Abstract
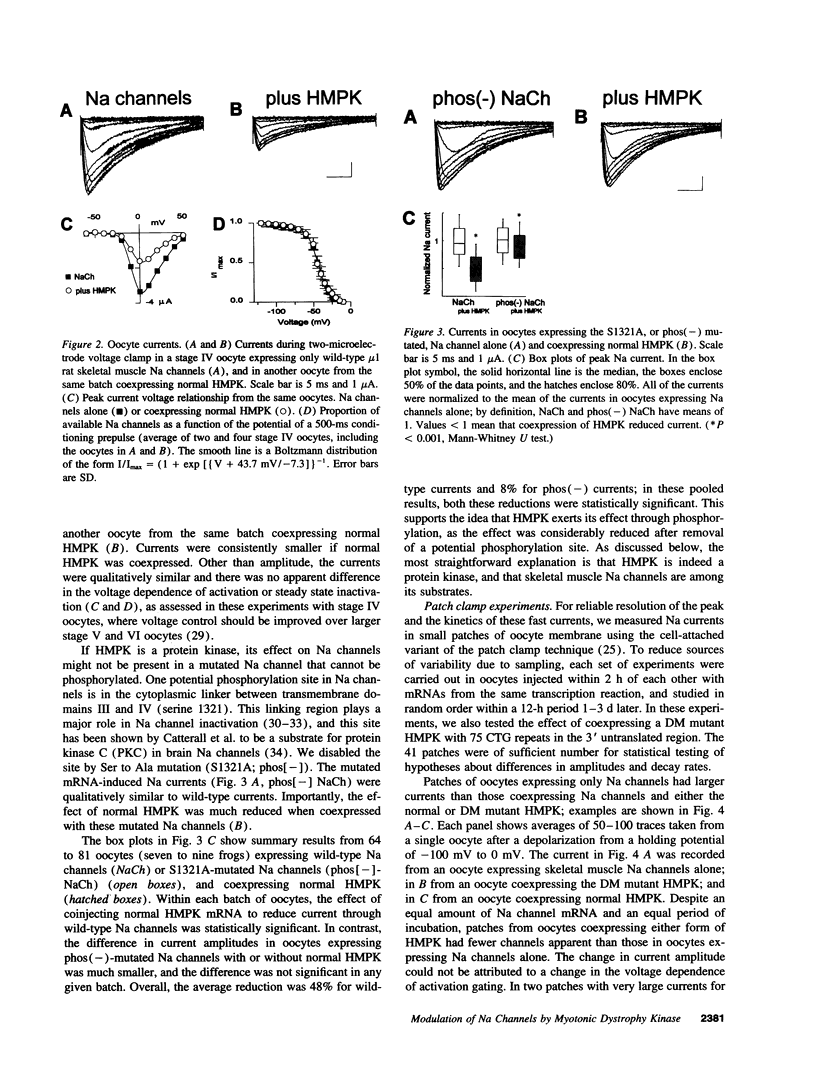
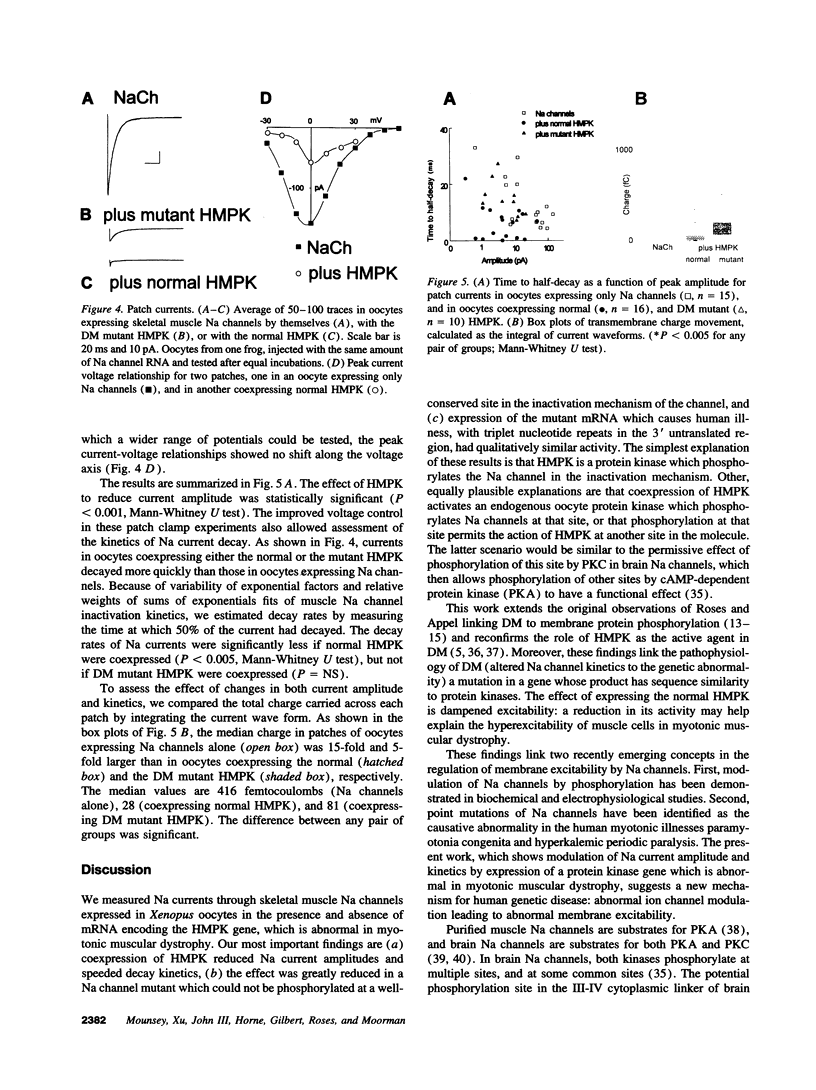
In myotonic muscular dystrophy, abnormal muscle Na currents underlie myotonic discharges. Since the myotonic muscular dystrophy gene encodes a product, human myotonin protein kinase, with structural similarity to protein kinases, we tested the idea that human myotonin protein kinase modulates skeletal muscle Na channels. Coexpression of human myotonin protein kinase with rat skeletal muscle Na channels in Xenopus oocytes reduced the amplitude of Na currents and accelerated current decay. The effect required the presence of a potential phosphorylation site in the inactivation mechanism of the channel. The mutation responsible for human disease, trinucleotide repeats in the 3' untranslated region, did not prevent the effect. The consequence of an abnormal amount of the kinase would be altered muscle cell excitability, consistent with the clinical finding of myotonia in myotonic dystrophy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aslanidis C., Jansen G., Amemiya C., Shutler G., Mahadevan M., Tsilfidis C., Chen C., Alleman J., Wormskamp N. G., Vooijs M. Cloning of the essential myotonic dystrophy region and mapping of the putative defect. Nature. 1992 Feb 6;355(6360):548–551. doi: 10.1038/355548a0. [DOI] [PubMed] [Google Scholar]
- Brewster B. S., Jeal S., Strong P. N. Identification of a protein product of the myotonic dystrophy gene using peptide specific antibodies. Biochem Biophys Res Commun. 1993 Aug 16;194(3):1256–1260. doi: 10.1006/bbrc.1993.1958. [DOI] [PubMed] [Google Scholar]
- Brook J. D., McCurrach M. E., Harley H. G., Buckler A. J., Church D., Aburatani H., Hunter K., Stanton V. P., Thirion J. P., Hudson T. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3' end of a transcript encoding a protein kinase family member. Cell. 1992 Feb 21;68(4):799–808. doi: 10.1016/0092-8674(92)90154-5. [DOI] [PubMed] [Google Scholar]
- Cannon S. C., Brown R. H., Jr, Corey D. P. Theoretical reconstruction of myotonia and paralysis caused by incomplete inactivation of sodium channels. Biophys J. 1993 Jul;65(1):270–288. doi: 10.1016/S0006-3495(93)81045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon S. C., Strittmatter S. M. Functional expression of sodium channel mutations identified in families with periodic paralysis. Neuron. 1993 Feb;10(2):317–326. doi: 10.1016/0896-6273(93)90321-h. [DOI] [PubMed] [Google Scholar]
