Abstract
Background and Aims
Nickel (Ni) hyperaccumulation is a rare form of physiological specialization shared by a small number of angiosperms growing on ultramafic soils. The evolutionary patterns of this feature among European members of tribe Alysseae (Brassicaceae) are investigated using a phylogenetic approach to assess relationships among Ni hyperaccumulators at the genus, species and below-species level.
Methods
Internal transcribed spacer (ITS) sequences were generated for multiple accessions of Alysseae. Phylogenetic trees were obtained for the genera of the tribe and Alyssum sect. Odontarrhena. All accessions and additional herbarium material were tested for Ni hyperaccumulation with the dimethylglyoxime colorimetric method.
Key Results
Molecular data strongly support the poorly known hyperaccumulator endemic Leptoplax (Peltaria) emarginata as sister to hyperaccumulator species of Bornmuellera within Alysseae. This is contrary to current assumptions of affinity between L. emarginata and the non-hyperaccumulator Peltaria in Thlaspideae. The lineage Bornmuellera–Leptoplax is, in turn, sister to the two non-hyperaccumulator Mediterranean endemics Ptilotrichum rupestre and P. cyclocarpum. Low ITS sequence variation was found within the monophyletic Alyssum sect. Odontarrhena and especially in A. murale sensu lato. Nickel hyperaccumulation was not monophyletic in any of three main clades retrieved, each consisting of hyperaccumulators and non-hyperaccumulators of different geographical origin.
Conclusions
Nickel hyperaccumulation in Alysseae has a double origin, but it did not evolve in Thlaspideae. In Bornmuellera–Leptoplax it represents an early synapomorphy inherited from an ancestor shared with the calcicolous, sister clade of Mediterranean Ptilotrichum. In Alyssum sect. Odontarrhena it has multiple origins even within the three European clades recognized. Lack of geographical cohesion suggests that accumulation ability has been lost or gained over the different serpentine areas of south Europe through independent events of microevolutionary adaptation and selection. Genetic continuity and strong phenotypic plasticity in the A. murale complex call for a reduction of the number of Ni hyperaccumulator taxa formally recognized.
Keywords: Alysseae, Alyssum sect, Odontarrhena, Bornmuellera, Brassicaceae, Leptoplax, molecular phylogeny, Ni hyperaccumulation
INTRODUCTION
Nickel (Ni) hyperaccumulation is the ability to uptake and store >1000 µg of Ni g−1 of leaf dry weight without toxicity symptoms, a relatively rare physiological trait shared by 340–390 plant taxa growing on metalliferous soils (Brooks et al., 1977; Reeves and Baker, 2000; Reeves, 2003; Reeves et al., 2007; Reeves and Adigüzel, 2008; Verbruggen et al., 2009; Krämer, 2010). This ability occurs in approx. 42 unrelated angiosperm families and is certainly of polyphyletic origin (Macnair, 2003; Krämer, 2010). Although most hyperaccumulators are endemic to Ni-rich serpentine soils, some species include both serpentine and non-serpentine populations, suggesting the lack of a strict Ni dependence but, rather, the capacity to take competitive advantage over other species within the local community (Reeves and Baker, 1984; Brooks, 1987). The ability for Ni accumulation is in fact mostly interpreted as an adaptive trait that serves as a herbivore deterrent against insects and pathogens and an allelopathic factor (Boyd and Martens, 1998; Boyd, 2004; Brady et al., 2005; Kazakou et al., 2008).
Metal hyperaccumulators have recently gained considerable interest because of their potential use in phytoremediation (Chaney et al., 2005; reviewed by Pilon-Smits, 2005), phytomining (Li et al., 2003) and food crop biofortification (Broadley et al., 2007; Palmgren et al., 2008). They also constitute an exceptional biological material and gene reservoir that can be exploited to understand metal homoeostasis and adaptation to extremely hostile environments. Classical physiological studies have recently been complemented by molecular research aiming at the identification, expression and transcription of functional genes in hyperaccumulators as compared with related control species (reviewed by Verbruggen et al., 2009). Such an approach implies the importance of phylogenetic evidence in selecting appropriate model systems including accumulating and non-accumulating taxa of clear evolutionary affinity. Indeed, mechanisms for the transport of metals across cell membranes and for their chelation in cell sap can be both specific and generic and thus subject to evolutionary constraints. Also, the differential expression of transport or chelating proteins may account for phylogenetic differences in metal accumulation between clades (Broadley et al., 2001). According to Krämer (2010), the clarification of the phylogenetic position and taxonomy of metal hyperaccumulator taxa will be necessary for comparative genomics and to prioritize among possible future model metal hyperaccumulators. However, only a few studies have explored the phylogenetic patterns of metal accumulation at the macroevolutionary scale, i.e. the species level or above (Broadley et al., 2001; Jansen et al., 2002, 2004; Mengoni et al., 2003).
