Abstract
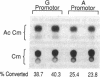
We investigated a common polymorphism in the human apolipoprotein A-I gene promoter at a position 76 bp upstream of the transcriptional start site. 54 human subjects, whose apoAI production rates had been determined by apoAI turnover studies, were genotyped at this polymorphic position by a novel technique using polymerase chain reaction followed by primer extension. 35 subjects were homozygous for a guanosine (G) at this locus and 19 were heterozygous with a guanosine and adenosine (A). The apoAI production rates were significantly lower (by 11%) in the G/A heterozygotes than in the G homozygotes (P = 0.025). In spite of the apparent effect of this apoAI gene promoter polymorphism on the apoAI production rate, there was no effect on HDL cholesterol or apoAI levels. To investigate whether the observed difference in apoAI production rates was related to differential gene expression of the two alleles, promoters containing either allele were linked to the reporter gene chloramphenicol acetyltransferase, and relative promoter efficiencies were determined after transfection into the human HepG2 hepatoma cell line. The A allele expressed only 68% +/- 5% as well as the G allele, a result consistent with the in vivo apoAI production rate data.
Full text
PDF
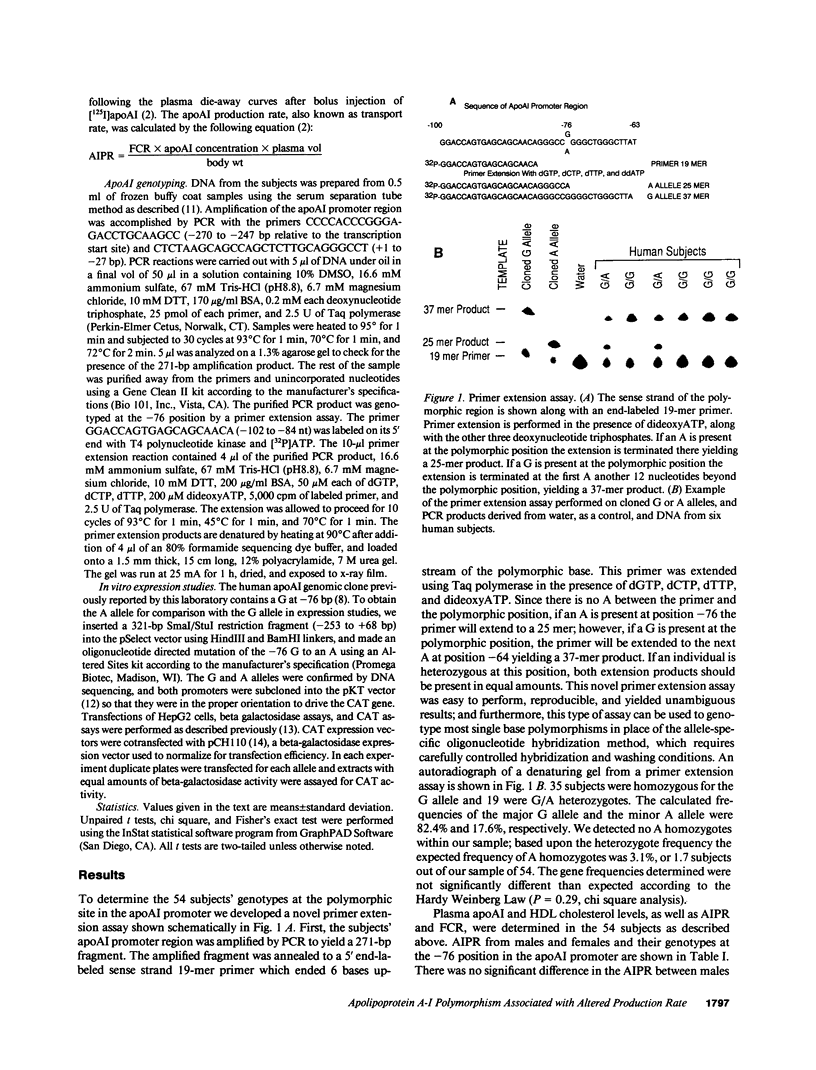
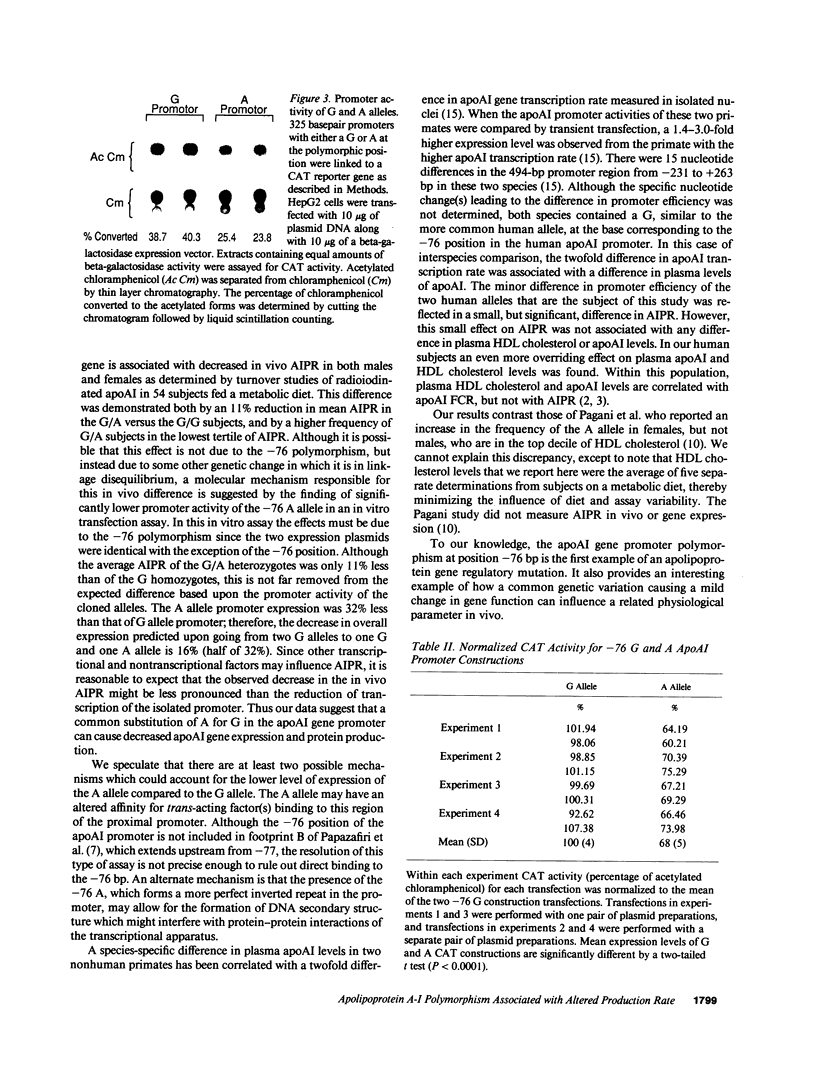

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinton E. A., Eisenberg S., Breslow J. L. A low-fat diet decreases high density lipoprotein (HDL) cholesterol levels by decreasing HDL apolipoprotein transport rates. J Clin Invest. 1990 Jan;85(1):144–151. doi: 10.1172/JCI114405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton E. A., Eisenberg S., Breslow J. L. Elevated high density lipoprotein cholesterol levels correlate with decreased apolipoprotein A-I and A-II fractional catabolic rate in women. J Clin Invest. 1989 Jul;84(1):262–269. doi: 10.1172/JCI114149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton E. A., Eisenberg S., Breslow J. L. Increased apo A-I and apo A-II fractional catabolic rate in patients with low high density lipoprotein-cholesterol levels with or without hypertriglyceridemia. J Clin Invest. 1991 Feb;87(2):536–544. doi: 10.1172/JCI115028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. V., Jacob P. E., Ringold G. M., Lee F. Expression and regulation of Escherichia coli lacZ gene fusions in mammalian cells. J Mol Appl Genet. 1983;2(1):101–109. [PubMed] [Google Scholar]
