Abstract
The Cut homeobox gene 1 (CUX1) codes for several homeodomain proteins that display distinct DNA binding and transcriptional properties. Some CUX1 isoforms were previously shown to stimulate entry into S phase. More recently, siRNA-mediated knockdown of CUX1 was shown to cause a decrease in cell migration and invasion. In contrast, ectopic expression of p110 or p75 CUX1 stimulated cell migration and invasion in tissue culture. Moreover, metastasis to the lung was observed in a few cases following development of mammary tumors in p75 CUX1 transgenic mice. Chromatin immunoprecipitation (ChIP) assays followed by hybridization on promoter arrays (ChIP-chip) led to the identification of more than 20 genes that are directly regulated by CUX1 and code for proteins involved in cytoskeleton remodeling, cell-cell and cell-matrix adhesion, epithelial to mesenchymal transition and transcriptional regulation. Many targets of CUX1 are regulators of Rho GTPases that play a role both in cell cycle progression and cell motility. Interestingly, some genes that promote cell motility are activated by CUX1, while some genes that inhibit cell motility are repressed by CUX1. The dual function of CUX1 as an activator and repressor is best exemplified by the regulatory cascade whereby CUX1 activates expression of the Snail and Slug transcription factors and then cooperates with them to repress the E-cadherin and occludin genes, thereby causing a severe disorganization of cellcell junctions. Together, these studies indicate that CUX1 stimulates cell motility by regulating a large number of genes involved in various molecular functions.
Key words: CUX1, transcriptional regulation, activation, repression, ChIP-chip, tumor progression, E-cadherin, occludin, vimentin
Cell Migratory Properties are Reduced in the Absence of CUX1
A role for the CUX1 homeodomain protein in cell motility was first revealed from a high-throughput RNA interference screen.1 In subsequent experiments using NIH3T3 fibroblastic cells and a panel of human cancer cell lines from several tissue origins (including HT1080, U87-MG, MDA-MB-231 and PANC1 cells), Michl and colleagues confirmed that siRNA-mediated knockdown of CUX1 caused a decrease in cell migration as measured in video time-lapse microscopy, two-chamber and wound healing assays.1 Transient knockdown of CUX1 also inhibited invasion through Matrigel.1 In agreement with these results, mouse embryonic fibroblasts (MEFs) from CUX1-knockout mice exhibited a defect in migration and invasion as compared to MEFs from wild type mice.2 These observations were extended using an in vivo invasion assay. Indeed, pulmonary colonization after tail vein injection was reduced following stable expression of CUX1 shRNA in HT1080 and MDA-MB-231.1 Together, these results clearly demonstrated a requirement for CUX1 in cell migration and invasion.
Elevated CUX1 Expression is Associated with Tumor Progression
Two sets of data suggested that elevated CUX1 expression may be associated with tumor progression.1 First, the addition of TGFb to NIH3T3 fibroblasts, AML12 hepatocytes and MDA-MB-231 breast tumor cells led to an increase in CUX1 mRNA and full-length protein steadystate levels. As TGFβ failed to stimulate migration of NIH3T3 and HT1080 cells when CUX1 was silenced by siRNA, the authors proposed that CUX1 functions as a downstream effector of the TGFβ-induced cell migration and invasion. Secondly, in cohorts of breast and pancreatic cancer patients, elevated expression of CUX1 was found to occur more frequently in tumors of higher histological grade. Furthermore, CUX1 expression inversely correlated with relapse-free survival and overall survival among patients with high-grade tumors. These results suggested that elevated CUX1 expression contributes to tumor progression.
