. A. Structure and developmental expression of the chicken CDC2 kinase. EMBO J. 1989 Oct;8(10):3071–3078. doi: 10.1002/j.1460-2075.1989.tb08458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee M. E., Temizer D. H., Clifford J. A., Quertermous T. Cloning of the GATA-binding protein that regulates endothelin-1 gene expression in endothelial cells. J Biol Chem. 1991 Aug 25;266(24):16188–16192. [PubMed] [Google Scholar]
- Lee M. G., Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987 May 7;327(6117):31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- Lee W. S., Smith M. S., Hoffman G. E. Luteinizing hormone-releasing hormone neurons express Fos protein during the proestrous surge of luteinizing hormone. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5163–5167. doi: 10.1073/pnas.87.13.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew D. J., Dulić V., Reed S. I. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell. 1991 Sep 20;66(6):1197–1206. doi: 10.1016/0092-8674(91)90042-w. [DOI] [PubMed] [Google Scholar]
- Marino T. A., Haldar S., Williamson E. C., Beaverson K., Walter R. A., Marino D. R., Beatty C., Lipson K. E. Proliferating cell nuclear antigen in developing and adult rat cardiac muscle cells. Circ Res. 1991 Nov;69(5):1353–1360. doi: 10.1161/01.res.69.5.1353. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Bernstein R. M., Franza B. R., Jr, Garrels J. I. Identity of the proliferating cell nuclear antigen and cyclin. Nature. 1984 May 24;309(5966):374–376. doi: 10.1038/309374a0. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Roussel M. F., Ashmun R. A., Sherr C. J. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991 May 17;65(4):701–713. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- Meikrantz W., Gisselbrecht S., Tam S. W., Schlegel R. Activation of cyclin A-dependent protein kinases during apoptosis. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3754–3758. doi: 10.1073/pnas.91.9.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H. T., Medford R. M., Nadal-Ginard B. Reversibility of muscle differentiation in the absence of commitment: analysis of a myogenic cell line temperature-sensitive for commitment. Cell. 1983 Aug;34(1):281–293. doi: 10.1016/0092-8674(83)90159-9. [DOI] [PubMed] [Google Scholar]
- Oshima J., Steinmann K. E., Campisi J., Schlegel R. Modulation of cell growth, p34cdc2 and cyclin A levels by SV-40 large T antigen. Oncogene. 1993 Nov;8(11):2987–2993. [PubMed] [Google Scholar]
- Osinska H. E., Lemanski L. F. Immunofluorescent localization of desmin and vimentin in developing cardiac muscle of Syrian hamster. Anat Rec. 1989 Apr;223(4):406–413. doi: 10.1002/ar.1092230409. [DOI] [PubMed] [Google Scholar]
- Pagano M., Pepperkok R., Verde F., Ansorge W., Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992 Mar;11(3):961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker T. G., Schneider M. D. Growth factors, proto-oncogenes, and plasticity of the cardiac phenotype. Annu Rev Physiol. 1991;53:179–200. doi: 10.1146/annurev.ph.53.030191.001143. [DOI] [PubMed] [Google Scholar]
- Perrella M. A., Yoshizumi M., Fen Z., Tsai J. C., Hsieh C. M., Kourembanas S., Lee M. E. Transforming growth factor-beta 1, but not dexamethasone, down-regulates nitric-oxide synthase mRNA after its induction by interleukin-1 beta in rat smooth muscle cells. J Biol Chem. 1994 May 20;269(20):14595–14600. [PubMed] [Google Scholar]
- Perrella M. A., Yoshizumi M., Fen Z., Tsai J. C., Hsieh C. M., Kourembanas S., Lee M. E. Transforming growth factor-beta 1, but not dexamethasone, down-regulates nitric-oxide synthase mRNA after its induction by interleukin-1 beta in rat smooth muscle cells. J Biol Chem. 1994 May 20;269(20):14595–14600. [PubMed] [Google Scholar]
