Abstract
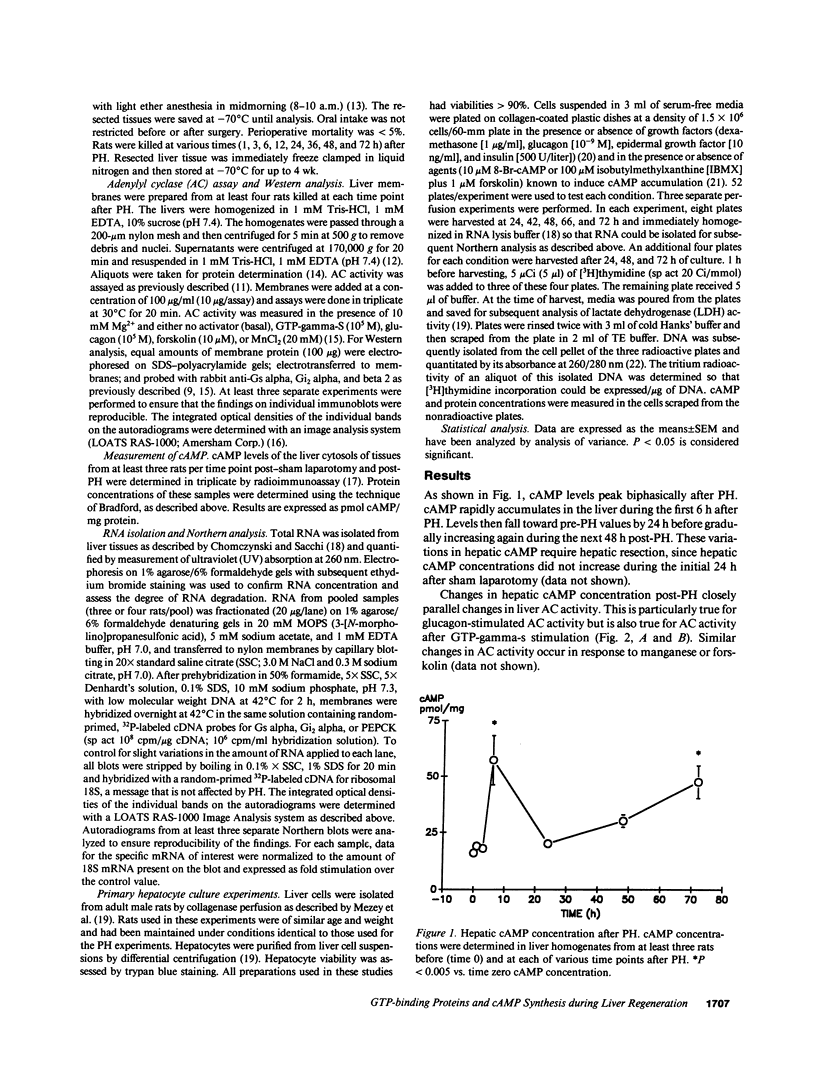
Events leading to cAMP accumulation after partial hepatectomy (PH) and effects of cAMP on hormonal induction of DNA synthesis in hepatocytes were characterized. Hepatic cAMP peaked biphasically post-PH and paralleled changes in adenylyl cyclase activity. Fluctuations in cyclase activity were not explained by variations in glucagon receptor kinetics, but reflected altered G-protein expression. Membrane levels of the stimulatory G-protein, Gs alpha, increased early after PH and were sustained. Levels of the inhibitory G-protein, Gi2 alpha, increased more slowly, peaked later, and quickly fell. Levels of both G-proteins correlated poorly with levels of their mRNAs, suggesting posttranscriptional factors modify their membrane concentrations. When growth factor-induced DNA synthesis was compared in hepatocyte cultures grown with or without agents that increase intracellular cAMP, DNA synthesis was inhibited by sustained high levels of cAMP but was enhanced when high cAMP levels fell. In both regenerating liver and hepatocyte cultures, the expression of a "differentiated" hepatocyte gene, phosphoenolpyruvate carboxykinase, correlated with elevated cAMP levels. These data suggest that the differential expression of G-proteins integrates signals initiated by several growth factors so that the accumulation of cAMP is tightly regulated post-PH. The ensuing variations in cAMP levels modulate both growth and differentiated functions during liver regeneration.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun L., Mead J. E., Panzica M., Mikumo R., Bell G. I., Fausto N. Transforming growth factor beta mRNA increases during liver regeneration: a possible paracrine mechanism of growth regulation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1539–1543. doi: 10.1073/pnas.85.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brønstad G., Christoffersen T. Increased effect of adrenaline on cyclic AMP formation and positive beta-adrenergic modulation of DNA-synthesis in regenerating hepatocytes. FEBS Lett. 1980 Oct 20;120(1):89–93. doi: 10.1016/0014-5793(80)81053-2. [DOI] [PubMed] [Google Scholar]
- Carter E. A., Wands J. R. Ethanol-induced inhibition of liver cell function: I. Effect of ethanol on hormone stimulated hepatocyte DNA synthesis and the role of ethanol metabolism. Alcohol Clin Exp Res. 1988 Aug;12(4):555–562. doi: 10.1111/j.1530-0277.1988.tb00241.x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cruise J. L. Alpha 1-adrenergic receptors in liver regeneration. Dig Dis Sci. 1991 Apr;36(4):485–488. doi: 10.1007/BF01298880. [DOI] [PubMed] [Google Scholar]