Most molecular research is currently carried out using a few members of the family Brassicaceae (order Brassicales, malvids clade of APG III, 2009) as model systems, especially Arabidopsis halleri and Noccaea (‘Thlaspi’) caerulescens (Verbruggen et al., 2009; Krämer, 2010). The remarkable proportion (approx. 2 %) of hyperaccumulators in the family (Table 1), however, offers still unexploited opportunities to investigate the expression, regulation and evolution of the genetic traits underlying metal accumulation. The search for new model systems in Brassicaceae consisting of taxa of clear phylogenetic affinity is one of the crucial aspects in current research on metal accumulation (Assunção et al., 2003; Peer et al., 2003, 2006). In spite of this, the evolutionary patterns of this ability in the family are still incompletely known.
Table 1.
List of known Ni hyperaccumulator tribes and genera of Brassicaceae, based on the most recent comprehensive treatment of the family (Koch and Al-Shehbaz, 2009)
| Taxa | Phytogeographical region | Literature sources* |
|---|---|---|
| Tribe Aethionemeae (1) | ||
| Aethionema (1) | Mediterranean | 16 |
| Tribe Alysseae (approx. 55) | ||
| Alyssum (approx. 50) | Circumboreal, Mediterranean, Irano-Turanian | 1–5, 7, 16–18 |
| Bornmuellera (5) | Euro-Mediterranean, Irano-Turanian | 11, 19 |
| Tribe Cardamineae (1) | ||
| Cardamine (1) | European | 6 |
| Tribe Noccaeeae (24) | ||
| Masmenia (1) | Irano-Turanian | 12 |
| Microthlaspi (1) | Euro-Mediterranean, Irano-Turanian | 15 |
| Noccaea (17)† | Circumboreal, Irano-turanian, Eastern Asian, Mediterranean, Rocky Mountains, Madrean | 10–15 |
| Pseudosempervivum (2) | Irano-Turanian | 12 |
| Raparia (1) | European | 10 |
| Thlaspiceras (2) | Mediterranean, Irano-Turanian | 12 |
| Tribe Schizopetaleae (1) | ||
| Streptanthus (1) | Madrean | 9 |
| Incertae sedis (Thlaspideae ?) (1) | ||
| Leptoplax (1) | Mediterranean | 8 |
Numbers of Ni hyperaccumulator species for tribes and genera are indicated in parentheses.
*The first report of hyperaccumulation for the species concerned: (1) Minguzzi and Vergnano (1948); (2) Doksopulo (1961); (3) Menezes de Sequeira (1969); (4) Brooks and Radford (1978); (5) Brooks et al. (1979); (6) Vergnano Gambi and Gabbrielli (1979); (7) Vergnano Gambi et al. (1979); (8) Reeves et al. (1980); (9) Reeves et al. (1981); (10) Reeves and Brooks (1983); (11) Reeves et al. (1983); (12) Reeves (1988); see Koch and Mummenhof (2001) for placement of Pseudosempervivum and Masmenia in Noccaeeae rather than in Cochlearieae as in Krämer (2010); (13) Mizuno et al. (2001); (14) Palmer et al. (2001); (15) Reeves et al. (2001); (16) Adigüzel and Reeves (2002); (17) Reeves and Adigüzel (2004); (18) Ghaderian et al. (2007); (19) Reeves and Adigüzel (2008).
†Including the two Ni hyperaccumulator species Thlaspi japonicum and T. jaubertii still not analysed phylogenetically; according to Koch and Al-Shehbaz (2009), Noccaea should include also Masmenia, Pseudosempervivum, Raparia and Thlaspiceras.
