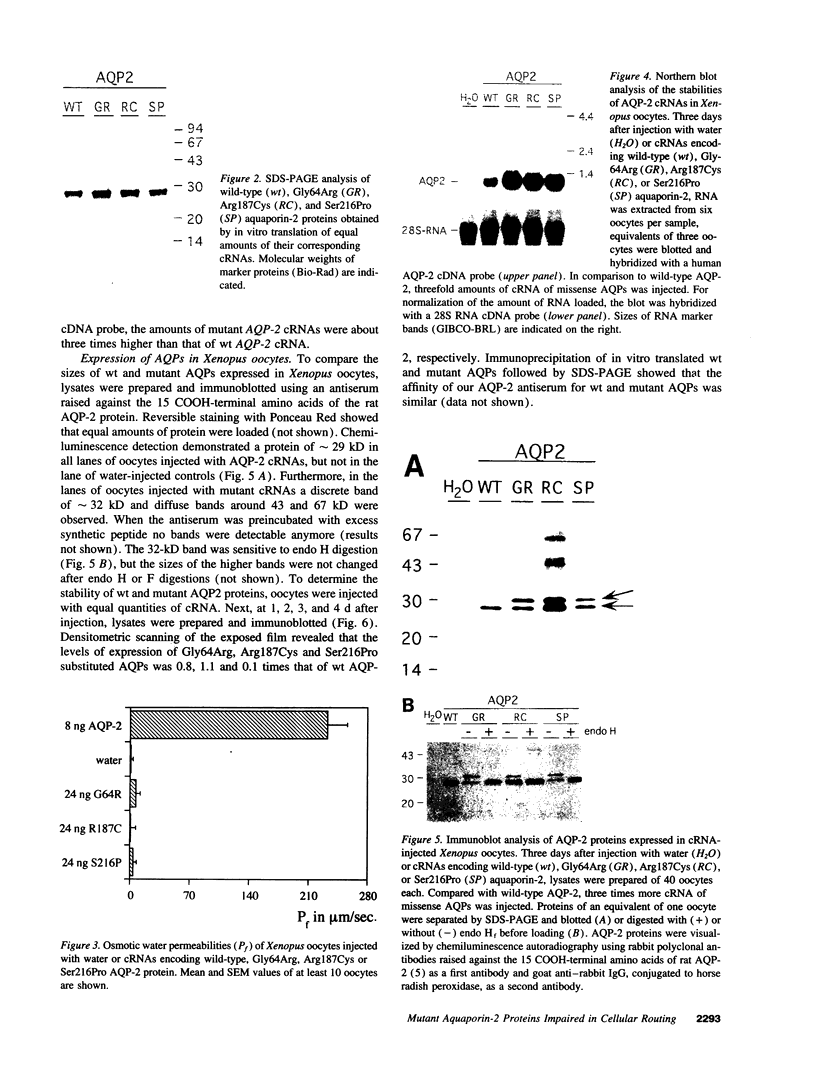
Abstract
Congenital nephrogenic diabetes insipidus is a recessive hereditary disorder characterized by the inability of the kidney to concentrate urine in response to vasopressin. Recently, we reported mutations in the gene encoding the water channel of the collecting duct, aquaporin-2 (AQP-2) causing an autosomal recessive form of nephrogenic diabetes insipidus (NDI). Expression of these mutant AQP-2 proteins (Gly64Arg, Arg187Cys, Ser216Pro) in Xenopus oocytes revealed nonfunctional water channels. Here we report further studies into the inability of these missense AQP-2 proteins to facilitate water transport in Xenopus oocytes. cRNAs encoding the missense AQPs were translated with equal efficiency as cRNAs encoding wild-type AQP-2 and were equally stable. Arg187Cys AQP2 was more stable and Gly6-4Arg and Ser216Pro AQP2 were less stable when compared to wild-type AQP2 protein. On immunoblots, oocytes expressing missense AQP-2 showed, besides the wild-type 29 kDa band, an endoplasmic reticulum-retarded form of AQP-2 of approximately 32 kD. Immunoblots and immunocytochemistry demonstrated only intense labeling of the plasma membranes of oocytes expressing wild-type AQP-2. Therefore, we conclude that in Xenopus oocytes the inability of Gly64-Arg, Arg187Cys or Ser216Pro substituted AQP-2 proteins to facilitate water transport is caused by an impaired routing to the plasma membrane.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Preston G. M., Smith B. L., Jung J. S., Raina S., Moon C., Guggino W. B., Nielsen S. Aquaporin CHIP: the archetypal molecular water channel. Am J Physiol. 1993 Oct;265(4 Pt 2):F463–F476. doi: 10.1152/ajprenal.1993.265.4.F463. [DOI] [PubMed] [Google Scholar]
- Amara J. F., Cheng S. H., Smith A. E. Intracellular protein trafficking defects in human disease. Trends Cell Biol. 1992 May;2(5):145–149. doi: 10.1016/0962-8924(92)90101-r. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., Lippincott-Schwartz J. Degradation of proteins within the endoplasmic reticulum. Curr Opin Cell Biol. 1991 Aug;3(4):592–600. doi: 10.1016/0955-0674(91)90028-w. [DOI] [PubMed] [Google Scholar]
- Cheng S. H., Gregory R. J., Marshall J., Paul S., Souza D. W., White G. A., O'Riordan C. R., Smith A. E. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990 Nov 16;63(4):827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Davis J. Q., Bennett V. Brain ankyrin. Purification of a 72,000 Mr spectrin-binding domain. J Biol Chem. 1984 Feb 10;259(3):1874–1881. [PubMed] [Google Scholar]
- Deen P. M., Dempster J. A., Wieringa B., Van Os C. H. Isolation of a cDNA for rat CHIP28 water channel: high mRNA expression in kidney cortex and inner medulla. Biochem Biophys Res Commun. 1992 Nov 16;188(3):1267–1273. doi: 10.1016/0006-291x(92)91368-z. [DOI] [PubMed] [Google Scholar]
