Abstract
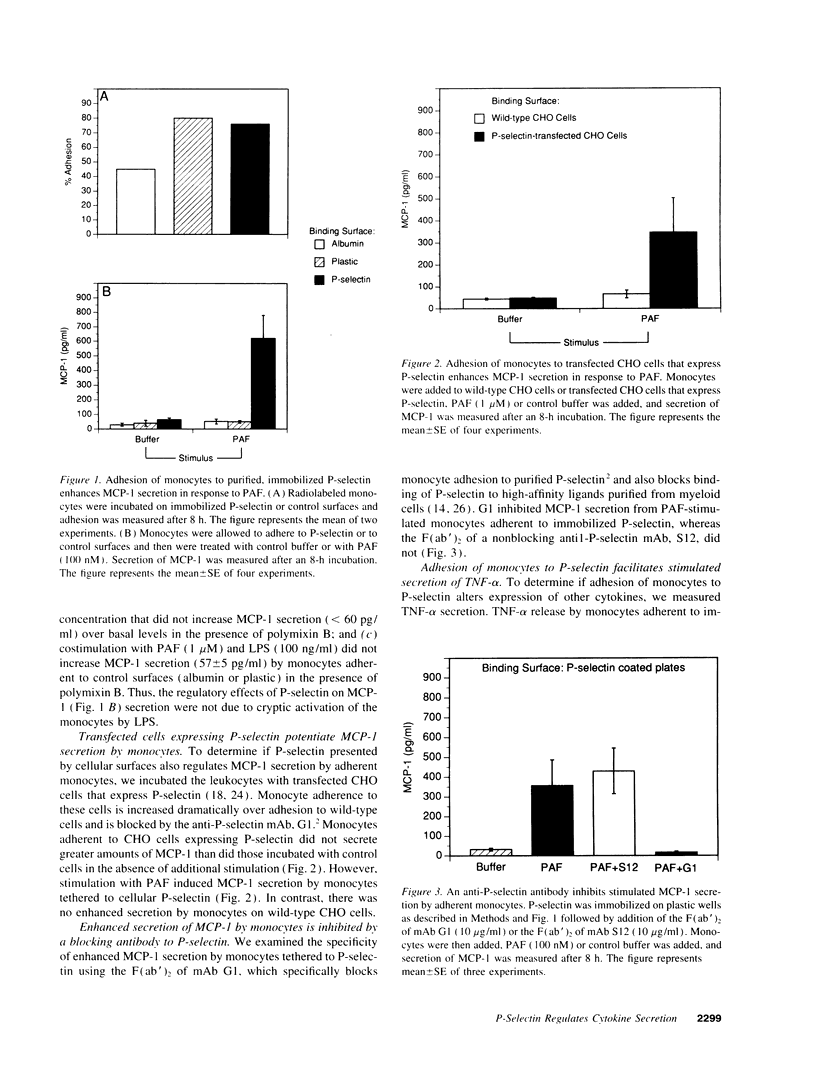
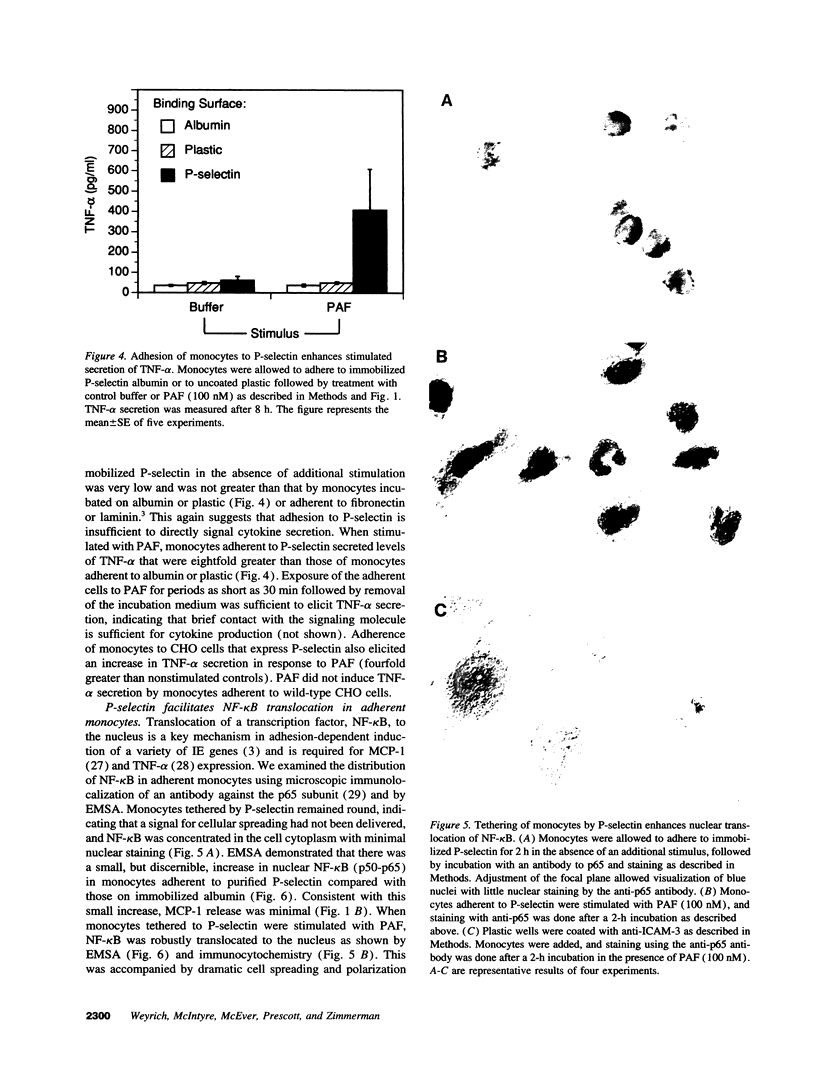
Adhesion molecules that tether circulating leukocytes to endothelial cells may also transduce or modulate outside-in signals for cellular activation, providing an initial regulatory point in the inflammatory response. Adhesion of human monocytes to P-selectin, the most rapidly expressed endothelial tethering factor, increased the secretion of monocyte chemotactic protein-1 (MCP-1) and tumor necrosis factor-alpha (TNF-alpha) by the leukocytes when they were stimulated with platelet-activating factor. Increased cytokine secretion was specifically inhibited by G1, an anti-P-selectin mAb that prevents P-selectin from binding to its ligand (P-selectin glycoprotein ligand-1) on myeloid cells. Moreover, tethering by P-selectin specifically enhanced nuclear translocation of nuclear factor-kappa B (NF-kappa B), a transcription factor required for expression of MCP-1, TNF-alpha, and other immediate-early genes. These results demonstrate that P-selectin, through its ligands on monocytes, may locally regulate cytokine secretion in inflamed tissues.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Celi A., Pellegrini G., Lorenzet R., De Blasi A., Ready N., Furie B. C., Furie B. P-selectin induces the expression of tissue factor on monocytes. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8767–8771. doi: 10.1073/pnas.91.19.8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damle N. K., Klussman K., Dietsch M. T., Mohagheghpour N., Aruffo A. GMP-140 (P-selectin/CD62) binds to chronically stimulated but not resting CD4+ T lymphocytes and regulates their production of proinflammatory cytokines. Eur J Immunol. 1992 Jul;22(7):1789–1793. doi: 10.1002/eji.1830220718. [DOI] [PubMed] [Google Scholar]
- Disdier M., Morrissey J. H., Fugate R. D., Bainton D. F., McEver R. P. Cytoplasmic domain of P-selectin (CD62) contains the signal for sorting into the regulated secretory pathway. Mol Biol Cell. 1992 Mar;3(3):309–321. doi: 10.1091/mbc.3.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstad M. R., Prescott S. M., McIntyre T. M., Zimmerman G. A. Synthesis and release of platelet-activating factor by stimulated human mononuclear phagocytes. J Immunol. 1988 Mar 1;140(5):1618–1624. [PubMed] [Google Scholar]
- Evanoff H. L., Burdick M. D., Moore S. A., Kunkel S. L., Strieter R. M. A sensitive ELISA for the detection of human monocyte chemoattractant protein-1 (MCP-1). Immunol Invest. 1992 Feb;21(1):39–45. doi: 10.3109/08820139209069361. [DOI] [PubMed] [Google Scholar]
- Feuillard J., Körner M., Fourcade C., Costa A., Binet J. L., Debré P., Raphael M. Visualization of the endogenous NF-kappa B p50 subunit in the nucleus of follicular dendritic cells in germinal centers. J Immunol. 1994 Jan 1;152(1):12–21. [PubMed] [Google Scholar]