According to recent molecular evidence, Mediterranean hyperaccumulators in Brassicaceae originated in only five out of the 35 monophyletic tribes that have been recognized (Reeves and Baker, 2000; Koch and Al-Shehbaz, 2009): Aethionemeae, Alysseae, Cardamineae, Noccaeeae and, putatively, Thlaspideae sensu stricto (s.s.) (Table 1). Alysseae include a number of hyperaccumulators (Table 1) and more or less closely related non-hyperaccumulators, therefore representing an ideal system for investigating the evolutionary dynamics of this ability. Previous investigations in this tribe have focused either on single genera (Mengoni et al., 2003) or on relationships between genera (Warwick et al., 2008). However, the limited taxon sampling of both studies left our knowledge incomplete. A powerful hyperaccumulator whose affinities remain enigmatic is Peltaria emarginata (synonym: Leptoplax emarginata), a serpentine endemic restricted to northern continental Greece (Reeves et al., 1980; Psaras et al., 2000; Chardot et al., 2005; Krämer, 2010; Redjala et al., 2010). Included by some taxonomists in Peltaria based on silicle morphology (Ball, 1993; Jalas and Suominen, 1996; Appel and Al-Shehbaz, 2003; Warwick et al., 2008), other authors have placed it in the monotypic genus Leptoplax due to its emarginate silicles, malpighiaceous pubescence and distinctive floral morphology (Greuter et al., 1986; Hartvig, 1986, 2002b; Stefanović et al., 2003). The problem remains unresolved and becomes even more relevant in the light of recent phylogenetic studies (Koch and Mummenhof, 2001; Appel and Al-Shehbaz, 2003; Al-Shehbaz et al., 2006; Koch and Al-Shehbaz, 2009) which support the removal of Peltaria from Alysseae and its placement in Thlaspideae. Thus, assessment of the ancestry and relationships of this species is crucial for understanding the evolution of Ni hyperaccumulation at deep taxonomic levels.
Alysseae also include one of the largest groups of Ni hyperaccumulators in Brassicaceae, Alyssum sect. Odontarrhena (Brooks et al., 1979). According to Mengoni et al. (2003) the capacity for Ni hyperaccumulation in this group has multiple origins, and it might have been gained or lost several times. Implementing the taxon sampling and the quality of DNA sequences used for that study (see Warwick et al., 2008), however, was relevant to gain a broader view of relationships at the inter- and infraspecific level and the geographical structure of the clades. Taxon sampling in Alyssum sect. Odontarrhena is not straightforward since species-level relationships and limits are still unclear as a consequence of an exceedingly narrow species concept and a redundant nomenclature (Nyárády, 1928, 1929a, b, 1949; Dudley, 1964b). As argued by Hartvig (2002a), such an approach overlooks the strong phenotypic polymorphism especially in A. murale sensu lato (s.l.), a complex of serpentine and non-serpentine populations from south-east Europe which was split into some 12 species and subspecies, mainly based on slight variations in silicle and trichome morphology (Nyárády, 1928; Dudley, 1964a, b). Consequently, different taxonomic concepts for this group led to different estimates of the number and identity of hyperaccumulators.
The goal of the present study is to shed further light on the evolutionary lineages of Ni hyperaccumulation in Brassicaceae, focusing on tribe Alysseae, through a phylogenetic approach based on non-coding nuclear ribosomal DNA (nrDNA) sequences. These were obtained from a broad taxonomic and geographical sampling of European species, which were also tested for hyperaccumulation using a standard method. Inclusion of serpentine and non-serpentine accessions of potential hyperaccumulators and non-hyperaccumulators allowed us to test previous hypotheses on the multiple origins of this trait at the genus, species and below-species level. Ultimately, the data emerging from this analysis may contribute to: (a) a deeper understanding of the evolutionary dynamics of Ni hyperaccumulation in Brassicaceae; (b) a clearer and more practical taxonomy of European hyperaccumulators in Alysseae; and (c) the identification of new model systems consisting of phylogenetically related taxa for comparative studies of the molecular mechanisms of metal hyperaccumulation and practical applications in the fields of phytoremediation, phytomining and food crop biofortification.
MATERIALS AND METHODS
Plant material
Samples and voucher specimens from wild populations of Bornmuellera, Leptoplax, Ptilotrichum, Alyssum sect. Alyssum, Alyssum sect. Odontarrhena, Aurinia and Aethionema were collected by the author L. Cecchi and F. Selvi on serpentine outcrops and other soil types in Italy, Albania and Greece. Additional material was obtained from herbarium specimens borrowed from European herbaria (CAT, C, FI, SOF, SOM; Table 2).
Table 2.