- Diamond I., Wrubel B., Estrin W., Gordon A. Basal and adenosine receptor-stimulated levels of cAMP are reduced in lymphocytes from alcoholic patients. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1413–1416. doi: 10.1073/pnas.84.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekanger R., Vintermyr O. K., Houge G., Sand T. E., Scott J. D., Krebs E. G., Eikhom T. S., Christoffersen T., Ogreid D., Døskeland S. O. The expression of cAMP-dependent protein kinase subunits is differentially regulated during liver regeneration. J Biol Chem. 1989 Mar 15;264(8):4374–4382. [PubMed] [Google Scholar]
- García-Sáinz J. A., Huerta-Bahena M. E., Malbon C. C. Hepatocyte beta-adrenergic responsiveness and guanine nucleotide-binding regulatory proteins. Am J Physiol. 1989 Feb;256(2 Pt 1):C384–C389. doi: 10.1152/ajpcell.1989.256.2.C384. [DOI] [PubMed] [Google Scholar]
- Granner D., Andreone T., Sasaki K., Beale E. Inhibition of transcription of the phosphoenolpyruvate carboxykinase gene by insulin. Nature. 1983 Oct 6;305(5934):549–551. doi: 10.1038/305549a0. [DOI] [PubMed] [Google Scholar]
- Krause D. S., Deutsch C. Cyclic AMP directly inhibits IL-2 receptor expression in human T cells: expression of both p55 and p75 subunits is affected. J Immunol. 1991 Apr 1;146(7):2285–2296. [PubMed] [Google Scholar]
- Leffert H., Alexander N. M., Faloona G., Rubalcava B., Unger R. Specific endocrine and hormonal receptor changes associated with liver regeneration in adult rats. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4033–4036. doi: 10.1073/pnas.72.10.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmanus J. P., Franks D. J., Youdale T., Braceland B. M. Increases in rat liver cyclic AMP concentrations prior to the initiation of DNA synthesis following partial hepatectomy or hormone infusion. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1201–1207. doi: 10.1016/0006-291x(72)90596-7. [DOI] [PubMed] [Google Scholar]
- Mezey E., Potter J. J., Diehl A. M. Depression of alcohol dehydrogenase activity in rat hepatocyte culture by dihydrotestosterone. Biochem Pharmacol. 1986 Jan 15;35(2):335–339. doi: 10.1016/0006-2952(86)90535-6. [DOI] [PubMed] [Google Scholar]
- Mohn K. L., Laz T. M., Melby A. E., Taub R. Immediate-early gene expression differs between regenerating liver, insulin-stimulated H-35 cells, and mitogen-stimulated Balb/c 3T3 cells. Liver-specific induction patterns of gene 33, phosphoenolpyruvate carboxykinase, and the jun, fos, and egr families. J Biol Chem. 1990 Dec 15;265(35):21914–21921. [PubMed] [Google Scholar]
- Morley C. G., Royse V. L. Adrenergic agents as possible regulators of liver regeneration. Int J Biochem. 1981;13(9):969–973. doi: 10.1016/0020-711x(81)90001-x. [DOI] [PubMed] [Google Scholar]