Not All CUX1 Isoforms Are Made Equal
In addition to the full-length protein, often called p200 CUX1, are a number of shorter isoforms with distinct DNA and transcriptional properties (reviewed in ref. 3). The p55 and p75 isoforms are encoded by tissue-specific transcripts respectively generated by alternative splicing or transcription initiation at an alternative site, whereas the p150, p110, p90 and p80 are the products of proteolytic processing events.4–9 While the major isoform, p200 CUX1, has been characterized as a transcriptional repressor, p150 CUX1 was described as a dominant-negative isoform and the p75 and p110 isoforms were found to function both as transcriptional activators or repressors depending on promoter context.10–12
Cell Motility Defect of Cux1Z/Z MEFs is Rescued by p110 CUX1, but Only Partially by the Full-Length Isoform, p200 CUX1
In order to establish the role of individual isoform, we investigated whether the defect of Cux1Z/Z MEFs in cell motility could be rescued by re-expressing one or the other CUX1 isoforms, using a panel of Adenovirus expressing either p200 CUX1, p110 CUX1 or an inactive mutant, CUX1(1–1109).2 The defect was rescued completely by p110 CUX1, but only partially by p200 CUX1. Subsequent experiments showed that cell migration was stimulated by treatments that increase proteolytic processing of p200 CUX1 into p110 CUX1, whereas cell migration was decreased in the presence of a protease inhibitor that prevents the generation of p110 CUX1. Together these results suggest that p200 CUX1 may not itself be active in this process but may mediate its effects on migration only indirectly through the generation of p110 CUX1.
Both p75 and p110 CUX1 Can Stimulate Motility of Epithelial Cells
The elevated levels of CUX1 in high grade tumors and the requirement for CUX1 in cell migration and invasion together suggest that CUX1 contributes to tumor progression by stimulating the migratory and invasive properties of tumor cells. To formally test this hypothesis, one would need to verify whether elevated CUX1 expression leads to an increase in cell motility. To address this issue, retroviral infections were performed in various cell lines to generate populations of cells stably expressing p110 CUX1. Cell migration and invasion were stimulated as judged from three assays: trans-well migration, invasion through matrigel and woundhealing assays.2 We noted that the stimulatory effect of p110 CUX1 was much more pronounced in epithelial cells than in fibroblastic cells. This observation will later find a mechanistic explanation with the identification of CUX1 transcriptional targets. In another study, stable expression of p75 CUX1 in a mouse mammary epithelial cell line was shown to stimulate cell migration in a two-chamber migration assay and a wound-healing assay.12 These results demonstrate that some CUX1 isoforms can stimulate cell migration and invasion.
Mammary Tumors from MMTV-p75 CUX1 Transgenic Mice Develop Lung Metastases
Transgenic mice expressing p75 or p110 CUX1 under the control of the Mouse Mammary Tumor Virus long terminal repeat (MMTV-LTR) were found to develop mammary tumors after a long latency period.12 Metastasis to the lung was observed in three p75 CUX1 transgenic mice. It should be noted that the sample size was too small to determine whether the metastasis rate is different between p75 and p110 CUX1 transgenic mice. Nevertheless, stable expression of p75 CUX1 in a mouse mammary epithelial cell line was shown to stimulate cell migration in tissue culture assays and to increase the formation of lung metastases following tail vein injection.12 Future studies should investigate whether various CUX1 isoforms differ in their ability to stimulate the invasive properties of cells. In this regards, it will be imperative to develop reagents and methods that can distinguish whether tumor progression is associated with an increase in the expression of specific CUX1 isoforms as previously proposed.6
Transcriptional Targets of CUX1 that Mediate its Effects on Cell Motility
In the original study of Michl et al. microarray gene expression profiling analysis of NIH3T3 CUX1 RNAi clones generated two lists of genes: those that were upregulated and those that were downregulated.1 It is reasonable to assume that some of these genes are direct targets of CUX1, while others represent indirect targets that are regulated in subsequent waves of gene regulation. Transcriptional targets of CUX1 were identified by performing genome-wide location analysis in several cell lines.2 Targets that play a role in cell migration, invasion and adhesion were then validated in independent chromatin immunoprecipitation (ChIP) experiments and their expression was measured in cells that do or do not overexpress p110 CUX1. This strategy led to the identification of 20 genes that are directly regulated by CUX1 and code for proteins involved in cytoskeleton formation and remodeling, cellcell and cell-matrix adhesion, epithelial to mesenchymal transition or transcriptional regulation.2 Interestingly, p110 CUX1 was found to activate genes promoting cell migration and invasion such as FAK, N-Cadherin and Vimentin, and to repress genes inhibiting the same process such as E-Cadherin, Occludin, RICS and EPLIN (Fig. 1). The dual role of p110 CUX1 as a transcriptional activator or repressor was confirmed in reporter assays: p110 CUX1 activated the promoters of the vimentin and N-cadherin genes, but repressed the promoters of the E-cadherin and occludin genes. In MDCK epithelial kidney cells, the regulation of these genes by p110 CUX1 led to a dramatic reduction of E-cadherin and occludin at cell-cell junctions thereby causing a severe disorganization of cell-cell junctions.2
Figure 1.