List of the investigated accessions, with vouchers (herbarium acronyms are according to Index Herbariorum), GenBank accession numbers and soil type
| Taxon | Voucher specimen | Soil type | GenBank accession no. |
|---|---|---|---|
| Aethionema arabicum (L.) Andrz. ex O.E.Schulz | Unknown | – | AY254539 |
| Aethionema saxatile (L.) R.Br. subsp. graecum (Boiss. & Sprun.) Hayek | Greece, Sterea Ellas, Mt. Kallidhromon; Cecchi & Selvi 08·11; FI | Serpentine | GQ284853 |
| Alyssoides utriculata (L.) Medik. | Swiss; Vautier 189; MO | Limestone | EF514593 |
| Alyssum alpestre L. | France, Hautes-Alpes, Col du Lautaret; Bruneau 1474 | Limestone | AY237957 |
| Alyssum argenteum All. | 1. Italy, Piedmont, Vallanta; Selvi 08·31; FI | Limestone | GQ284855 |
| 2. Italy, Piedmont, Molette; Siniscalco s.n.; TO | Serpentine | GQ284854 | |
| Alyssum aureum (Fenzl) Boiss. | Israel; Samuelsson s.n.; MO | – | EF514601 |
| Alyssum baldaccii Nyár. (= A. fallacinum auct. non Hausskn.) | 1. Greece, Sterea Ellas, Fourka pass; Bigazzi & Selvi 01·02; FI | Serpentine | GQ284858 |
| 2. Greece, Sterea Ellas, Kedrhos; Cecchi & Selvi 08·08; FI | Serpentine | GQ284856 | |
| 3. Greece, Sterea Ellas, Mt. Kallidhromon; Cecchi & Selvi 08·12; FI | Serpentine | GQ284857 | |
| 4. Greece, Crete, Gonies; Baker s.n. | Serpentine | AY237946 | |
| Alyssum bertolonii Desv. subsp. bertolonii | Italia, Tuscany, Mt. Ferrato; Cecchi & Cecchi 08·35; FI | Serpentine | GQ284859 |
| Alyssum bertolonii Desv. subsp. scutarinum Nyár. | 1. Albania, Shkodër, Mt. Bardanjolt; Cecchi et al. 06·13; FI (= A. janchenii Nyár.) | Serpentine | GQ284867 |
| 2. Yugoslavia, Kosovo, E of Prizren; Metlesics s.n. (= A. punctatum Nyár.) | Serpentine | AY237930 | |
| Alyssum borzaeanum Nyár. | Ucraina; Vakarenko & Mosyakin s.n.; MO | Serpentine | EF514603 |
| Alyssum corymbosoides Form. | 1. Greece, Western Macedonia, Mt. Vourinos; Cecchi & Selvi 08·25; FI | Schist | GQ284878 |
| 2. Republic of Macedonia, Prilep; Cernoch 27287 | Limestone | GQ284892 | |
| Alyssum desertorum Stapf | Albania, Korçë, Bitincka; Hasko 05·07; FI | Serpentine | GQ284881 |
| Alyssum densistellatum T.R.Dudley | 1. Greece, Sterea Ellas, Evia; Cecchi & Selvi 08·17; FI | Serpentine | GQ284879 |
| 2. Greece, Sterea Ellas, Mt. Kallidhromon; Cecchi & Selvi 08·14; FI | Serpentine | GQ284880 | |
| Alyssum euboeum Halácsy | Greece, Sterea Ellas, Evia; Cecchi & Selvi 08·20; FI | Serpentine | GQ284882 |
| Alyssum fragillimum (Baldacci) Rech.f. | Greece, Crete, Lefka Ori; Bergmeier & Matthäs 3254; C | – | GQ284883 |
| Alyssum heldreichii Hausskn. | Greece, Western Macedonia, Mt. Vourinos; Cecchi & Selvi 08·26; FI | Serpentine | GQ284884 |
| Alyssum klimesii Al-Shehbaz | India, Kashmir, Ladak; Klimes 38361; MO | – | EF514608 |
| Alyssum meniocoides Boiss. | Syria; Samuelsson s.n.; MO | – | EF514612 |
| Alyssum montanum L. | Italy, Tuscany, Monterufoli; FI | Serpentine | AY237938 |
| Alyssum murale Waldst. & Kit. s.l. | 1. Albania, Berat; Hasko 07·24; FI (= A. chalcidicum Janka) | Serpentine | GQ284860 |
| 2. Albania, Elbasan, Librazhd; Hasko 06·19; FI (= A. markgrafii O.E.Schulz) | Serpentine | GQ284864 | |
| 3. Albania, Elbasan, Librazhd; Cecchi et al. 07·21; FI (= A. markgrafii) | Serpentine | GQ284863 | |
| 4. Albania, Elbasan, Librazhd; Hasko 3303; FI (= A. markgrafii) | Serpentine | GQ284865 | |
| 5. Albania, Elbasan, Librazhd; Hasko 3306; FI (= A. markgrafii) | Serpentine | GQ284866 | |
