Abstract
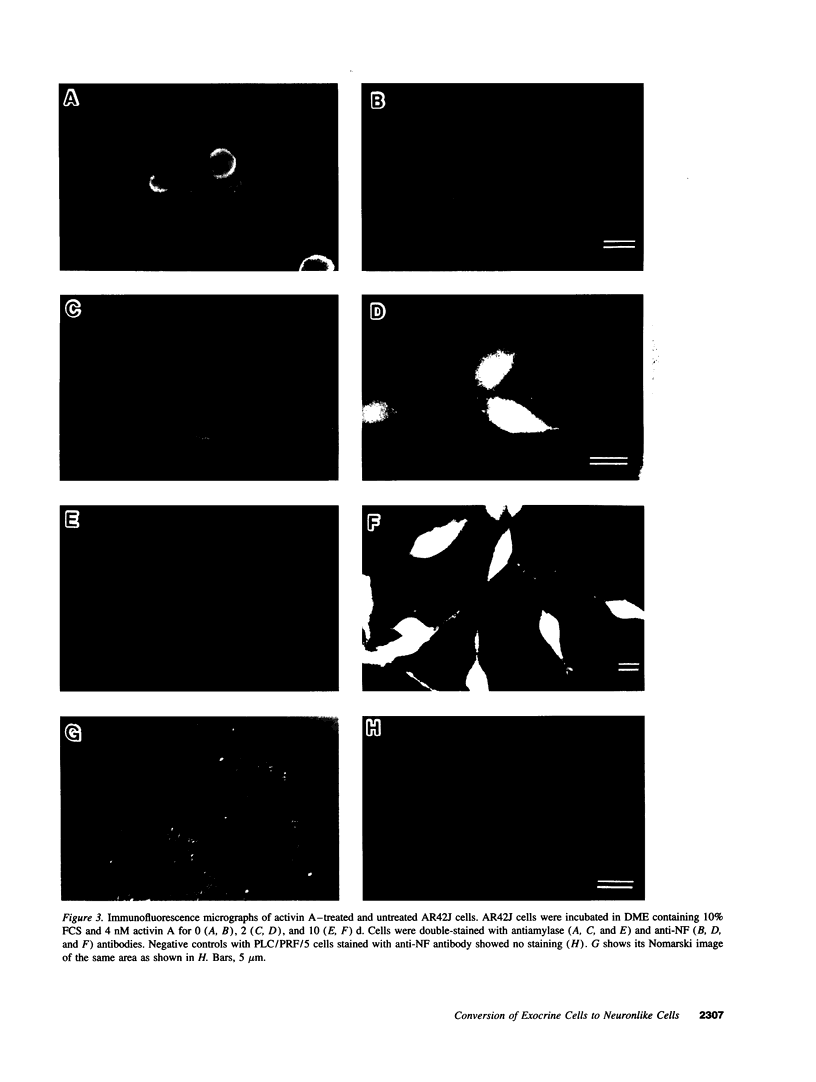
When AR42J cells, an amylase-secreting pancreatic exocrine cell line, were treated with activin A, cells extended neuritelike processes, and, concomitantly, amylase-containing vesicles disappeared. Immunofluorescence and immunoelectron microscopy revealed that these processes had neurite-specific cytoskeletal architectures: neurofilaments and microtubule bundles with cross-bridges of microtubule-associated protein 2. In addition to such morphological changes, activin-treated cells exhibited a marked increase in cytoplasmic free calcium concentration in response to depolarizing concentration of potassium. Moreover, activin-treated AR42J cells expressed mRNA for alpha 1 subunit of the neuroendocrine/beta cell-type voltage-dependent calcium channel. In naive AR42J cells, a sulfonylurea compound, tolbutamide, did not affect free calcium concentration, while it induced a marked elevation of free calcium in activin-treated cells. Single channel recording of the membrane patch revealed the existence of ATP-sensitive potassium channel in activin-treated cells. These results indicate that activin A converts amylase-secreting AR42J cells to neuronlike cells. Given that pancreatic endocrine cells possess neuronlike properties and express ATP-sensitive potassium channel as well as neuroendocrine/beta cell-type voltage-dependent calcium channel, activin treatment of AR42J cells may provide an in vitro model system to study the conversion of pancreatic exocrine cells to endocrine cells in islets.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. J., Bey E. M., Geddes E. W., Lecatsas G. Establishment of a continuously growing cell line from primary carcinoma of the liver. S Afr Med J. 1976 Dec 18;50(54):2124–2128. [PubMed] [Google Scholar]
- Baas P. W., Pienkowski T. P., Kosik K. S. Processes induced by tau expression in Sf9 cells have an axon-like microtubule organization. J Cell Biol. 1991 Dec;115(5):1333–1344. doi: 10.1083/jcb.115.5.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt R., Matus A. Light and electron microscopic studies of the distribution of microtubule-associated protein 2 in rat brain: a difference between dendritic and axonal cytoskeletons. J Comp Neurol. 1984 Jun 20;226(2):203–221. doi: 10.1002/cne.902260205. [DOI] [PubMed] [Google Scholar]
- Black M. M., Baas P. W. The basis of polarity in neurons. Trends Neurosci. 1989 Jun;12(6):211–214. doi: 10.1016/0166-2236(89)90124-0. [DOI] [PubMed] [Google Scholar]
- Brugg B., Matus A. PC12 cells express juvenile microtubule-associated proteins during nerve growth factor-induced neurite outgrowth. J Cell Biol. 1988 Aug;107(2):643–650. doi: 10.1083/jcb.107.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Kanai Y., Cowan N. J., Hirokawa N. Projection domains of MAP2 and tau determine spacings between microtubules in dendrites and axons. Nature. 1992 Dec 17;360(6405):674–677. doi: 10.1038/360674a0. [DOI] [PubMed] [Google Scholar]