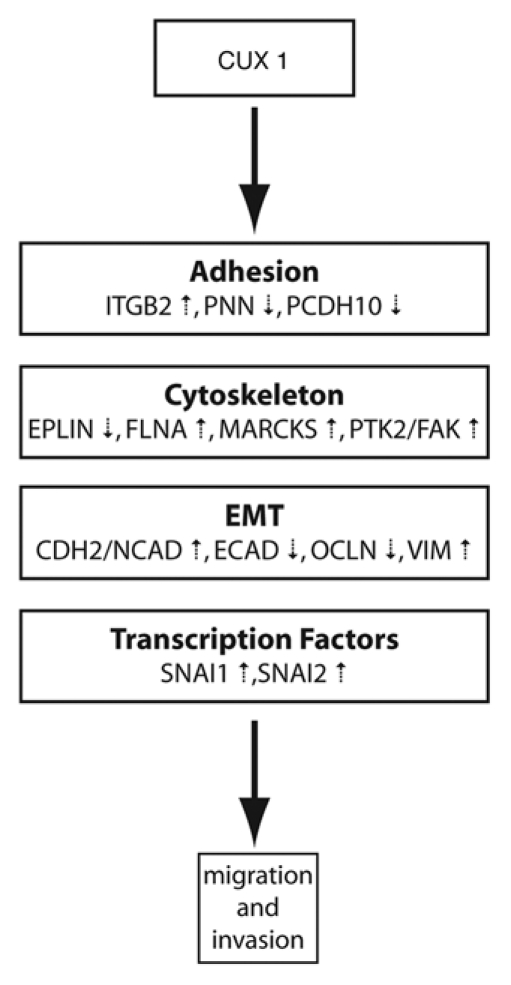
Transcriptional targets of CUX1 that mediates its effect on cell motility. p110 CUX1 regulates distinct classes of genes, either by activating or by repressing their expression, which culminates in increased cell motility and invasion.
Activation and Repression of Transcription by CUX1: How?
CUX1 represses transcription by two distinct mechanisms: competition for binding site occupancy and active repression.13 The carboxy-terminal region of CUX1, which contains two active repression domains, can bind the histone deacetylase HDAC1.14 In addition, CUX1 can recruit the G9a histone lysine methyltransferase.15 No transactivation domain have been identified in CUX1. Therefore, the dual activity of CUX1 as a transcriptional activator or repressor is likely to involve its interaction with different partners on distinct promoters. The regulatory effect would be determined by the nature of the protein complex that is formed on each promoter (Fig. 2). Several reports have documented interactions between CUX1 and other transcription factors and co-factors. Van Wijnen and colleagues demonstrated that CUX1 can associate with pRB on the histone H4 gene promoter whereas it interacts with p107 on a non-histone gene promoter.16 A recombinant p110 CUX1 protein was shown to cooperate with the rITF2 factor to activate the rat tyrosine hydroxylase gene enhancer.17 More recently, p110 CUX1 was found to cooperate with E2F1 and E2F2 in the transcriptional activation of several genes involved in DNA replication and progression through S phase and mitosis.11,18 Chromatin immunoprecipitation assays revealed that CUX1 enhanced the recruitment of E2F factors to the promoter of these genes.