- Deen P. M., Verdijk M. A., Knoers N. V., Wieringa B., Monnens L. A., van Os C. H., van Oost B. A. Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science. 1994 Apr 1;264(5155):92–95. doi: 10.1126/science.8140421. [DOI] [PubMed] [Google Scholar]
- Drumm M. L., Wilkinson D. J., Smit L. S., Worrell R. T., Strong T. V., Frizzell R. A., Dawson D. C., Collins F. S. Chloride conductance expressed by delta F508 and other mutant CFTRs in Xenopus oocytes. Science. 1991 Dec 20;254(5039):1797–1799. doi: 10.1126/science.1722350. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fushimi K., Uchida S., Hara Y., Hirata Y., Marumo F., Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature. 1993 Feb 11;361(6412):549–552. doi: 10.1038/361549a0. [DOI] [PubMed] [Google Scholar]
- Halban P. A., Irminger J. C. Sorting and processing of secretory proteins. Biochem J. 1994 Apr 1;299(Pt 1):1–18. doi: 10.1042/bj2990001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J. S., Preston G. M., Smith B. L., Guggino W. B., Agre P. Molecular structure of the water channel through aquaporin CHIP. The hourglass model. J Biol Chem. 1994 May 20;269(20):14648–14654. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langley J. M., Balfe J. W., Selander T., Ray P. N., Clarke J. T. Autosomal recessive inheritance of vasopressin-resistant diabetes insipidus. Am J Med Genet. 1991 Jan;38(1):90–94. doi: 10.1002/ajmg.1320380120. [DOI] [PubMed] [Google Scholar]
- Nielsen S., DiGiovanni S. R., Christensen E. I., Knepper M. A., Harris H. W. Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11663–11667. doi: 10.1073/pnas.90.24.11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura H., Pallardo F. V., Seidner G. A., Vannucci S., Simpson I. A., Birnbaum M. J. Kinetics of GLUT1 and GLUT4 glucose transporters expressed in Xenopus oocytes. J Biol Chem. 1993 Apr 25;268(12):8514–8520. [PubMed] [Google Scholar]
- Pan Y., Metzenberg A., Das S., Jing B., Gitschier J. Mutations in the V2 vasopressin receptor gene are associated with X-linked nephrogenic diabetes insipidus. Nat Genet. 1992 Oct;2(2):103–106. doi: 10.1038/ng1092-103. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Control of protein exit from the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:1–23. doi: 10.1146/annurev.cb.05.110189.000245. [DOI] [PubMed] [Google Scholar]
- Preston G. M., Agre P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11110–11114. doi: 10.1073/pnas.88.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston G. M., Carroll T. P., Guggino W. B., Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992 Apr 17;256(5055):385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Preston G. M., Jung J. S., Guggino W. B., Agre P. The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J Biol Chem. 1993 Jan 5;268(1):17–20. [PubMed] [Google Scholar]