- Drubin D. G., Feinstein S. C., Shooter E. M., Kirschner M. W. Nerve growth factor-induced neurite outgrowth in PC12 cells involves the coordinate induction of microtubule assembly and assembly-promoting factors. J Cell Biol. 1985 Nov;101(5 Pt 1):1799–1807. doi: 10.1083/jcb.101.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto Y., Tsuji T., Takezawa M., Takano S., Yokogawa Y., Shibai H. Purification and characterization of erythroid differentiation factor (EDF) isolated from human leukemia cell line THP-1. Biochem Biophys Res Commun. 1987 Feb 13;142(3):1095–1103. doi: 10.1016/0006-291x(87)91528-2. [DOI] [PubMed] [Google Scholar]
- Furukawa M., Eto Y., Kojima I. Expression of immunoreactive activin A in fetal rat pancreas. Endocr J. 1995 Feb;42(1):63–68. doi: 10.1507/endocrj.42.63. [DOI] [PubMed] [Google Scholar]
- Goedert M., Crowther R. A., Garner C. C. Molecular characterization of microtubule-associated proteins tau and MAP2. Trends Neurosci. 1991 May;14(5):193–199. doi: 10.1016/0166-2236(91)90105-4. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hashimoto M., Kondo S., Sakurai T., Etoh Y., Shibai H., Muramatsu M. Activin/EDF as an inhibitor of neural differentiation. Biochem Biophys Res Commun. 1990 Nov 30;173(1):193–200. doi: 10.1016/s0006-291x(05)81040-x. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A., Melton D. A. Inhibition of activin receptor signaling promotes neuralization in Xenopus. Cell. 1994 Apr 22;77(2):273–281. doi: 10.1016/0092-8674(94)90319-0. [DOI] [PubMed] [Google Scholar]
- Hirokawa N., Hisanaga S., Shiomura Y. MAP2 is a component of crossbridges between microtubules and neurofilaments in the neuronal cytoskeleton: quick-freeze, deep-etch immunoelectron microscopy and reconstitution studies. J Neurosci. 1988 Aug;8(8):2769–2779. doi: 10.1523/JNEUROSCI.08-08-02769.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. N., Lasek R. J. The slow component of axonal transport. Identification of major structural polypeptides of the axon and their generality among mammalian neurons. J Cell Biol. 1975 Aug;66(2):351–366. doi: 10.1083/jcb.66.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashima Y., Kondoh-Abiko A., Seino S., Takeda J., Eto M., Polonsky K. S., Makino I. Reduced levels of messenger ribonucleic acid for calcium channel, glucose transporter-2, and glucokinase are associated with alterations in insulin secretion in fasted rats. Endocrinology. 1994 Sep;135(3):1010–1017. doi: 10.1210/endo.135.3.8070343. [DOI] [PubMed] [Google Scholar]
- Kanai Y., Takemura R., Oshima T., Mori H., Ihara Y., Yanagisawa M., Masaki T., Hirokawa N. Expression of multiple tau isoforms and microtubule bundle formation in fibroblasts transfected with a single tau cDNA. J Cell Biol. 1989 Sep;109(3):1173–1184. doi: 10.1083/jcb.109.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama T., Shiota K., Takahashi M. Activin A increases the number of follicle-stimulating hormone cells in anterior pituitary cultures. Mol Cell Endocrinol. 1990 Mar 5;69(2-3):179–185. doi: 10.1016/0303-7207(90)90011-v. [DOI] [PubMed] [Google Scholar]
- Kojima I., Mogami H., Ogata E. Oscillation of cytoplasmic free calcium concentration induced by insulin-like growth factor I. Am J Physiol. 1992 Mar;262(3 Pt 1):E307–E311. doi: 10.1152/ajpendo.1992.262.3.E307. [DOI] [PubMed] [Google Scholar]