| 6. Albania, Elbasan, Perrënjas; Hasko 06·20; FI (= A. chalcidicum) | Serpentine | GQ284871 | |
| 7. Albania, Elbasan, Perrënjas; Cecchi et al. 07·22; FI (= A. chalcidicum) | Serpentine | GQ284869 | |
| 8. Albania, Elbasan, Perrënjas; Hasko 3334; FI (= A. chalcidicum) | Serpentine | GQ284870 | |
| 9. Albania, Korçë, Bitincka; Hasko 04·01; FI (= A. chalcidicum) | Serpentine | GQ284861 | |
| 10. Albania, Korçë, Pogradeč; Hasko 06·21; FI (= A. chalcidicum) | Serpentine | GQ284875 | |
| 11. Albania, Korçë, Pogradeč; Cecchi et al. 07·23; FI (= A. chalcidicum) | Serpentine | GQ284872 | |
| 12. Albania, Korçë, Pogradeč; Hasko 04·02; FI (= A. chalcidicum) | Serpentine | GQ284873 | |
| 13. Albania, Korçë, Pogradeč; Hasko 05·10; FI (= A. chalcidicum) | Serpentine | GQ284874 | |
| 14. Albania, Kukës, Mt. Paštrik; Cecchi et al. 06·17; FI (= A. kosaninum Nyár.) | Serpentine | GQ284868 | |
| 15. Albania, Lezhë, Rubik; Hasko 3355; FI (= A. chalcidicum) | Copper mine | GQ284862 | |
| 16. Bulgaria, Blagoevgrad, Mt. Belasica; Pavlova et al.; SOF 104630 | Limestone | GQ284885 | |
| 17. Greece, Anatolic Macedonia, Mt. Pangeon; Univ. Copenhagen Excurs. 226; C | Limestone | GQ284886 | |
| 18. Greece, Epirus, Metsovo; Cecchi & Selvi 08·04; FI (= A. chlorocarpum Hausskn.) | Schist | GQ284877 | |
| 19. Greece, Central Macedonia, Drosia; Bigazzi & Selvi 01·01; FI (= A. subvirescens Form.) | Serpentine | GQ284876 | |
| 20. Italy, Piedmont, naturalized near Casteldelfino; Selvi 08·32; FI | Schist | GQ284887 | |
| Alyssum nebrodense Tineo | Italy, Sicily, Piano della Battaglia; FI | Limestone | AY237935 |
| Alyssum obovatum (C.A.Mey.) Turcz. | Russia, Amur, Boyko & Starchenko s.n.; MO | – | EF514617 |
| Alyssum orbelicum Ančev & Uzunov | L | Limestone | GQ284888 |
| Alyssum robertianum Bernard ex Gren. & Godr. | Italy, Sardinia, Monte Novo San Giovanni; Charpin et al.; FI | Limestone | GQ284889 |
| Alyssum serpyllifolium Desf. | Portugal, Auriault 13088; MO | – | EF514623 |
| Alyssum sibiricum Willd. | Greece, Sterea Ellas, Evia; Cecchi & Selvi 08·21; FI | Serpentine | GQ284890 |
| Alyssum smolikanum Nyár. | Greece, Epirus, Mt. Smolikas; Cecchi & Selvi 08·28; FI | Serpentine | GQ284891 |
| Alyssum tenium Halácsy | Greece, Cyclades, Tinos; Baker s.n. | Serpentine | AY237926 |
| Alyssum tortuosum Waldst. & Kit. ex Willd. | Turkmenistan; Kurbanov 391; MO | – | EF514625 |
| Alyssum troodi Boiss. ex Boiss. | Cyprus, Mt. Troodos, Mt. Khionistra; Brullo et al. s.n.; CAT | Serpentine | GQ284893 |
| Aurinia saxatilis (L.) Desv. | 1. Unknown | – | AF401115 |
| Aurinia saxatilis (L.) Desv. subsp. orientalis (Ard.) T.R.Dudley | 2. Greece, Sterea Ellas, Aliartos; Cecchi & Selvi 08·15; FI | Limestone | GQ284894 |
| Berteroa orbiculata DC. | Greece; Rechinger 22481; MO | – | EF514634 |
| Bornmuellera baldaccii (Degen) Heywood | 1. Greece, Epirus, Mt. Smolikas; Cecchi & Selvi 08·29; FI | Serpentine | GQ284895 |
| 2. Greece; Rechinger 20986; MO | Serpentine | EF514635 | |
| Bornmuellera tymphaea (Hausskn.) Hausskn. | Greece; Rechinger 18407; MO | Serpentine | EF514640 |
| Clastophus vestitus (Desv.) Boiss. | Iran; Rechinger 6461; MO | – | EF514641 |
| Clypeola jonthlaspi L. | Turkmenistan; Kurbanov 1887; MO | – | EF514644 |
| Degenia velebitica (Degen) Hayek | Croatia, Dalmatia | Limestone | DQ249857 |
| Farsetia aegyptia Turra | Mauritania; Adam 13237; MO | – | EF514649 |