Figure 2.
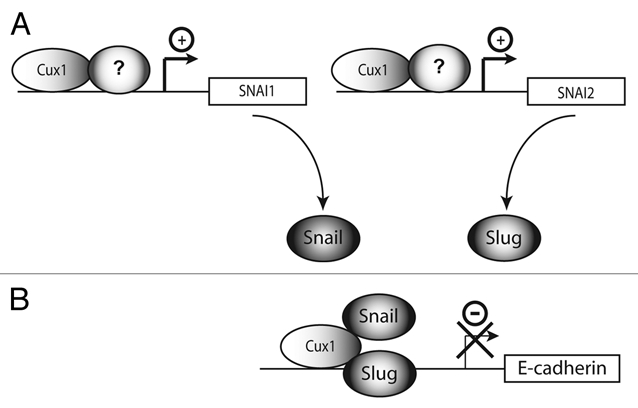
Transcriptional activation and repression by CUX1. p110 and p75 CUX1 can activate or repress transcription depending on promoter context. These opposite regulatory effects can be best explained if we envision that CUX1 interacts with different partners on each class of genes. (A) CUX1 cooperates with yet to be identified transcriptional activators to activate expression of the Snail and Slug genes. (B) CUX1 cooperates with Snail and Slug to repress transcription of the E-Cadherin gene.
Transcriptional Regulatory Cascade from p110 CUX1 to SNAI1/SNAI2 to CDH1
Expression of the E-cadherin gene was found to be reduced in cells stably expressing p110 CUX1. Among transcriptional targets that are activated by p110 CUX1 are two genes that code for the transcription factors SNAI1 (Snail homologue 1) and SNAI2 (Snail homologue 2, also called Slug). SNAI1 and SNAI2 have been characterized as key factors that induce the process of epithelial-mesenchymal transition (EMT) at various developmental stages as well as during tumor invasion.19 One mechanism by which these factors stimulate EMT is through the transcriptional repression of the CDH1 gene which codes for E-cadherin. The finding that p110 CUX1 activates the expression of two factors that repress the E-cadherin gene raised the possibility that p110 CUX1 only indirectly regulates the E-cadherin gene. Yet, this gene had also been identified as a direct target of p110 CUX1. To verify whether p110 CUX1 can directly repress the E-cadherin gene, we tested its effect in a reporter assay using a truncated version of the E-cadherin gene promoter carrying replacement mutations at all E boxes, the consensus binding site for Snail factors.2 Chromatin immunoprecipitation confirmed that Snail did not bind to the mutated version of the reporter plasmid. This reporter was still repressed by p110 CUX1, whereas it was not affected by Snail. However, the wild type E-cadherin gene reporter was repressed by either p110 CUX1, SNAI1 or SNAI2 and greater repression was achieved when p110 CUX1 was co-expressed with SNAI1 or SNAI2. Altogether these results depict a regulatory cascade whereby p110 CUX1 activates the expression of SNAI1 and SNAI2 and then cooperates with these factors in the repression of the E-cadherin and occludin genes (Fig. 2). At the cellular level, transcriptional repression of these genes causes a severe disorganization of cell-cell junctions, as observed in MDCK epithelial kidney cells.2 In parallel, transcriptional activation of the vimentin, N-cadherin and FAK genes by p110 CUX1 contributes to change the cell intermediate filament status to a vimentin-rich network connecting to focal adhesion.
