Abstract
Plants generally react to the attack of non-host and incompatible host microorganisms by inducing pathogenesis-related (PR) genes and localised cell death (LCD) at the site of infection, a process collectively known as the hypersensitive response (HR). Reactive oxygen species (ROS) are generated in various sub-cellular compartments shortly after pathogen recognition, and proposed to cue subsequent orchestration of the HR. Although apoplast-associated ROS production by plasma membrane NADPH oxidases have been most thoroughly studied, recent observations suggest that ROS are generated in chloroplasts earlier in the response and play a key role in execution of LCD. A model is presented in which the initial outcome of successful pathogen detection is ROS accumulation in plastids, likely mediated by mitogen-activated protein kinases and caused by dysfunction of the photosynthetic electron transport chain. ROS signaling is proposed to spread from plastids to the apoplast, through the activation of NADPH oxidases, and from there to adjacent cells, leading to suicidal death in the region of attempted infection.
Key words: biotic stress, chloroplasts, flavodoxin, hypersensitive response (HR), reactive oxygen species (ROS), ROS signaling
Introduction
The hypersensitive response (HR), first described by Stakmann1 in 1915, is the landmark of successful pathogen recognition during non-host and incompatible host plant-microbe interactions.2 It is a multicomponent response involving increased expression of defence-associated genes (pathogenesis-related or PR genes), synthesis of antimicrobial secondary metabolites and a form of localised cell death (LCD) at the site of infection, purportedly designed to restrict further advance of biotrophic or hemi-biotrophic microorganisms.2 Although the HR has been usually employed as a visual marker of biotic interactions, some of its features, including LCD and induction of PR genes, are shared by plant responses to a number of abiotic stresses such as excess of excitation energy (EEE),3 and exposure to ozone.4 A biphasic oxidative burst leading to the generation of reactive oxygen species (ROS) commonly precedes cell death, and the signaling role played by these oxidants in the orchestration of the HR has long been recognised.5–8 The link between ROS and the HR was established more than twenty years ago, when Doke9 reported superoxide production prior to HR elicited by Phytophthora infestans and tobacco mosaic virus on potato and tobacco, respectively. However, many aspects of ROS function remain obscure. For instance, it is not clear if they participate in triggering LCD, in the induction of PR genes, or in both pathways. Also, the relative contribution of ROS produced in different compartments has yet to be established. These reactive species can be synthesised in the apoplast by NADPH oxidases bound to the plasma membrane, or intracellularly in chloroplasts, mitochondria and peroxisomes, as byproducts of metabolic processes such as photosynthesis and respiration.8 Although the contribution of organellar ROS is increasingly appreciated, most researches have focused on NADPH oxidases as the HR-relevant ROS generating system. We will briefly review these contributions and propose an integrated working hypothesis for future developments.
Apoplastic ROS Production and its Contribution to the HR
By comparison with mammalian systems, ROS production in the apoplast, mediated by NADPH oxidase activities encoded by the Rboh gene family, has been long considered as a central feature of the HR. Plants usually contain several Rboh genes (ten in Arabidopsis) which are transcriptionally upregulated by pathogens, and whose products display a certain degree of functional overlap.8 Genetic proof of the role played by NADPH oxidase isoforms in the pathogen-induced oxidative burst has been obtained by the use of Rboh mutants and antisense lines.10 Extracellular ROS production has been linked to direct lipid peroxidation, to the alkalinisation of the apoplast, thereby propagating the signal by alkali-responsive peroxidases, or to alterations in the levels and/or redox status of antioxidant pools.2 Interestingly, downregulation or elimination of Rboh genes could lead to variable effects on the HR. For example, although Arabidopsis RbohD and RbohF mutants exhibited lower ROS accumulation, they displayed enhanced HR when introduced into a lesion stimulating disease 1 mutant background, or when challenged with avirulent bacteria.10 These results indicate that while NADPH oxidase activity is required for pathogen-induced ROS production in the apoplast, these ROS might serve different signaling purposes during the HR.10
Mitochondria and LCD
Mitochondria play an important role in ROS generation leading to LCD in mammalian cells, and recent observations suggest a similar role in plants.11–13 For instance, treatment of Arabidopsis leaves with bacterial elicitors results in rapid ROS generation in mitochondria, followed by membrane pore formation, dissipation of membrane potential and decline of ATP levels. The results indicate that oxidative phosphorylation is uncoupled early after the challenge, leading to runaway oxygen consumption and ROS propagation.14 Salicylic acid, which is induced in many plant-microbe interactions and is involved in the deployment of systemic acquired resistance, has been shown to act as uncoupler of oxidative phosphorylation and, at higher levels, inhibitor of the respiratory chain, leading to ROS generation in mitochondria.13 It is likely that changes in the redox status of this organelle result in cellular metabolic dysfunction, and significantly contribute to the establishment of the HR-associated LCD.2,11
Chloroplasts Shed Light on the HR