| Fibigia clypeata (L.) Medik. | Unknown | – | DQ249852 |
| Galitzkya macrocarpa (Ikonn.-Gal.) V.V.Botschantz. | Mongolia; Guricheva & Rachkovskaya 2499; MO | – | EF514655 |
| Hormathophylla longicaulis (Boiss.) Cullen & T.R.Dudley | Spain; Charpin et al. 2504; MO | – | EF514659 |
| Leptoplax emarginata (Boiss.) O.E.Schulz | 1. Greece, Thessaly, Kedrhos; Cecchi & Selvi 08·10; FI | Serpentine | GQ284897 |
| 2. Greece, Sterea Ellas, Evia; Cecchi & Selvi 08·18; FI | Serpentine | GQ284896 | |
| Lobularia maritima (L.) Desv. | 1. Italy, Tuscany | – | AY237920 |
| 2. Spain; Borgen 3607; O | – | EF514681 | |
| Peltaria alliacea Jacq. | Unknown | – | DQ289855 |
| Physoptychis caspica (Hablitz) V.V.Botschantz. | Iraq; Rechinger 11159; MO | – | EF514682 |
| Ptilotrichum canescens (DC.) C.A.Mey | Cina, Xinjiang; Bartholomew et al. 8657; MO | – | EF514683 |
| Ptilotrichum cyclocarpum Boiss. subsp. pindicum Hartvig | Greece, Epirus, Mt. Timfi; Cecchi & Selvi 08·30; FI | Limestone | GQ284851 |
| Ptilotrichum rupestre (Ten.) Boiss. | Italy, Abruzzo, Mt. Cavallo; Conti et al. s.n.; APP | Limestone | GQ284852 |
The genera of Alysseae are according to Warwick et al. (2008), except for Leptoplax and Ptilotrichum (Hartvig, 2002).
Complete sequences from the internal transcribed spacer region of nuclear DNA (ITS) were generated for two accessions of L. emarginata, Ptilotrichum rupestre, P. cyclocarpum and a few other taxa of Brassicaceae, in order to examine their position within the framework of the recently published phylogenetic analysis of Alysseae (Warwick et al., 2008).
Sampling in Alyssum was more intensive, and included three outgroup accessions of Alyssum sect. Alyssum and 45 ingroup accessions, of which 37 were original. Together these represent 17 of 19 species of Alyssum sect. Odontarrhena native to the Euro-Mediterranean region as currently accepted in Flora Europaea (Ball and Dudley, 1993) (all except A. obtusifolium and A. caliacrae). Special emphasis was given to the A. murale complex, represented by 22 samples and 14 different geographical accessions, from both ultramafic and non-ultramafic soils. Some of these accessions can be referred to doubtful taxa of Alyssum described from southeast Europe, and were included to examine their position in the ITS phylogenetic analysis.
Test of Ni hyperaccumulation
All accessions of Alyssum, Leptoplax and Bornmuellera that were included in the phylogenetic analysis were tested for Ni hyperaccumulation in leaf dry weight using the standard colorimetric method proposed by Tschugaeff (1905). In spite of some shortcomings, the dimethylglyoxime (DMG) method is widely utilized and accepted in the literature as a useful screen for Ni, especially in the field or on herbarium material (Brooks, 1998; Reeves et al., 1999; Whiting et al., 2004). Three leaves for each dry collection were screened with DMG (Tschugaeff reagent), a selective reagent turning red when forming nickel complexes. Filter paper slices were firstly impregnated with a 1 % DMG solution in absolute ethanol, then dried at room temperature, rehydrated with sterile water and rubbed against leaf fragments. In such a semi-quantitative test, a negative result generally indicates an Ni concentration of <1000 µg g−1 in dry leaf mass (Reeves et al., 1999). The individual plant collections for which a clear change of colour was obtained were considered Ni hyperaccumulators.