- Rosenthal W., Seibold A., Antaramian A., Lonergan M., Arthus M. F., Hendy G. N., Birnbaumer M., Bichet D. G. Molecular identification of the gene responsible for congenital nephrogenic diabetes insipidus. Nature. 1992 Sep 17;359(6392):233–235. doi: 10.1038/359233a0. [DOI] [PubMed] [Google Scholar]
- Sasaki S., Fushimi K., Saito H., Saito F., Uchida S., Ishibashi K., Kuwahara M., Ikeuchi T., Inui K., Nakajima K. Cloning, characterization, and chromosomal mapping of human aquaporin of collecting duct. J Clin Invest. 1994 Mar;93(3):1250–1256. doi: 10.1172/JCI117079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherly D., Boelens W., van Venrooij W. J., Dathan N. A., Hamm J., Mattaj I. W. Identification of the RNA binding segment of human U1 A protein and definition of its binding site on U1 snRNA. EMBO J. 1989 Dec 20;8(13):4163–4170. doi: 10.1002/j.1460-2075.1989.tb08601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schielen W. J., Voskuilen M., Tesser G. I., Nieuwenhuizen W. The sequence A alpha-(148-160) in fibrin, but not in fibrinogen, is accessible to monoclonal antibodies. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8951–8954. doi: 10.1073/pnas.86.22.8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel E. Use of Xenopus oocytes for the functional expression of plasma membrane proteins. J Membr Biol. 1990 Sep;117(3):201–221. doi: 10.1007/BF01868451. [DOI] [PubMed] [Google Scholar]
- Wall D. A., Patel S. Isolation of plasma membrane complexes from Xenopus oocytes. J Membr Biol. 1989 Feb;107(2):189–201. doi: 10.1007/BF01871724. [DOI] [PubMed] [Google Scholar]
- Ware V. C., Tague B. W., Clark C. G., Gourse R. L., Brand R. C., Gerbi S. A. Sequence analysis of 28S ribosomal DNA from the amphibian Xenopus laevis. Nucleic Acids Res. 1983 Nov 25;11(22):7795–7817. doi: 10.1093/nar/11.22.7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. F., Kahn C. R. The insulin signaling system. J Biol Chem. 1994 Jan 7;269(1):1–4. [PubMed] [Google Scholar]
- van Lieburg A. F., Verdijk M. A., Knoers V. V., van Essen A. J., Proesmans W., Mallmann R., Monnens L. A., van Oost B. A., van Os C. H., Deen P. M. Patients with autosomal nephrogenic diabetes insipidus homozygous for mutations in the aquaporin 2 water-channel gene. Am J Hum Genet. 1994 Oct;55(4):648–652. [PMC free article] [PubMed] [Google Scholar]
- van den Ouweland A. M., Dreesen J. C., Verdijk M., Knoers N. V., Monnens L. A., Rocchi M., van Oost B. A. Mutations in the vasopressin type 2 receptor gene (AVPR2) associated with nephrogenic diabetes insipidus. Nat Genet. 1992 Oct;2(2):99–102. doi: 10.1038/ng1092-99. [DOI] [PubMed] [Google Scholar]