Initial reports implicating chloroplasts as sources of ROS signaling for the HR were based on the observation that at least some forms of this response required light.15–17 Indeed, chloroplasts are the major ROS source in plant cells (over mitochondria and peroxisomes), largely through overreduction of the photosynthetic electron transport chain (PETC) under EEE conditions, when photon input exceeds that required for photochemistry.3,18 ROS production in chloroplasts is also a common theme in many situations of abiotic stress, significantly contributing to the damage undergone by the plant.19
The role of chloroplast-generated ROS in the HR has been recently evaluated using tobacco plants which expressed a plastid-targeted flavodoxin. Flavodoxins are electron shuttle proteins present in photosynthetic microorganisms, but not in plants, which can engage in several plant-borne electron transport processes in vivo.20,21 When expressed in transgenic plants, they specifically prevent ROS formation in chloroplasts, without affecting extra-plastidial sources.19,22 Transformants displayed enhanced tolerance to iron deficit, to multiple sources of abiotic stress and to xenobiotics.20,23,24
Infiltration of flavodoxin-expressing plants with a non-host pathogen resulted in lower ROS accumulation in chloroplasts compared to non-transformed siblings, whereas apoplastic ROS production was hardly affected.22 Noteworthy, LCD symptoms were largely prevented in the infected flavodoxin transformants, correlating with preservation of metabolic routes normally inhibited by pathogen infection, such as photosynthesis, amino acid synthesis and antioxidant metabolism. Other aspects of the HR were instead not affected by flavodoxin expression. Induction of PR genes, and synthesis of salicylic and jasmonic acid proceeded as in inoculated non-transformed plants, and infection did not spread to tissues adjacent to the infiltrated regions. The results indicated that chloroplast-derived ROS were essential for the progress of LCD during the HR, but did not contribute to induction of defence-associated genes or other signaling components of the response.
Integration of ROS Signaling During the HR
How the various extracellular and intracellular sources of ROS integrate to trigger the HR? Which are the signals that initiate ROS production in the different compartments upon recognition of an invading pathogen? These questions remain largely unanswered, but recent developments have provided promising clues to understand the succession of events that lead from ROS build-up to full manifestation of the HR.
An increasing number of reports have shown that plant mitogen-activated protein kinases (MAPKs) are converging nodes after perception of pathogens and elicitors.25–27 ROS-induced activation of MAPKs had generally been taken as evidence that ROS act upstream of the MAPK cascade.25 However, recent investigations on different plant-pathogen interactions showed that various MAPK and Ca++-dependent kinase pathways might be part of an amplification network upstream of rboh genes, which are in turn responsible for producing the secondary peak of the biphasic ROS burst in response to pathogen infection.25,27–29 Liu et al.17 have studied a conditional gain-of-function mutant of tobacco MEK2, a protein kinase kinase that, when expressed under control of a steroid-inducible promoter, activates downstream kinases and induces LCD lesions in the absence of pathogen. Constitutive expression of MEK2 caused loss of membrane potential, electrolyte leakage and ROS generation in both chloroplasts and mitochondria, which was preceded by disruption of metabolic activities in these organelles.17 Consistent with a role of chloroplast-generated ROS in MAPK-mediated LCD, plants kept in the dark failed to accumulate peroxides in plastids after MEK2 activation and cell death was considerably delayed.17
Another piece of evidence of the role played by chloroplasts in the early signaling for the HR came from studies on the Arabidopsis response to ozone which, as indicated before, displays many features in common with pathogen-induced HR, including biphasic oxidative burst. In a time-course analysis of the response, Joo et al.4 were able to demonstrate that the early phase of ROS accumulation was confined to chloroplasts of the guard cells, followed by extracellular ROS production in the plasma membrane of the same cells (dependent on NADPH oxidase activity), and subsequent spread to adjacent tissues. Ozone did not penetrate the cytosol but triggered the response by an unknown mechanism which involves membrane-bound heterotrimetic G proteins.4
Based on these recent contributions,4,17,22 we propose that chloroplasts are the initial source of ROS during the HR, resulting from shutdown of electron utilisation in the chloroplast stroma, and leading to over-reduction of the photosynthetic electron transport chain and EEE in the thylakoids. The ROS-linked signals are then somehow communicated to the plasma membrane for apoplastic oxidative burst and spread to adjacent cells, and eventually also to mitochondria. The comprehensive model depicted in Figure 1 is intended as a working hypothesis and includes many unknown stages and open questions, providing direction for future research. For instance, the similarities between the ozone and pathogen responses need to be further studied. Inhibition of chloroplast ROS build-up in flavodoxin-expressing plants was measured at 19 hours post infiltration, when both phases of the oxidative burst are expected to have occurred.22 Determination of the kinetics of the response in these plants is mandatory. The role of lipid peroxides in transmitting the signal from chloroplasts to other ROS sources should be investigated, as well as the clues that promote plastid ROS production in the first place. Finally, it should be borne in mind that even when ROS production is abolished by mutation, transgenic or pharmacological approaches, some aspects of the HR proceed unaffected and LCD is only ameliorated or delayed,22 indicating that ROS-independent processes also contribute to the HR. Distinction between these two types of mechanisms and the possible cross-talk between them constitutes yet another important task to understand this very complex plant response.