- Carango P., Noble J. E., Marks H. G., Funanage V. L. Absence of myotonic dystrophy protein kinase (DMPK) mRNA as a result of a triplet repeat expansion in myotonic dystrophy. Genomics. 1993 Nov;18(2):340–348. doi: 10.1006/geno.1993.1474. [DOI] [PubMed] [Google Scholar]
- Caskey C. T., Pizzuti A., Fu Y. H., Fenwick R. G., Jr, Nelson D. L. Triplet repeat mutations in human disease. Science. 1992 May 8;256(5058):784–789. doi: 10.1126/science.1589758. [DOI] [PubMed] [Google Scholar]
- D'Arcangelo G., Paradiso K., Shepherd D., Brehm P., Halegoua S., Mandel G. Neuronal growth factor regulation of two different sodium channel types through distinct signal transduction pathways. J Cell Biol. 1993 Aug;122(4):915–921. doi: 10.1083/jcb.122.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux M. E., Salafranca M. N., Lynch K. R., Moorman J. R. Lysophosphatidic acid induces a pertussis toxin-sensitive Ca(2+)-activated Cl- current in Xenopus laevis oocytes. Am J Physiol. 1992 Oct;263(4 Pt 1):C896–C900. doi: 10.1152/ajpcell.1992.263.4.C896. [DOI] [PubMed] [Google Scholar]
- Franke C., Hatt H., Iaizzo P. A., Lehmann-Horn F. Characteristics of Na+ channels and Cl- conductance in resealed muscle fibre segments from patients with myotonic dystrophy. J Physiol. 1990 Jun;425:391–405. doi: 10.1113/jphysiol.1990.sp018110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y. H., Friedman D. L., Richards S., Pearlman J. A., Gibbs R. A., Pizzuti A., Ashizawa T., Perryman M. B., Scarlato G., Fenwick R. G., Jr Decreased expression of myotonin-protein kinase messenger RNA and protein in adult form of myotonic dystrophy. Science. 1993 Apr 9;260(5105):235–238. doi: 10.1126/science.8469976. [DOI] [PubMed] [Google Scholar]
- Fu Y. H., Pizzuti A., Fenwick R. G., Jr, King J., Rajnarayan S., Dunne P. W., Dubel J., Nasser G. A., Ashizawa T., de Jong P. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992 Mar 6;255(5049):1256–1258. doi: 10.1126/science.1546326. [DOI] [PubMed] [Google Scholar]
- Ginty D. D., Fanger G. R., Wagner J. A., Maue R. A. The activity of cAMP-dependent protein kinase is required at a posttranslational level for induction of voltage-dependent sodium channels by peptide growth factors in PC12 cells. J Cell Biol. 1992 Mar;116(6):1465–1473. doi: 10.1083/jcb.116.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harley H. G., Rundle S. A., Reardon W., Myring J., Crow S., Brook J. D., Harper P. S., Shaw D. J. Unstable DNA sequence in myotonic dystrophy. Lancet. 1992 May 9;339(8802):1125–1128. doi: 10.1016/0140-6736(92)90729-m. [DOI] [PubMed] [Google Scholar]