- Kusano K., Gainer H. Whole cell current analyses of pancreatic acinar AR42J cells. I. Voltage- and Ca(2+)-activated currents. Am J Physiol. 1991 May;260(5 Pt 1):C934–C948. doi: 10.1152/ajpcell.1991.260.5.C934. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liem R. K., Hutchison S. B. Purification of individual components of the neurofilament triplet: filament assembly from the 70 000-dalton subunit. Biochemistry. 1982 Jun 22;21(13):3221–3226. doi: 10.1021/bi00256a029. [DOI] [PubMed] [Google Scholar]
- Logsdon C. D., Moessner J., Williams J. A., Goldfine I. D. Glucocorticoids increase amylase mRNA levels, secretory organelles, and secretion in pancreatic acinar AR42J cells. J Cell Biol. 1985 Apr;100(4):1200–1208. doi: 10.1083/jcb.100.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata F., Tsuyama S., Ihida K., Kashio N., Kawano M., Li Z. Z. Sulfated glycoconjugates demonstrated in combination with high iron diamine thiocarbohydrazide-silver proteinate and silver acetate physical development. J Electron Microsc (Tokyo) 1992 Feb;41(1):14–20. [PubMed] [Google Scholar]
- Nishimura M., Kaku K., Azuno Y., Okafuji K., Etoh Y., Shiozaki M., Sasaki H., Inoue T., Kaneko T. Effect of erythroid differentiation factor on megakaryocytic differentiation of L8057, a murine megakaryoblastic leukemia cell line. Biochem Biophys Res Commun. 1991 Dec 31;181(3):1042–1047. doi: 10.1016/0006-291x(91)92042-i. [DOI] [PubMed] [Google Scholar]
- Rae J., Cooper K., Gates P., Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991 Mar;37(1):15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Rosewicz S., Vogt D., Harth N., Grund C., Franke W. W., Ruppert S., Schweitzer E., Riecken E. O., Wiedenmann B. An amphicrine pancreatic cell line: AR42J cells combine exocrine and neuroendocrine properties. Eur J Cell Biol. 1992 Oct;59(1):80–91. [PubMed] [Google Scholar]
- Schlaepfer W. W., Lynch R. G. Immunofluorescence studies of neurofilaments in the rat and human peripheral and central nervous system. J Cell Biol. 1977 Jul;74(1):241–250. doi: 10.1083/jcb.74.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Kimura H., LaCorbiere M., Vaughan J., Karr D., Fischer W. H. Activin is a nerve cell survival molecule. Nature. 1990 Apr 26;344(6269):868–870. doi: 10.1038/344868a0. [DOI] [PubMed] [Google Scholar]
- Seino S., Chen L., Seino M., Blondel O., Takeda J., Johnson J. H., Bell G. I. Cloning of the alpha 1 subunit of a voltage-dependent calcium channel expressed in pancreatic beta cells. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):584–588. doi: 10.1073/pnas.89.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G. A., Weber K., Osborn M. Centriole number and process formation in established neuroblastoma cells and primary dorsal root ganglion neurones. Eur J Cell Biol. 1982 Nov;29(1):97–103. [PubMed] [Google Scholar]