- Gamble J. R., Skinner M. P., Berndt M. C., Vadas M. A. Prevention of activated neutrophil adhesion to endothelium by soluble adhesion protein GMP140. Science. 1990 Jul 27;249(4967):414–417. doi: 10.1126/science.1696029. [DOI] [PubMed] [Google Scholar]
- Geng J. G., Bevilacqua M. P., Moore K. L., McIntyre T. M., Prescott S. M., Kim J. M., Bliss G. A., Zimmerman G. A., McEver R. P. Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature. 1990 Feb 22;343(6260):757–760. doi: 10.1038/343757a0. [DOI] [PubMed] [Google Scholar]
- Grober J. S., Bowen B. L., Ebling H., Athey B., Thompson C. B., Fox D. A., Stoolman L. M. Monocyte-endothelial adhesion in chronic rheumatoid arthritis. In situ detection of selectin and integrin-dependent interactions. J Clin Invest. 1993 Jun;91(6):2609–2619. doi: 10.1172/JCI116500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Lander A. D. Contact and adhesive specificities in the associations, migrations, and targeting of cells and axons. Cell. 1992 Jan 24;68(2):303–322. doi: 10.1016/0092-8674(92)90472-o. [DOI] [PubMed] [Google Scholar]
- Johnson-Tidey R. R., McGregor J. L., Taylor P. R., Poston R. N. Increase in the adhesion molecule P-selectin in endothelium overlying atherosclerotic plaques. Coexpression with intercellular adhesion molecule-1. Am J Pathol. 1994 May;144(5):952–961. [PMC free article] [PubMed] [Google Scholar]
- Juliano R. L., Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993 Feb;120(3):577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchens R. L., Ulevitch R. J., Munford R. S. Lipopolysaccharide (LPS) partial structures inhibit responses to LPS in a human macrophage cell line without inhibiting LPS uptake by a CD14-mediated pathway. J Exp Med. 1992 Aug 1;176(2):485–494. doi: 10.1084/jem.176.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. H., Yurochko A., Kornberg L., Morris J., Walker J. J., Haskill S., Juliano R. L. The role of protein tyrosine phosphorylation in integrin-mediated gene induction in monocytes. J Cell Biol. 1994 Sep;126(6):1585–1593. doi: 10.1083/jcb.126.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou H. C., Baltimore D. Regulation of the NF-kappa B/rel transcription factor and I kappa B inhibitor system. Curr Opin Cell Biol. 1993 Jun;5(3):477–487. doi: 10.1016/0955-0674(93)90014-h. [DOI] [PubMed] [Google Scholar]
- Locati M., Zhou D., Luini W., Evangelista V., Mantovani A., Sozzani S. Rapid induction of arachidonic acid release by monocyte chemotactic protein-1 and related chemokines. Role of Ca2+ influx, synergism with platelet-activating factor and significance for chemotaxis. J Biol Chem. 1994 Feb 18;269(7):4746–4753. [PubMed] [Google Scholar]