- Mumby S. M., Kahn R. A., Manning D. R., Gilman A. G. Antisera of designed specificity for subunits of guanine nucleotide-binding regulatory proteins. Proc Natl Acad Sci U S A. 1986 Jan;83(2):265–269. doi: 10.1073/pnas.83.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen N. E. Effect of prostaglandin E1 on DNA synthesis in vascular smooth muscle cells. Am J Physiol. 1986 Apr;250(4 Pt 1):C584–C588. doi: 10.1152/ajpcell.1986.250.4.C584. [DOI] [PubMed] [Google Scholar]
- Patten J. L., Johns D. R., Valle D., Eil C., Gruppuso P. A., Steele G., Smallwood P. M., Levine M. A. Mutation in the gene encoding the stimulatory G protein of adenylate cyclase in Albright's hereditary osteodystrophy. N Engl J Med. 1990 May 17;322(20):1412–1419. doi: 10.1056/NEJM199005173222002. [DOI] [PubMed] [Google Scholar]
- Ransnäs L. A., Svoboda P., Jasper J. R., Insel P. A. Stimulation of beta-adrenergic receptors of S49 lymphoma cells redistributes the alpha subunit of the stimulatory G protein between cytosol and membranes. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7900–7903. doi: 10.1073/pnas.86.20.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. S., Hsieh L. L., Peraino C., Weinstein I. B. Isolation of a complementary DNA encoding the catalytic subunit of protein kinase A and studies on the expression of this sequence in rat hepatomas and regenerating liver. Cancer Res. 1990 Mar 15;50(6):1675–1680. [PubMed] [Google Scholar]
- Russell W. E., Coffey R. J., Jr, Ouellette A. J., Moses H. L. Type beta transforming growth factor reversibly inhibits the early proliferative response to partial hepatectomy in the rat. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5126–5130. doi: 10.1073/pnas.85.14.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K., Cripe T. P., Koch S. R., Andreone T. L., Petersen D. D., Beale E. G., Granner D. K. Multihormonal regulation of phosphoenolpyruvate carboxykinase gene transcription. The dominant role of insulin. J Biol Chem. 1984 Dec 25;259(24):15242–15251. [PubMed] [Google Scholar]
- Sibley D. R., Lefkowitz R. J. Molecular mechanisms of receptor desensitization using the beta-adrenergic receptor-coupled adenylate cyclase system as a model. Nature. 1985 Sep 12;317(6033):124–129. doi: 10.1038/317124a0. [DOI] [PubMed] [Google Scholar]
- Wand G. S. Ethanol differentially regulates proadrenocorticotropin/endorphin production and corticosterone secretion in LS and SS lines of mice. Endocrinology. 1989 Jan;124(1):518–526. doi: 10.1210/endo-124-1-518. [DOI] [PubMed] [Google Scholar]
- Wand G. S., Levine M. A. Hormonal tolerance to ethanol is associated with decreased expression of the GTP-binding protein, Gs alpha, and adenylyl cyclase activity in ethanol-treated LS mice. Alcohol Clin Exp Res. 1991 Aug;15(4):705–710. doi: 10.1111/j.1530-0277.1991.tb00583.x. [DOI] [PubMed] [Google Scholar]