- Shiomura Y., Hirokawa N. Colocalization of microtubule-associated protein 1A and microtubule-associated protein 2 on neuronal microtubules in situ revealed with double-label immunoelectron microscopy. J Cell Biol. 1987 Jun;104(6):1575–1578. doi: 10.1083/jcb.104.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C., Price B. M., Van Nimmen K., Huylebroeck D. Identification of a potent Xenopus mesoderm-inducing factor as a homologue of activin A. Nature. 1990 Jun 21;345(6277):729–731. doi: 10.1038/345729a0. [DOI] [PubMed] [Google Scholar]
- Sokol S., Melton D. A. Pre-existent pattern in Xenopus animal pole cells revealed by induction with activin. Nature. 1991 May 30;351(6325):409–411. doi: 10.1038/351409a0. [DOI] [PubMed] [Google Scholar]
- Sugino H., Nakamura T., Hasegawa Y., Miyamoto K., Abe Y., Igarashi M., Eto Y., Shibai H., Titani K. Erythroid differentiation factor can modulate follicular granulosa cell functions. Biochem Biophys Res Commun. 1988 May 31;153(1):281–288. doi: 10.1016/s0006-291x(88)81219-1. [DOI] [PubMed] [Google Scholar]
- Takasawa S., Nata K., Yonekura H., Okamoto H. Cyclic ADP-ribose in insulin secretion from pancreatic beta cells. Science. 1993 Jan 15;259(5093):370–373. doi: 10.1126/science.8420005. [DOI] [PubMed] [Google Scholar]
- Teitelman G. Insulin cells of pancreas extend neurites but do not arise from the neuroectoderm. Dev Biol. 1990 Dec;142(2):368–379. doi: 10.1016/0012-1606(90)90357-o. [DOI] [PubMed] [Google Scholar]
- Teitelman G., Lee J. K. Cell lineage analysis of pancreatic islet development: glucagon and insulin cells arise from catecholaminergic precursors present in the pancreatic duct. Dev Biol. 1987 Jun;121(2):454–466. doi: 10.1016/0012-1606(87)90182-5. [DOI] [PubMed] [Google Scholar]
- Vale W., Rivier J., Vaughan J., McClintock R., Corrigan A., Woo W., Karr D., Spiess J. Purification and characterization of an FSH releasing protein from porcine ovarian follicular fluid. Nature. 1986 Jun 19;321(6072):776–779. doi: 10.1038/321776a0. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B., Franke W. W., Kuhn C., Moll R., Gould V. E. Synaptophysin: a marker protein for neuroendocrine cells and neoplasms. Proc Natl Acad Sci U S A. 1986 May;83(10):3500–3504. doi: 10.1073/pnas.83.10.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H., Mine T., Shibata H., Eto Y., Hasegawa Y., Takeuchi T., Asano S., Kojima I. Activin A: an autocrine inhibitor of initiation of DNA synthesis in rat hepatocytes. J Clin Invest. 1993 Sep;92(3):1491–1496. doi: 10.1172/JCI116727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H., Tanaka S., Ohnishi H., Mashima H., Ogushi N., Mine T., Kojima I. Activin A: negative regulator of amylase secretion and cell proliferation in rat pancreatic acinar AR42J cells. Am J Physiol. 1994 Aug;267(2 Pt 1):G220–G226. doi: 10.1152/ajpgi.1994.267.2.G220. [DOI] [PubMed] [Google Scholar]
- Ying S. Y. Inhibins, activins, and follistatins: gonadal proteins modulating the secretion of follicle-stimulating hormone. Endocr Rev. 1988 May;9(2):267–293. doi: 10.1210/edrv-9-2-267. [DOI] [PubMed] [Google Scholar]
- Yu J., Shao L. E., Lemas V., Yu A. L., Vaughan J., Rivier J., Vale W. Importance of FSH-releasing protein and inhibin in erythrodifferentiation. Nature. 1987 Dec 24;330(6150):765–767. doi: 10.1038/330765a0. [DOI] [PubMed] [Google Scholar]