- Jansen G., Mahadevan M., Amemiya C., Wormskamp N., Segers B., Hendriks W., O'Hoy K., Baird S., Sabourin L., Lennon G. Characterization of the myotonic dystrophy region predicts multiple protein isoform-encoding mRNAs. Nat Genet. 1992 Jul;1(4):261–266. doi: 10.1038/ng0792-261. [DOI] [PubMed] [Google Scholar]
- Kalman D., Wong B., Horvai A. E., Cline M. J., O'Lague P. H. Nerve growth factor acts through cAMP-dependent protein kinase to increase the number of sodium channels in PC12 cells. Neuron. 1990 Mar;4(3):355–366. doi: 10.1016/0896-6273(90)90048-k. [DOI] [PubMed] [Google Scholar]
- Kowdley G. C., Ackerman S. J., John J. E., 3rd, Jones L. R., Moorman J. R. Hyperpolarization-activated chloride currents in Xenopus oocytes. J Gen Physiol. 1994 Feb;103(2):217–230. doi: 10.1085/jgp.103.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krafte D. S., Lester H. A. Expression of functional sodium channels in stage II-III Xenopus oocytes. J Neurosci Methods. 1989 Jan;26(3):211–215. doi: 10.1016/0165-0270(89)90118-0. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., West J. W., Numann R., Murphy B. J., Scheuer T., Catterall W. A. Convergent regulation of sodium channels by protein kinase C and cAMP-dependent protein kinase. Science. 1993 Sep 10;261(5127):1439–1442. doi: 10.1126/science.8396273. [DOI] [PubMed] [Google Scholar]
- Mahadevan M., Tsilfidis C., Sabourin L., Shutler G., Amemiya C., Jansen G., Neville C., Narang M., Barceló J., O'Hoy K. Myotonic dystrophy mutation: an unstable CTG repeat in the 3' untranslated region of the gene. Science. 1992 Mar 6;255(5049):1253–1255. doi: 10.1126/science.1546325. [DOI] [PubMed] [Google Scholar]
- McClatchey A. I., Van den Bergh P., Pericak-Vance M. A., Raskind W., Verellen C., McKenna-Yasek D., Rao K., Haines J. L., Bird T., Brown R. H., Jr Temperature-sensitive mutations in the III-IV cytoplasmic loop region of the skeletal muscle sodium channel gene in paramyotonia congenita. Cell. 1992 Feb 21;68(4):769–774. doi: 10.1016/0092-8674(92)90151-2. [DOI] [PubMed] [Google Scholar]
- Methfessel C., Witzemann V., Takahashi T., Mishina M., Numa S., Sakmann B. Patch clamp measurements on Xenopus laevis oocytes: currents through endogenous channels and implanted acetylcholine receptor and sodium channels. Pflugers Arch. 1986 Dec;407(6):577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- Moorman J. R., Kirsch G. E., Brown A. M., Joho R. H. Changes in sodium channel gating produced by point mutations in a cytoplasmic linker. Science. 1990 Nov 2;250(4981):688–691. doi: 10.1126/science.2173138. [DOI] [PubMed] [Google Scholar]