Figure 1.
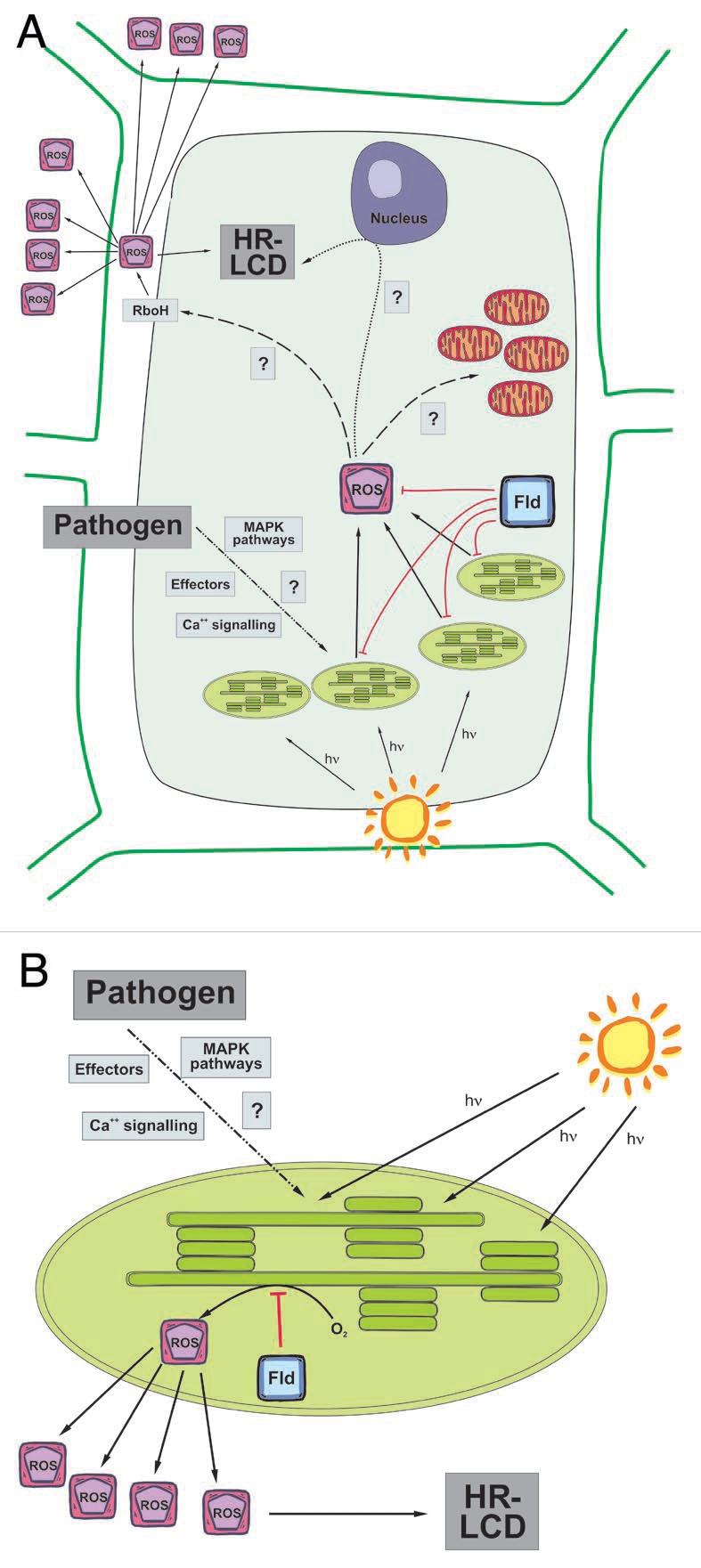
Schematic diagram of ROS signaling for the LCD associated to the HR in a plant-pathogen interaction. (A) Recognition of an invading pathogen triggers light-dependent ROS production in chloroplasts. Chloroplast-generated ROS then signal for further ROS production in the apoplast by directly or indirectly activating RboH-type NADPH oxidases, which are involved in propagation of the signal to adjacent cells. There is also a relay of information to the nucleus and mitochondria. Altogether, the different signaling factors lead to the establishment of LCD. (B) Flavodoxin (Fld) expression in chloroplasts specifically blocks ROS generation in this organelle, delaying the appearance of LCD symptoms. This experimental evidence supports the central role played by chloroplasts in the signaling pathways for HR-associated LCD.