Cellular Processes That Are Commonly Used for Cell Cycling and Cell Motility
In addition to its role in cell migration and invasion, p110 CUX1 has previously been implicated in cell cycle progression.10,20 Cell motility and cell proliferation both require remodeling of the actin cytoskeleton, a process that involves the Rho GTPases proteins Rac, Cdc42 and RhoA.21 These GTPases play a role in cell migration by promoting actin polymerization at the front of migrating cells and by generating contractile forces that move the cell body forward. A role in cell cycle progression has also been demonstrated for Rho GTPases, as inhibition of Rho, Rac or Cdc42 blocks G1 progression in a variety of mammary cell types.21,22 Many effects of Rho GTPases on G1 progression are thought to reflect the crucial role of anchorage- or adhesion-dependent signals necessary for cell proliferation. Genome-wide location arrays identified several targets of CUX1 that code for regulators of Rho GTPases.2 These regulators belong to three categories: guanine nucleotide exchange factors (GEFs) catalyze exchange of GDP for GTP to activate the switch, GTPase-activating proteins (GAPs) stimulate the intrinsic GTPase activity to inactivate the switch and guanine nucleotide dissociation inhibitors (GDIs) block spontaneous activation. In a separate study, Seguin and colleagues identified two other targets of CUX1, Ect2 and MgcRacGAP, that respectively code for a GEF and a GAP.18 CUX1 was shown to cooperate with E2F1 to induce the expression of Ect2 and MgcRacGAP in S phase, leading to a peak of expression in mitosis where these proteins interact with the kinesin KKLP1 to regulate cytokinesis.18 While Ect2 is known to play a role in mitosis, its ortholog Pebble has been shown to regulate cell migration and epithelial-mesenchymal transition, highlighting again the inter-relationship between these two cellular processes.23,24 The importance of transcriptional regulation for Rho GTPases activity was confirmed both from siRNA-mediated knockdown of CUX1 as well as overexpression of p110 CUX1. Stable knockdown of CUX1 in two cell lines caused a reduction in the activity of RhoA, Cdc42 and Rac1.25 In contrast, forced expression of p110 CUX1 lead to an increase in Rac and Cdc42 GTPase activity (Fig. 3). Together these results indicate that two distinct cellular processes, cell migration and cell cycle progression, exploit the regulatory properties of CUX1 to control the expression and activity of effector molecules that play a role in cytoskeleton remodeling.
Figure 3.
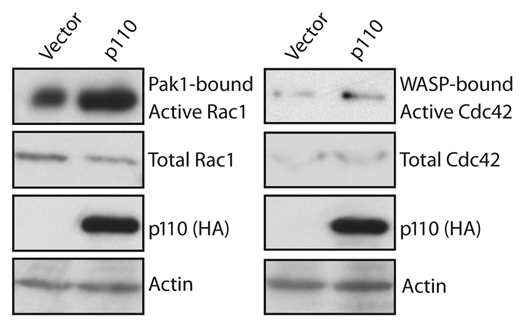
Rho GTPase activity assays. Lysates from NMuMG-NYPD/vector or NMuMG-NYPD/p110 cells were incubated with GST fusion protein containing the binding domain of Rac1 or Cdc42 and bound to Glutathione Sepharose beads. Active Rac1 and Cdc42 specifically bound to the beads were eluted in Laemmli sample buffer and analyzed by western blotting using anti-Rac1 or anti-Cdc42 antibodies. The amount of total protein was determined by analysis of an aliquot of total lysate, and equal loading was verified with actin.
Another Target of CUX1, p27kip1, Plays a Dual Role in Cell Proliferation and Migration
Another known target of CUX1 that has been implicated in both cell cycle progression and motility is the cyclin dependent kinase inhibitor p27kip126–28 While the role of p27kip1 as an inhibitor of cell cycle progression is well established, its regulatory function in cell motility is still debated. Several studies convincingly demonstrated that a portion of p27kip1 protein relocalizes from the nucleus to the cytoplasm where it inhibits RhoA activity by preventing its interaction with GEFs.29–33 These findings led the authors to propose that p27kip1 functions as a tumor suppressor in the nucleus by inhibiting cyclin-dependent kinases, whereas in the cytoplasm p27kip1 functions as an oncogene by regulating the cytoskeleton structure to enable cell migration.34 Other studies, however, found that p27kip1 depletion in glioblastoma and fibroblastic cell lines was associated with increased cell migration.35,36 It remains to be verified whether the role of CUX1 in cell motility also involves its function as a regulator of p27kip1 expression.