To test the constancy of Ni hyperaccumulation in different accessions of serpentine-tolerant species, the same test was also applied to an additional sample of 90 herbarium specimens representing 18 taxa of Alyssum sect. Odontarrhena, Bornmuellera and Leptoplax (Table 3, Appendix).
Table 3.
Results of Tschugaeff reactive (dimethylglyoxime) test of Ni hyperaccumulation performed on 90 herbarium accessions from ultramafic substrates of European (including Cyprus) serpentine-tolerant taxa of Alyssum sect. Odontarrhena, Bornmuellera and Leptoplax
| Genus, section and clade | Species | No. of accessions* | Origin† |
|---|---|---|---|
| Alyssum sect. Odontarrhena | |||
| Clade O1 | A. euboeum | 2/2 | Greece (Evia) |
| Clade O2 | A. argenteum | 11/11 | Italy |
| A. tenium | 3/3 | Greece (Tinos) | |
| A. serpyllifolium | 5/5 | Portugal, Spain | |
| A. baldaccii | 5/5 | Greece (including Crete) | |
| A. murale | 35/35 | Bosnia-Hercegovina, Albania, Kosovo, Macedonia, Greece | |
| Clade O3 | A. bertolonii | 11/11 | Italy |
| A. sibiricum | 0/1 | Greece (Evia) | |
| A. robertianum | 2/2 | France (Corsica) | |
| A. heldreichii | 4/4 | Greece | |
| A. smolikanum | 1/1 | Greece | |
| A. troodi | 2/2 | Cyprus | |
| Incertae sedis | A. akamasicum | 2/2 | Cyprus |
| A. cypricum | 1/1 | Cyprus | |
| A. lesbiacum | 1/1 | Greece (Lesbos) | |
| Bornmuellera (Alysseae clade C) | B. baldaccii | 2/2 | Greece |
| B. tymphaea | 1/1 | Greece | |
| Leptoplax (Alysseae clade C) | L. emarginata | 2/2 | Greece (including Evia) |
Data are shown as in Jansen et al. (2004).
*Ni hyperaccumulator accessions/total accessions.
†See Appendix for details on vouchers and localities.
DNA extraction and amplification
Genomic DNA was extracted following a modified 2× CTAB (cetyltrimethylammonium bromide) protocol (Doyle and Doyle, 1990) using silica-gel dried samples of leaf tissue or herbarium material. The extracted DNA was quantified by spectrophotometric analysis (Biophotometer, Eppendorf).
For amplification of the ITS region, including ITS1, 5·8S and ITS2, the primers ITS4 and ITS5 of White et al. (1990) were used.
PCRs were performed in a total volume of 25 µL containing 2·5 µL of reaction buffer (Dynazyme II, Finnzyme, Espoo, Finland), 1·5 mm MgCl2, 10 pmol of each primer, 200 µm dNTPs, 1 U of Taq DNA polymerase (Dynazyme II, Finnzyme, Espoo, Finland) and 10 ng of template DNA. Reactions were performed in an MJ PTC-100 thermocycler (Peltier Thermal Cycler, MJ Research). Subsequently, 5 µL of each amplification mixture was analysed by agarose gel electrophoresis in TAE buffer (1·5 % w/v) containing 1 µg mL−1 ethidium bromide, by comparison with a known mass standard. The PCR products were purified of excess salts and primers with a PCR Purification Kit (Roche, Mannheim, Germany) and quantified by spectrophotometry (Biophotometer, Eppendorf). Automated DNA sequencing was performed directly from the purified PCR products using BigDye Terminator v.2 chemistry and an ABI310 sequencer (PE-Applied Biosystems, Norwalk, CT, USA). Sequences are deposited in EMBL (accession numbers are given in Table 2).