- Moorman J. R., Kirsch G. E., Lacerda A. E., Brown A. M. Angiotensin II modulates cardiac Na+ channels in neonatal rat. Circ Res. 1989 Dec;65(6):1804–1809. doi: 10.1161/01.res.65.6.1804. [DOI] [PubMed] [Google Scholar]
- Moorman J. R., Kirsch G. E., VanDongen A. M., Joho R. H., Brown A. M. Fast and slow gating of sodium channels encoded by a single mRNA. Neuron. 1990 Feb;4(2):243–252. doi: 10.1016/0896-6273(90)90099-2. [DOI] [PubMed] [Google Scholar]
- Murphy B. J., Catterall W. A. Phosphorylation of purified rat brain Na+ channel reconstituted into phospholipid vesicles by protein kinase C. J Biol Chem. 1992 Aug 15;267(23):16129–16134. [PubMed] [Google Scholar]
- Nilius B., Tytgat J., Albitz R. Modulation of cardiac Na channels by angiotensin II. Biochim Biophys Acta. 1989 Dec 14;1014(3):259–262. doi: 10.1016/0167-4889(89)90221-8. [DOI] [PubMed] [Google Scholar]
- Numann R., Catterall W. A., Scheuer T. Functional modulation of brain sodium channels by protein kinase C phosphorylation. Science. 1991 Oct 4;254(5028):115–118. doi: 10.1126/science.1656525. [DOI] [PubMed] [Google Scholar]
- Pancrazio J. J. PCS: an IBM-compatible microcomputer program for the analysis and display of voltage-clamp data. Comput Methods Programs Biomed. 1993 Jul;40(3):175–180. doi: 10.1016/0169-2607(93)90055-p. [DOI] [PubMed] [Google Scholar]
- Ptacek L. J., Gouw L., Kwieciński H., McManis P., Mendell J. R., Barohn R. J., George A. L., Jr, Barchi R. L., Robertson M., Leppert M. F. Sodium channel mutations in paramyotonia congenita and hyperkalemic periodic paralysis. Ann Neurol. 1993 Mar;33(3):300–307. doi: 10.1002/ana.410330312. [DOI] [PubMed] [Google Scholar]
- Qu Y., Rogers J., Tanada T., Scheuer T., Catterall W. A. Modulation of cardiac Na+ channels expressed in a mammalian cell line and in ventricular myocytes by protein kinase C. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3289–3293. doi: 10.1073/pnas.91.8.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roses A. D., Appel S. H. Muscle membrane protein kinase in myotonic muscular dystrophy. Nature. 1974 Jul 19;250(463):245–247. doi: 10.1038/250245a0. [DOI] [PubMed] [Google Scholar]
- Roses A. D., Appel S. H. Phosphorylation of component a of the human erythrocyte membrane in myotonic muscular dystrophy. J Membr Biol. 1975;20(1-2):51–58. doi: 10.1007/BF01870627. [DOI] [PubMed] [Google Scholar]