Concluding Remarks
Cell migration and invasion are complex processes that take place during embryonic morphogenesis, tissue repair and regeneration. However, aberrant activation of these processes in cancer cells can also contribute to cancer progression and cell dissemination necessary for metastasis. Cell-based assays provided the demonstration not only that CUX1 is required for cell migration and invasion but also that elevated CUX1 expression as seen in cancer cells can increase the migratory properties of cells. Genome-wide location arrays and expression studies showed that CUX1 plays an important role in the establishment of a transcriptional program that enables cell movements. To do so, short CUX1 isoforms cooperate with other transcription factors to regulate a large number of genes that belong to various functional categories.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/11407 DOI: 10.4161/cam.4.3.11407
References
- 1.Michl P, Ramjaun AR, Pardo OE, Warne PH, Wagner M, Poulsom R, et al. CUTL1 is a target of TGF(beta) signaling that enhances cancer cell motility and invasiveness. Cancer Cell. 2005;7:521–532. doi: 10.1016/j.ccr.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 2.Kedinger V, Sansregret L, Harada R, Vadnais C, Cadieux C, Fathers K, et al. p110 CUX1 homeodomain protein stimulates cell migration and invasion in part through a regulatory cascade culminating in the repression of E-cadherin and occludin. J Biol Chem. 2009;284:27701–27711. doi: 10.1074/jbc.M109.031849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sansregret L, Nepveu A. The multiple roles of CUX1: Insights from mouse models and cell-based assays. Gene. 2008;412:84–94. doi: 10.1016/j.gene.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Truscott M, Denault JB, Goulet B, Leduy L, Salvesen GS, Nepveu A. Carboxyl-terminal proteolytic processing of CUX1 by a caspase enables transcriptional activation in proliferating cells. J Biol Chem. 2007;282:30216–30226. doi: 10.1074/jbc.M702328200. [DOI] [PubMed] [Google Scholar]
- 5.Vandenheuvel GB, Quaggin SE, Igarashi P. A unique variant of a homeobox gene related to Drosophila cut is expressed in mouse testis. Biol Reprod. 1996;55:731–739. doi: 10.1095/biolreprod55.4.731. [DOI] [PubMed] [Google Scholar]
- 6.Goulet B, Watson P, Poirier M, Leduy L, Berube G, Meterissian S, et al. Characterization of a tissue-specific CDP/Cux isoform, p75, activated in breast tumor cells. Cancer Res. 2002;62:6625–6633. [PubMed] [Google Scholar]
- 7.Goulet B, Baruch A, Moon NS, Poirier M, Sansregret LL, Erickson A, et al. A cathepsin L isoform that is devoid of a signal peptide localizes to the nucleus in S phase and processes the CDP/Cux transcription factor. Mol Cell. 2004;14:207–219. doi: 10.1016/s1097-2765(04)00209-6. [DOI] [PubMed] [Google Scholar]
- 8.Moon NS, Premdas P, Truscott M, Leduy L, Berube G, Nepveu A. S phase-specific proteolytic cleavage is required to activate stable DNA binding by the CDP/Cut homeodomain protein. Mol Cell Biol. 2001;21:6332–6345. doi: 10.1128/MCB.21.18.6332-6345.