- Peter M., Herskowitz I. Joining the complex: cyclin-dependent kinase inhibitory proteins and the cell cycle. Cell. 1994 Oct 21;79(2):181–184. doi: 10.1016/0092-8674(94)90186-4. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell. 1989 Sep 8;58(5):833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- Polyak K., Kato J. Y., Solomon M. J., Sherr C. J., Massague J., Roberts J. M., Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994 Jan;8(1):9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- Polyak K., Lee M. H., Erdjument-Bromage H., Koff A., Roberts J. M., Tempst P., Massagué J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994 Jul 15;78(1):59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- Reiss K., Meggs L. G., Li P., Olivetti G., Capasso J. M., Anversa P. Upregulation of IGF1, IGF1-receptor, and late growth related genes in ventricular myocytes acutely after infarction in rats. J Cell Physiol. 1994 Jan;158(1):160–168. doi: 10.1002/jcp.1041580120. [DOI] [PubMed] [Google Scholar]
- Rumyantsev P. P. Interrelations of the proliferation and differentiation processes during cardiact myogenesis and regeneration. Int Rev Cytol. 1977;51:186–273. [PubMed] [Google Scholar]
- Schneider M. D., Payne P. A., Ueno H., Perryman M. B., Roberts R. Dissociated expression of c-myc and a fos-related competence gene during cardiac myogenesis. Mol Cell Biol. 1986 Nov;6(11):4140–4143. doi: 10.1128/mcb.6.11.4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M., Hannon G. J., Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993 Dec 16;366(6456):704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Sherr C. J. G1 phase progression: cycling on cue. Cell. 1994 Nov 18;79(4):551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- Sherr C. J. Mammalian G1 cyclins. Cell. 1993 Jun 18;73(6):1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- Simpson P. C. Proto-oncogenes and cardiac hypertrophy. Annu Rev Physiol. 1989;51:189–202. doi: 10.1146/annurev.ph.51.030189.001201. [DOI] [PubMed] [Google Scholar]
- Sobczak-Thepot J., Harper F., Florentin Y., Zindy F., Brechot C., Puvion E. Localization of cyclin A at the sites of cellular DNA replication. Exp Cell Res. 1993 May;206(1):43–48. doi: 10.1006/excr.1993.1118. [DOI] [PubMed] [Google Scholar]
- Speir E., Tanner V., Gonzalez A. M., Farris J., Baird A., Casscells W. Acidic and basic fibroblast growth factors in adult rat heart myocytes. Localization, regulation in culture, and effects on DNA synthesis. Circ Res. 1992 Aug;71(2):251–259. doi: 10.1161/01.res.71.2.251. [DOI] [PubMed] [Google Scholar]
- Toyoshima H., Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994 Jul 15;78(1):67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- Tsai L. H., Harlow E., Meyerson M. Isolation of the human cdk2 gene that encodes the cyclin A- and adenovirus E1A-associated p33 kinase. Nature. 1991 Sep 12;353(6340):174–177. doi: 10.1038/353174a0. [DOI] [PubMed] [Google Scholar]
- Ueno H., Perryman M. B., Roberts R., Schneider M. D. Differentiation of cardiac myocytes after mitogen withdrawal exhibits three sequential states of the ventricular growth response. J Cell Biol. 1988 Nov;107(5):1911–1918. doi: 10.1083/jcb.107.5.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Chenivesse X., Henglein B., Bréchot C. Hepatitis B virus integration in a cyclin A gene in a hepatocellular carcinoma. Nature. 1990 Feb 8;343(6258):555–557. doi: 10.1038/343555a0. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Connolly T., Futcher B., Beach D. Human D-type cyclin. Cell. 1991 May 17;65(4):691–699. doi: 10.1016/0092-8674(91)90100-d. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Yoshida M., Ono K., Fujita T., Ohtani-Fujita N., Sakai T., Nikaido T. Effect of tumor suppressors on cell cycle-regulatory genes: RB suppresses p34cdc2 expression and normal p53 suppresses cyclin A expression. Exp Cell Res. 1994 Jan;210(1):94–101. doi: 10.1006/excr.1994.1014. [DOI] [PubMed] [Google Scholar]
- Yoshizumi M., Kourembanas S., Temizer D. H., Cambria R. P., Quertermous T., Lee M. E. Tumor necrosis factor increases transcription of the heparin-binding epidermal growth factor-like growth factor gene in vascular endothelial cells. J Biol Chem. 1992 May 15;267(14):9467–9469. [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]