- Lorant D. E., Patel K. D., McIntyre T. M., McEver R. P., Prescott S. M., Zimmerman G. A. Coexpression of GMP-140 and PAF by endothelium stimulated by histamine or thrombin: a juxtacrine system for adhesion and activation of neutrophils. J Cell Biol. 1991 Oct;115(1):223–234. doi: 10.1083/jcb.115.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorant D. E., Topham M. K., Whatley R. E., McEver R. P., McIntyre T. M., Prescott S. M., Zimmerman G. A. Inflammatory roles of P-selectin. J Clin Invest. 1993 Aug;92(2):559–570. doi: 10.1172/JCI116623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs N. W., Strieter R. M., Elner V. M., Evanoff H. L., Burdick M., Kunkel S. L. Intercellular adhesion molecule-1 mediates the expression of monocyte-derived MIP-1 alpha during monocyte-endothelial cell interactions. Blood. 1994 Mar 1;83(5):1174–1178. [PubMed] [Google Scholar]
- Mayadas T. N., Johnson R. C., Rayburn H., Hynes R. O., Wagner D. D. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993 Aug 13;74(3):541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- McEver R. P. Selectins. Curr Opin Immunol. 1994 Feb;6(1):75–84. doi: 10.1016/0952-7915(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Moore K. L., Eaton S. F., Lyons D. E., Lichenstein H. S., Cummings R. D., McEver R. P. The P-selectin glycoprotein ligand from human neutrophils displays sialylated, fucosylated, O-linked poly-N-acetyllactosamine. J Biol Chem. 1994 Sep 16;269(37):23318–23327. [PubMed] [Google Scholar]
- Moore K. L., Stults N. L., Diaz S., Smith D. F., Cummings R. D., Varki A., McEver R. P. Identification of a specific glycoprotein ligand for P-selectin (CD62) on myeloid cells. J Cell Biol. 1992 Jul;118(2):445–456. doi: 10.1083/jcb.118.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard K. E., Moore K. L., Diaz S., Stults N. L., Ushiyama S., McEver R. P., Cummings R. D., Varki A. Characterization of a specific ligand for P-selectin on myeloid cells. A minor glycoprotein with sialylated O-linked oligosaccharides. J Biol Chem. 1993 Jun 15;268(17):12764–12774. [PubMed] [Google Scholar]
- Patel K. D., Zimmerman G. A., Prescott S. M., McEver R. P., McIntyre T. M. Oxygen radicals induce human endothelial cells to express GMP-140 and bind neutrophils. J Cell Biol. 1991 Feb;112(4):749–759. doi: 10.1083/jcb.112.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako D., Chang X. J., Barone K. M., Vachino G., White H. M., Shaw G., Veldman G. M., Bean K. M., Ahern T. J., Furie B. Expression cloning of a functional glycoprotein ligand for P-selectin. Cell. 1993 Dec 17;75(6):1179–1186. doi: 10.1016/0092-8674(93)90327-m. [DOI] [PubMed] [Google Scholar]
- Ueda A., Okuda K., Ohno S., Shirai A., Igarashi T., Matsunaga K., Fukushima J., Kawamoto S., Ishigatsubo Y., Okubo T. NF-kappa B and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J Immunol. 1994 Sep 1;153(5):2052–2063. [PubMed] [Google Scholar]