Acknowledgements
This research was supported by grants and funding from the National Agency for the Promotion of Science and Technology (ANPCyT, Argentina), the National Research Council (CONICET, Argentina), the German Academic Exchange Service (DAAD, Germany), the Leibniz-Institute of Plant Genetics and Crop Plant Research (Leibniz-IPK), the German Research Foundation (DFG) and the European Molecular Biology Organization (EMBO, European Union).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/10793
References
- 1.Stakman EC. Relation between Puccinia graminis and plants highly resistant to its attack. J Agric Res. 1915;4:193–200. [Google Scholar]
- 2.Mur LA, Kenton P, Lloyd AJ, Ougham H, Prats E. The hypersensitive response; the centenary is upon us but how much do we know? J Exp Bot. 2008;59:501–520. doi: 10.1093/jxb/erm239. [DOI] [PubMed] [Google Scholar]
- 3.Mühlenbock P, Szechynska-Hebda M, Plaszczyca M, Baudo M, Mullineaux PM, Parker JE, et al. Chloroplast signaling and LESION SIMULATING DISEASE1 regulate crosstalk between light acclimation and immunity in Arabidopsis. Plant Cell. 2008;20:2339–2356. doi: 10.1105/tpc.108.059618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joo JH, Wang S, Chen JG, Jones AM, Fedoroff NV. Different signaling and cell death roles of heterotrimeric G protein alpha and beta subunits in the Arabidopsis oxidative stress response to ozone. Plant Cell. 2005;17:957–970. doi: 10.1105/tpc.104.029603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fobert PR, Després C. Redox control of systemic acquired resistance. Curr Opin Plant Biol. 2005;8:378–382. doi: 10.1016/j.pbi.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Mittler R, Herr EH, Orvar BL, van Camp W, Willekens H, Inzé D, Ellis BE. Transgenic tobacco plants with reduced capability to detoxify reactive oxygen intermediates are hyperresponsive to pathogen infection. Proc Natl Acad Sci USA. 1999;96:14165–14170. doi: 10.1073/pnas.96.24.14165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavet V, Olmos E, Kiddle G, Mowla S, Kumar S, Antoniw J, et al. Ascorbic acid deficiency activates cell death and disease resistance responses in Arabidopsis. Plant Physiol. 2005;139:1291–1303. doi: 10.1104/pp.105.067686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torres MA, Dangl JL. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol. 2005;8:397–403. doi: 10.1016/j.pbi.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Doke N. Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiol Mol Plant Pathol. 1983;23:345–357. [Google Scholar]
- 10.Torres MA, Jones JD, Dangl JL. Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat Genet. 2005;37:1130–1134. doi: 10.1038/ng1639. [DOI] [PubMed] [Google Scholar]
- 11.Maxwell DP, Nickels R, McIntosh L. Evidence of mitochondrial involvement in the transduction of signals required for the induction of genes associated with pathogen attack and senescence. Plant J. 2002;29:269–279. doi: 10.1046/j.1365-313x.2002.01216.x. [DOI] [PubMed] [Google Scholar]
- 12.Amirsadeghi S, Robson CA, McDonald AE, Vanlerberghe GC. Changes in plant mitochondrial electron transport alter cellular levels of reactive oxygen species and susceptibility to cell death signaling molecules. Plant Cell Physiol. 2006;47:1509–1519. doi: 10.1093/pcp/pcl016. [DOI] [PubMed] [Google Scholar]
- 13.Love AJ, Milner JJ, Sadanandom A. Timing is everything: regulatory overlap in plant cell death. Trends Plant Sci. 2008;13:589–595. doi: 10.1016/j.tplants.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Yao N, Greenberg JT. Arabidopsis ACCELERATED CELL DEATH2 modulates programmed cell death. Plant Cell. 2006;18:397–411. doi: 10.1105/tpc.105.036251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karpinski S, Gabrys H, Mateo A, Karpinska B, Mullineaux PM. Light perception in plant disease defence signalling. Curr Opin Plant Biol. 2003;6:390–396. doi: 10.1016/s1369-5266(03)00061-x. [DOI] [PubMed] [Google Scholar]
