Abstract
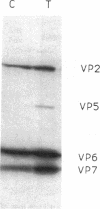
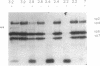
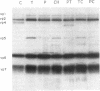
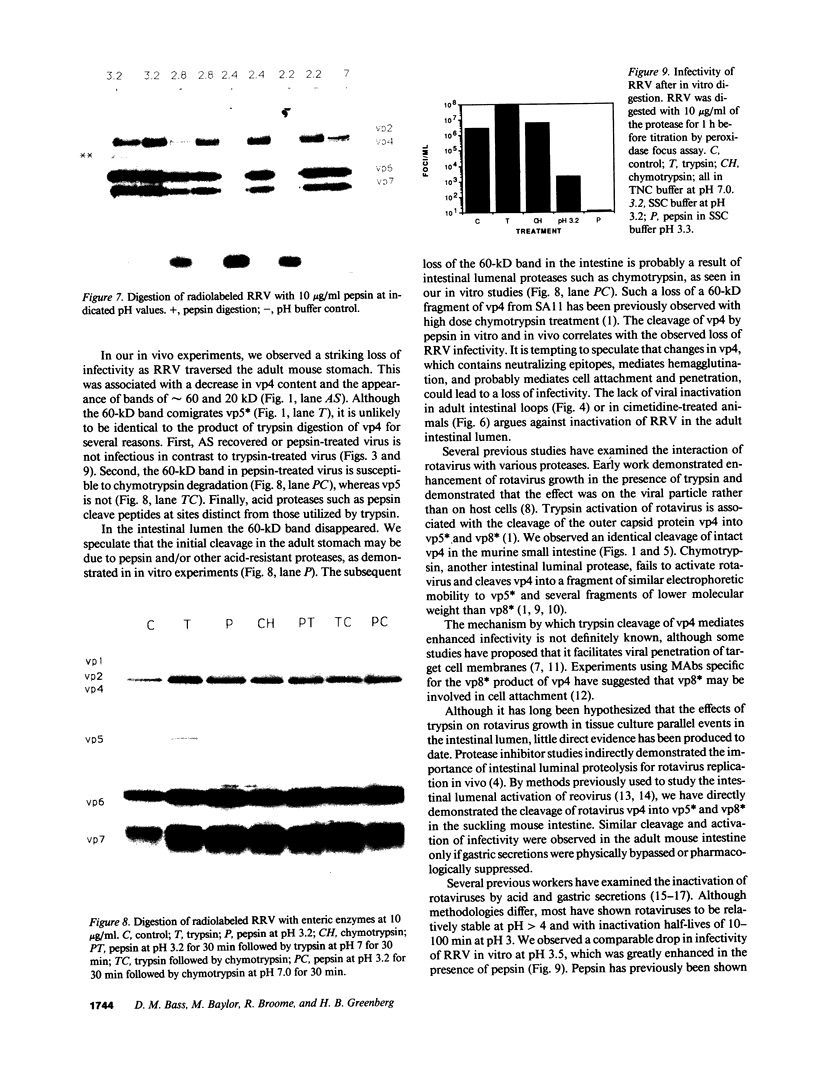
Rotavirus requires specific proteolytic activation by trypsin for efficient replication in tissue culture. To observe the nature of intestinal proteolytic activation of rotavirus in vivo, metabolically labeled rhesus rotavirus (RRV) grown in the presence of trypsin inhibitors was administered to adult and 10-d-old suckling mice by gavage. In the adult stomach, vp4 was cleaved in a manner distinct from in vitro trypsin cleavage. In the suckling stomach, RRV vp4 remains largely uncleaved. The alternative cleavage in the adult stomach was associated with a profound decrease in viral infectivity. vp4 from RRV recovered from the suckling small intestinal lumen was cleaved in a pattern similar or identical to in vitro trypsin-activated virus with bands comigrating with vp5* and vp8*. In contrast, vp4 was not observed in any recognizable form in RRV recovered from adult intestines. Comparison of infectivity of virus recovered from suckling and adult intestines revealed a 10,000-fold decrease in titer in the virus recovered from the adult intestine. In vitro digestions of RRV revealed that pepsin digestion can cleave RRV vp4 and markedly enhance acid-induced loss of rotavirus infectivity. Subsequent digestion with chymotrypsin removes most of the pepsin cleavage products of vp4. Virus injected directly into jejunal loops of adult mice and virus administered orally to adult mice pretreated with antiacid drugs retained infectivity. These studies indicate the development of gastric acid and pepsin secretion may be an important host defense factor in rotavirus gastroenteritis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass D. M., Bodkin D., Dambrauskas R., Trier J. S., Fields B. N., Wolf J. L. Intraluminal proteolytic activation plays an important role in replication of type 1 reovirus in the intestines of neonatal mice. J Virol. 1990 Apr;64(4):1830–1833. doi: 10.1128/jvi.64.4.1830-1833.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass D. M., Mackow E. R., Greenberg H. B. Identification and partial characterization of a rhesus rotavirus binding glycoprotein on murine enterocytes. Virology. 1991 Aug;183(2):602–610. doi: 10.1016/0042-6822(91)90989-o. [DOI] [PubMed] [Google Scholar]
- Bass D. M., Mackow E. R., Greenberg H. B. NS35 and not vp7 is the soluble rotavirus protein which binds to target cells. J Virol. 1990 Jan;64(1):322–330. doi: 10.1128/jvi.64.1.322-330.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodkin D. K., Nibert M. L., Fields B. N. Proteolytic digestion of reovirus in the intestinal lumens of neonatal mice. J Virol. 1989 Nov;63(11):4676–4681. doi: 10.1128/jvi.63.11.4676-4681.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. M., Roth J. R., Clark M. L., Barnett B. B., Spendlove R. S. Trypsin enhancement of rotavirus infectivity: mechanism of enhancement. J Virol. 1981 Sep;39(3):816–822. doi: 10.1128/jvi.39.3.816-822.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Mason B. B. Proteolytic enhancement of rotavirus infectivity: molecular mechanisms. J Virol. 1981 Sep;39(3):879–888. doi: 10.1128/jvi.39.3.879-888.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Smith E. M., Gerba C. P. Rotavirus stability and inactivation. J Gen Virol. 1979 May;43(2):403–409. doi: 10.1099/0022-1317-43-2-403. [DOI] [PubMed] [Google Scholar]
- Gilman R. H., Partanen R., Brown K. H., Spira W. M., Khanam S., Greenberg B., Bloom S. R., Ali A. Decreased gastric acid secretion and bacterial colonization of the stomach in severely malnourished Bangladeshi children. Gastroenterology. 1988 Jun;94(6):1308–1314. doi: 10.1016/0016-5085(88)90668-3. [DOI] [PubMed] [Google Scholar]