- Roses A. D., Appel S. H. Protein kinase activity in erythrocyte ghosts of patients with myotonic muscular dystrophy. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1855–1859. doi: 10.1073/pnas.70.6.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossie S., Gordon D., Catterall W. A. Identification of an intracellular domain of the sodium channel having multiple cAMP-dependent phosphorylation sites. J Biol Chem. 1987 Dec 25;262(36):17530–17535. [PubMed] [Google Scholar]
- Sabouri L. A., Mahadevan M. S., Narang M., Lee D. S., Surh L. C., Korneluk R. G. Effect of the myotonic dystrophy (DM) mutation on mRNA levels of the DM gene. Nat Genet. 1993 Jul;4(3):233–238. doi: 10.1038/ng0793-233. [DOI] [PubMed] [Google Scholar]
- Stühmer W., Conti F., Suzuki H., Wang X. D., Noda M., Yahagi N., Kubo H., Numa S. Structural parts involved in activation and inactivation of the sodium channel. Nature. 1989 Jun 22;339(6226):597–603. doi: 10.1038/339597a0. [DOI] [PubMed] [Google Scholar]
- Trimmer J. S., Cooperman S. S., Tomiko S. A., Zhou J. Y., Crean S. M., Boyle M. B., Kallen R. G., Sheng Z. H., Barchi R. L., Sigworth F. J. Primary structure and functional expression of a mammalian skeletal muscle sodium channel. Neuron. 1989 Jul;3(1):33–49. doi: 10.1016/0896-6273(89)90113-x. [DOI] [PubMed] [Google Scholar]
- Vassilev P. M., Scheuer T., Catterall W. A. Identification of an intracellular peptide segment involved in sodium channel inactivation. Science. 1988 Sep 23;241(4873):1658–1661. doi: 10.1126/science.241.4873.1658. [DOI] [PubMed] [Google Scholar]
- West J. W., Numann R., Murphy B. J., Scheuer T., Catterall W. A. A phosphorylation site in the Na+ channel required for modulation by protein kinase C. Science. 1991 Nov 8;254(5033):866–868. doi: 10.1126/science.1658937. [DOI] [PubMed] [Google Scholar]
- West J. W., Patton D. E., Scheuer T., Wang Y., Goldin A. L., Catterall W. A. A cluster of hydrophobic amino acid residues required for fast Na(+)-channel inactivation. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10910–10914. doi: 10.1073/pnas.89.22.10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Barchi R. Phosphorylation of the rat skeletal muscle sodium channel by cyclic AMP-dependent protein kinase. J Neurochem. 1990 Mar;54(3):954–962. doi: 10.1111/j.1471-4159.1990.tb02343.x. [DOI] [PubMed] [Google Scholar]
- Yang X. C., Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1989 Feb 24;243(4894 Pt 1):1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]