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maitra U, Seo J, Lozano MM, Dudley JP. Differentiation-induced cleavage of Cutl1/CDP generates a novel dominant-negative isoform that regulates mammary gene expression. Mol Cell Biol. 2006;26:7466–7478. doi: 10.1128/MCB.01083-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harada R, Vadnais C, Sansregret L, Leduy L, Berube G, Robert F, et al. Genome-wide location analysis and expression studies reveal a role for p110 CUX1 in the activation of DNA replication genes. Nucleic Acids Res. 2008;36:189–202. doi: 10.1093/nar/gkm970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Truscott M, Harada R, Vadnais C, Nepveu A. The p110 isoform of CUX1 cooperates with E2F transcription factors in the transcriptional activation of cell cycle-regulated gene promoters. Molecular and Cellular Biology. 2008;28:3127–3138. doi: 10.1128/MCB.02089-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cadieux C, Kedinger V, Yao L, Vadnais C, Drossos M, Paquet M, et al. Mouse mammary tumor virus p75 and p110 CUX1 transgenic mice develop mammary tumors of various histologic types. Cancer Res. 2009;69:7188–7197. doi: 10.1158/0008-5472.CAN-08-4899. [DOI] [PubMed] [Google Scholar]
- 13.Mailly F, Berube G, Harada R, Mao PL, Phillips S, Nepveu A. The human cut homeodomain protein can repress gene expression by two distinct mechanisms: active repression and competition for binding site occupancy. Mol Cell Biol. 1996;16:5346–5357. doi: 10.1128/mcb.16.10.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S, Moy L, Pittman N, Shue G, Aufiero B, Neufeld EJ, et al. Transcriptional repression of the cystic fibrosis transmembrane conductance regulator gene, mediated by CCAAT displacement protein/cut homolog, is associated with histone deacetylation. J Biol Chem. 1999;274:7803–7815. doi: 10.1074/jbc.274.12.7803. [DOI] [PubMed] [Google Scholar]
- 15.Nishio H, Walsh MJ. CCAAT displacement protein/cut homolog recruits G9a histone lysine methyltransferase to repress transcription. Proc Natl Acad Sci USA. 2004;101:11257–11262. doi: 10.1073/pnas.0401343101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Wijnen AJ, van Gurp MF, de Ridder MC, Tufarelli C, Last TJ, Birnbaum M, et al. CDP/cut is the DNA-binding subunit of histone gene transcription factor HiNF-D: a mechanism for gene regulation at the G1/S phase cell cycle transition point independent of transcription factor E2F. Proc Natl Acad Sci USA. 1996;93:11516–11521. doi: 10.1073/pnas.93.21.11516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon SO, Chikaraishi DM. Isolation of two E-box binding factors that interact with the rat tyrosine hydroxylase enhancer. J Biol Chem. 1994;269:18453–18462. [PubMed] [Google Scholar]
- 18.Seguin L, Liot C, Mzali R, Harada R, Siret A, Nepveu A, et al. CUX1 and E2F1 regulate coordinated expression of the mitotic complex genes Ect2, MgcRacGAP and MKLP1 in S phase. Mol Cell Biol. 2009;29:570–581. doi: 10.1128/MCB.01275-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 20.Sansregret L, Goulet B, Harada R, Wilson B, Leduy L, Bertoglio J, et al. The p110 isoform of the CDP/Cux transcription factor accelerates entry into S phase. Mol Cell Biol. 2006;26:2441–2455. doi: 10.1128/MCB.26.6.2441-2455.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaffe AB, Hall A. Rho GTPases: Biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 22.Olson MF, Ashworth A, Hall A. An essential role for Rho, Rac and Cdc42 GTPases in cell cycle progression through G1. Science. 1995;269:1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- 23.Schumacher S, Gryzik T, Tannebaum S, Muller HA. The RhoGEF Pebble is required for cell shape changes during cell migration triggered by the Drosophila FGF receptor Heartless. Development. 2004;131:2631–2640. doi: 10.1242/dev.01149. [DOI] [PubMed] [Google Scholar]