- Karathanasis S. K., Norum R. A., Zannis V. I., Breslow J. L. An inherited polymorphism in the human apolipoprotein A-I gene locus related to the development of atherosclerosis. Nature. 1983 Feb 24;301(5902):718–720. doi: 10.1038/301718a0. [DOI] [PubMed] [Google Scholar]
- Karathanasis S. K., Zannis V. I., Breslow J. L. Isolation and characterization of the human apolipoprotein A-I gene. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6147–6151. doi: 10.1073/pnas.80.20.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. J., Miller N. E. Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet. 1975 Jan 4;1(7897):16–19. doi: 10.1016/s0140-6736(75)92376-4. [DOI] [PubMed] [Google Scholar]
- Moreno R. F., Booth F. R., Hung I. H., Tilzer L. L. Efficient DNA isolation within a single gel barrier tube. Nucleic Acids Res. 1989 Oct 25;17(20):8393–8393. doi: 10.1093/nar/17.20.8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani F., Sidoli A., Giudici G. A., Barenghi L., Vergani C., Baralle F. E. Human apolipoprotein A-I gene promoter polymorphism: association with hyperalphalipoproteinemia. J Lipid Res. 1990 Aug;31(8):1371–1377. [PubMed] [Google Scholar]
- Papazafiri P., Ogami K., Ramji D. P., Nicosia A., Monaci P., Cladaras C., Zannis V. I. Promoter elements and factors involved in hepatic transcription of the human ApoA-I gene positive and negative regulators bind to overlapping sites. J Biol Chem. 1991 Mar 25;266(9):5790–5797. [PubMed] [Google Scholar]
- Reue K., Leff T., Breslow J. L. Human apolipoprotein CIII gene expression is regulated by positive and negative cis-acting elements and tissue-specific protein factors. J Biol Chem. 1988 May 15;263(14):6857–6864. [PubMed] [Google Scholar]
- Shoulders C. C., Kornblihtt A. R., Munro B. S., Baralle F. E. Gene structure of human apolipoprotein A1. Nucleic Acids Res. 1983 May 11;11(9):2827–2837. doi: 10.1093/nar/11.9.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. D., Melián A., Leff T., Breslow J. L. Expression of the human apolipoprotein E gene is regulated by multiple positive and negative elements. J Biol Chem. 1988 Jun 15;263(17):8300–8308. [PubMed] [Google Scholar]
- Sorci-Thomas M., Kearns M. W. Transcriptional regulation of the apolipoprotein A-I gene. Species-specific expression correlates with rates of gene transcription. J Biol Chem. 1991 Sep 25;266(27):18045–18050. [PubMed] [Google Scholar]
- Walsh A., Ito Y., Breslow J. L. High levels of human apolipoprotein A-I in transgenic mice result in increased plasma levels of small high density lipoprotein (HDL) particles comparable to human HDL3. J Biol Chem. 1989 Apr 15;264(11):6488–6494. [PubMed] [Google Scholar]