- 16.Montillet JL, Chamnongpol S, Rusterucci C, Dat J, van de Cotte B, Agnel JP, et al. Fatty acid hydroperoxides and H2O2 in the execution of hypersensitive cell death in tobacco leaves. Plant Physiol. 2005;138:1516–1526. doi: 10.1104/pp.105.059907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Ren D, Pike S, Pallardy S, Gassmann W, Zhang S. Chloroplast-generated reactive oxygen species are involved in hypersensitive response-like cell death mediated by a mitogen-activated protein kinase cascade. Plant J. 2007;51:941–954. doi: 10.1111/j.1365-313X.2007.03191.x. [DOI] [PubMed] [Google Scholar]
- 18.Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006;141:391–396. doi: 10.1104/pp.106.082040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zurbriggen MD, Tognetti VB, Fillat MF, Hajirezaei MR, Valle EM, Carrillo N. Combating stress with flavodoxin: a promising route for crop improvement. Trends Biotechnol. 2008;26:531–537. doi: 10.1016/j.tibtech.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Tognetti VB, Palatnik JF, Fillat MF, Melzer M, Hajirezaei MR, Valle EM, Carrillo N. Functional replacement of ferredoxin by a cyanobacterial flavodoxin in tobacco confers broad-range stress tolerance. Plant Cell. 2006;18:2035–2050. doi: 10.1105/tpc.106.042424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zurbriggen MD, Tognetti VB, Carrillo N. Stress-inducible flavodoxin from photosynthetic microorganisms. The mystery of flavodoxin loss from the plant genome. IUBMB Life. 2007;59:355–360. doi: 10.1080/15216540701258744. [DOI] [PubMed] [Google Scholar]
- 22.Zurbriggen MD, Carrillo N, Tognetti VB, Melzer M, Peisker M, Hause B, Hajirezaei M-R. Chloroplast-generated reactive oxygen species play a major role in localised cell death during the non-host interaction between tobacco and Xanthomonas campestris pv. vesicatoria. Plant J. 2009;60:962–973. doi: 10.1111/j.1365-313X.2009.04010.x. [DOI] [PubMed] [Google Scholar]
- 23.Tognetti VB, Zurbriggen MD, Morandi EN, Fillat MF, Valle EM, Hajirezaei MR, Carrillo N. Enhanced plant tolerance to iron starvation by functional substitution of chloroplast ferredoxin with a bacterial flavodoxin. Proc Natl Acad Sci USA. 2007;104:11495–11500. doi: 10.1073/pnas.0704553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tognetti VB, Monti MR, Valle EM, Carrillo N, Smania AM. Detoxification of 2,4-dinitrotoluene by transgenic tobacco plants expressing a bacterial flavodoxin. Environ Sci Technol. 2007;41:4071–4076. doi: 10.1021/es070015y. [DOI] [PubMed] [Google Scholar]
- 25.Nakagami H, Pitzschke A, Hirt H. Emerging MAP kinase pathways in plant stress signalling. Trends Plant Sci. 2005;10:339–346. doi: 10.1016/j.tplants.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 26.del Pozo O, Pedley KF, Martin GB. MAPKKKalpha is a positive regulator of cell death associated with both plant immunity and disease. EMBO J. 2004;23:3072–3082. doi: 10.1038/sj.emboj.7600283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren D, Yang KY, Li GJ, Liu Y, Zhang S. Activation of Ntf4, a tobacco mitogen-activated protein kinase, during plant defense response and its involvement in hypersensitive response-like cell death. Plant Physiol. 2006;141:1482–1493. doi: 10.1104/pp.106.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshioka H, Asai S, Yoshioka M, Kobayashi M. Molecular mechanisms of generation for nitric oxide and reactive oxygen species, and role of the radical burst in plant immunity. Mol Cells. 2009;28:321–329. doi: 10.1007/s10059-009-0156-2. [DOI] [PubMed] [Google Scholar]
- 29.Asai S, Yoshioka H. The role of radical burst via MAPK signaling in plant immunity. Plant Signal Behav. 2008;3:920–922. doi: 10.4161/psb.6601. [DOI] [PMC free article] [PubMed] [Google Scholar]