- 24.Smallhorn M, Murray MJ, Saint R. The epithelial-mesenchymal transition of the Drosophila mesoderm requires the Rho GTP exchange factor Pebble. Development. 2004;131:2641–2651. doi: 10.1242/dev.01150. [DOI] [PubMed] [Google Scholar]
- 25.Aleksic T, Bechtel M, Krndija D, von Wichert G, Knobel B, Giehl K, et al. CUTL1 promotes tumor cell migration by decreasing proteasome-mediated Src degradation. Oncogene. 2007;26:5939–5949. doi: 10.1038/sj.onc.1210398. [DOI] [PubMed] [Google Scholar]
- 26.Alcalay NI, Sharma M, Vassmer D, Chapman B, Paul B, Zhou J, et al. Acceleration of polycystic kidney disease progression in cpk mice carrying a deletion in the homeodomain protein Cux1. Am J Physiol. 2008;295:1725–1734. doi: 10.1152/ajprenal.90420.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma M, Brantley JG, Vassmer D, Chaturvedi G, Baas J, Vanden Heuvel GB. The homeodomain protein Cux1 interacts with Grg4 to repress p27 kip1 expression during kidney development. Gene. 2009;439:87–94. doi: 10.1016/j.gene.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cadieux C, Fournier S, Peterson AC, Bédard C, Bedell BJ, Nepveu A. Transgenic mice expressing the p75 CCAAT-displacement protein/cut homeobox isoform develop a myeloproliferative disease-like myeloid Leukemia. Cancer Res. 2006;66:9492–9501. doi: 10.1158/0008-5472.CAN-05-4230. [DOI] [PubMed] [Google Scholar]
- 29.Besson A, Gurian-West M, Chen X, Kelly-Spratt KS, Kemp CJ, Roberts JM. A pathway in quiescent cells that controls p27Kip1 stability, subcellular localization and tumor suppression. Genes Dev. 2006;20:47–64. doi: 10.1101/gad.1384406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Besson A, Gurian-West M, Schmidt A, Hall A, Roberts JM. p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev. 2004;18:862–876. doi: 10.1101/gad.1185504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Larrea MD, Hong F, Wander SA, da Silva TG, Helfman D, Lannigan D, et al. RSK1 drives p27Kip1 phosphorylation at T198 to promote RhoA inhibition and increase cell motility. Proc Natl Acad Sci USA. 2009;106:9268–9273. doi: 10.1073/pnas.0805057106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee EY, Jeon MJ, Yang W, Shin I. Effect of p27 on motility of MDA-MB-231 breast cancer cells. Oncol Rep. 2009;21:1621–1625. doi: 10.3892/or_00000396. [DOI] [PubMed] [Google Scholar]
- 33.Wang XQ, Lui EL, Cai Q, Ching WY, Liu KS, Poon RT, et al. p27Kip1 promotes migration of metastatic hepatocellular carcinoma cells. Tumour Biol. 2008;29:217–223. doi: 10.1159/000152939. [DOI] [PubMed] [Google Scholar]
- 34.Besson A, Assoian RK, Roberts JM. Regulation of the cytoskeleton: an oncogenic function for CDK inhibitors? Nat Rev Cancer. 2004;4:948–955. doi: 10.1038/nrc1501. [DOI] [PubMed] [Google Scholar]
- 35.Berton S, Belletti B, Wolf K, Canzonieri V, Lovat F, Vecchione A, et al. The tumor suppressor functions of p27(kip1) include control of the mesenchymal/amoeboid transition. Mol Cell Biol. 2009;29:5031–5045. doi: 10.1128/MCB.00144-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schiappacassi M, Lovat F, Canzonieri V, Belletti B, Berton S, Di Stefano D, et al. p27Kip1 expression inhibits glioblastoma growth, invasion and tumor-induced neoangiogenesis. Mol Cancer Ther. 2008;7:1164–1175. doi: 10.1158/1535-7163.MCT-07-2154. [DOI] [PubMed] [Google Scholar]