- Gorziglia M., Hoshino Y., Buckler-White A., Blumentals I., Glass R., Flores J., Kapikian A. Z., Chanock R. M. Conservation of amino acid sequence of VP8 and cleavage region of 84-kDa outer capsid protein among rotaviruses recovered from asymptomatic neonatal infection. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7039–7043. doi: 10.1073/pnas.83.18.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracey M. Intestinal microflora and bacterial overgrowth in early life. J Pediatr Gastroenterol Nutr. 1982;1(1):13–22. doi: 10.1097/00005176-198201010-00005. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Dufour G. R., Estes M. K. Minimal infective dose of rotavirus. Arch Virol. 1987;92(3-4):261–271. doi: 10.1007/BF01317483. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Valdesuso J., van Wyke K., Midthun K., Walsh M., McAuliffe V., Wyatt R. G., Kalica A. R., Flores J., Hoshino Y. Production and preliminary characterization of monoclonal antibodies directed at two surface proteins of rhesus rotavirus. J Virol. 1983 Aug;47(2):267–275. doi: 10.1128/jvi.47.2.267-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaljot K. T., Shaw R. D., Rubin D. H., Greenberg H. B. Infectious rotavirus enters cells by direct cell membrane penetration, not by endocytosis. J Virol. 1988 Apr;62(4):1136–1144. doi: 10.1128/jvi.62.4.1136-1144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaoka S., Suzuki H., Sato T., Numazaki Y., Konno T., Ishida N. Failure in chymotrypsin enhancement of human rotavirus infectivity. Tohoku J Exp Med. 1987 Jan;151(1):127–128. doi: 10.1620/tjem.151.127. [DOI] [PubMed] [Google Scholar]
- Mackow E. R., Shaw R. D., Matsui S. M., Vo P. T., Dang M. N., Greenberg H. B. The rhesus rotavirus gene encoding protein VP3: location of amino acids involved in homologous and heterologous rotavirus neutralization and identification of a putative fusion region. Proc Natl Acad Sci U S A. 1988 Feb;85(3):645–649. doi: 10.1073/pnas.85.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno S., Inouye S., Kono R. Plaque assay of neonatal calf diarrhea virus and the neutralizing antibody in human sera. J Clin Microbiol. 1977 Jan;5(1):1–4. doi: 10.1128/jcm.5.1.1-4.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichichero M. E., Losonsky G. A., Rennels M. B., Disney F. A., Green J. L., Francis A. B., Marsocci S. M. Effect of dose and a comparison of measures of vaccine take for oral rhesus rotavirus vaccine. The Maryland Clinical Studies Group. Pediatr Infect Dis J. 1990 May;9(5):339–344. doi: 10.1097/00006454-199005000-00007. [DOI] [PubMed] [Google Scholar]
- Riepenhoff-Talty M., Lee P. C., Carmody P. J., Barrett H. J., Ogra P. L. Age-dependent rotavirus-enterocyte interactions. Proc Soc Exp Biol Med. 1982 Jun;170(2):146–154. doi: 10.3181/00379727-170-41410. [DOI] [PubMed] [Google Scholar]
- Ruggeri F. M., Greenberg H. B. Antibodies to the trypsin cleavage peptide VP8 neutralize rotavirus by inhibiting binding of virions to target cells in culture. J Virol. 1991 May;65(5):2211–2219. doi: 10.1128/jvi.65.5.2211-2219.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Kitaoka S., Suzuki H., Konno T., Ishida N. Effect of trypsin and chymotrypsin on polypeptides of human rotavirus KUN strain. Med Microbiol Immunol. 1987;176(2):65–73. doi: 10.1007/BF00200676. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Isolauri E., D'Hondt E., Delem A., André F. E. Increased "take" rate of oral rotavirus vaccine in infants after milk feeding. Lancet. 1984 Sep 22;2(8404):700–700. doi: 10.1016/s0140-6736(84)91262-5. [DOI] [PubMed] [Google Scholar]
- Vonderfecht S. L., Miskuff R. L., Wee S. B., Sato S., Tidwell R. R., Geratz J. D., Yolken R. H. Protease inhibitors suppress the in vitro and in vivo replication of rotavirus. J Clin Invest. 1988 Dec;82(6):2011–2016. doi: 10.1172/JCI113821. [DOI] [PMC free article] [PubMed] [Google Scholar]