- Ushiyama S., Laue T. M., Moore K. L., Erickson H. P., McEver R. P. Structural and functional characterization of monomeric soluble P-selectin and comparison with membrane P-selectin. J Biol Chem. 1993 Jul 15;268(20):15229–15237. [PubMed] [Google Scholar]
- Vazeux R., Hoffman P. A., Tomita J. K., Dickinson E. S., Jasman R. L., St John T., Gallatin W. M. Cloning and characterization of a new intercellular adhesion molecule ICAM-R. Nature. 1992 Dec 3;360(6403):485–488. doi: 10.1038/360485a0. [DOI] [PubMed] [Google Scholar]
- Webb D. S., Shimizu Y., Van Seventer G. A., Shaw S., Gerrard T. L. LFA-3, CD44, and CD45: physiologic triggers of human monocyte TNF and IL-1 release. Science. 1990 Sep 14;249(4974):1295–1297. doi: 10.1126/science.1697984. [DOI] [PubMed] [Google Scholar]
- Weyrich A. S., Buerke M., Albertine K. H., Lefer A. M. Time course of coronary vascular endothelial adhesion molecule expression during reperfusion of the ischemic feline myocardium. J Leukoc Biol. 1995 Jan;57(1):45–55. doi: 10.1002/jlb.57.1.45. [DOI] [PubMed] [Google Scholar]
- Weyrich A. S., Ma X. Y., Lefer D. J., Albertine K. H., Lefer A. M. In vivo neutralization of P-selectin protects feline heart and endothelium in myocardial ischemia and reperfusion injury. J Clin Invest. 1993 Jun;91(6):2620–2629. doi: 10.1172/JCI116501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C. S., Gamble J. R., Skinner M. P., Lucas C. M., Berndt M. C., Vadas M. A. Adhesion protein GMP140 inhibits superoxide anion release by human neutrophils. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2397–2401. doi: 10.1073/pnas.88.6.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurochko A. D., Liu D. Y., Eierman D., Haskill S. Integrins as a primary signal transduction molecule regulating monocyte immediate-early gene induction. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9034–9038. doi: 10.1073/pnas.89.19.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Sternsdorf T., Liese J., Belohradsky B., Weber C., Wedel A., Schreck R., Bäuerle P., Ströbel M. Pyrrolidine dithiocarbamate inhibits NF-kappa B mobilization and TNF production in human monocytes. J Immunol. 1993 Dec 15;151(12):6986–6993. [PubMed] [Google Scholar]
- Zimmerman G. A., Prescott S. M., McIntyre T. M. Endothelial cell interactions with granulocytes: tethering and signaling molecules. Immunol Today. 1992 Mar;13(3):93–100. doi: 10.1016/0167-5699(92)90149-2. [DOI] [PubMed] [Google Scholar]
- el-Gabalawy H., Gallatin M., Vazeux R., Peterman G., Wilkins J. Expression of ICAM-R (ICAM-3), a novel counter-receptor for LFA-1, in rheumatoid and nonrheumatoid synovium. Comparison with other adhesion molecules. Arthritis Rheum. 1994 Jun;37(6):846–854. doi: 10.1002/art.1780370612. [DOI] [PubMed] [Google Scholar]