Sequence alignment and phylogenetic analyses
New sequences of Leptoplax, Alyssum sect. Alyssum, Alyssum sect. Odontarrhena and other members of Alysseae and Aethionema were checked for orthology to the sequences of Bornmuellera baldaccii and Alyssum montanum. Multiple alignments of the ITS data sets of Alysseae and Alyssum sect. Odontarrhena were performed separately with Multalin (Corpet, 1988), then carefully examined and slightly corrected by eye. Using separate alignments allowed us to explore and describe Ni hyperaccumulation in two steps representing different phylogenetic depths, the genus and the species level, and to avoid problems with treatments of gaps. For analyses in Alysseae, these were coded as separate characters according to the ‘simple gap coding’ method (Simmons and Ochoterena, 2000) as implemented in FastGap (Borchsenius, 2009), and appended to the end of the matrices. Because insertions/deletions in the aligned data set of Alyssum sect. Odontarrhena were introduced only as a result of the accessions retrieved from GenBank, gaps were treated as missing data in order not to give excessive weight to possible sequencing artefacts. For the phylogenetic analysis of Alysseae, members of Aethionemeae, Camelineae, Thlaspideae, Arabideae and Malcolmieae were used as outgroups based on their position in the recent study by Warwick et al. (2008). The type species of the genera Aurinia, Ptilotrichum and Peltaria were included to evaluate without ambiguity the position of Leptoplax and the other critical taxa analysed for the first time here. Representatives of Alyssum sect. Alyssum were used as outgroups in the analysis of Alyssum sect. Odontarrhena, based on evidence from the previous analysis of Alysseae. Most of the ITS sequences of section Odontarrhena already available from GenBank were not used for phylogenetic reconstructions, as these were judged of ‘poor quality’ (Bailey et al., 2006; Warwick et al., 2008).
Phylogenetic trees for both data sets were first generated using maximum parsimony (MP) as performed in PAUP 4·0 (Swofford, 2000). Heuristic searches were run with ‘tree–bisection–reconnection’ (TBR) branch-swapping with accelerated transformation (ACCTRAN) optimization to infer branch (edge) lengths; MULTREES option on, ADDSEQ = random, ten randomized replicates. All characters were weighted equally, and character state transitions were treated as unordered. Most-parsimonious trees were summarized in a strict consensus, that was viewed and edited with TreeView (Page, 1996). Bootstrap support (BS) for clades was obtained, performing the analysis with 1000 replicates, using TBR branch-swapping, ten random taxon entries per replicate and MULTREES option on; search = FASTSTEP.
Both data sets were also analysed using Bayesian inference (BI) with MrBayes 3·1.2 (Ronquist and Huelsenbeck, 2003), with a General Time Reversible (GTR) model and γ-distributed rate variation across sites. Analyses were performed using four incrementally heated Markov chains (one cold, three heated) simultaneously started from random trees, and run for 1 million cycles sampling a tree every 100 generations. The stationary phase was reached when the average standard deviation of split frequencies approached 0·01. Trees that preceded the stabilization of the likelihood value (the burn-in) were discarded, and the remaining trees were used to calculate a 50 % majority-rule consensus phylogram that was viewed and edited with TreeView, with Bayesian posterior probability (PP) values for the internal tree nodes.
RESULTS
Ni hyperaccumulation test
All leaf samples of each specimen gave the same result in the DMG test, i.e. they consistently either produced or did not produce a change to red in the reagent. With the exception of A. sibiricum, all serpentine accessions of 18 species of Alyssum sect. Odontarrhena, Leptoplax and Bornmuellera gave a positive result and showed the potential for Ni hyperaccumulation (Table 3).
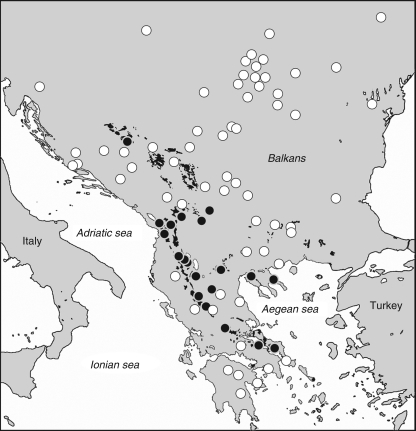
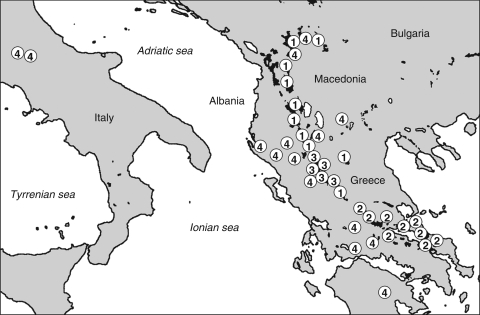
The DMG results for 35 specimens of A. murale s.l. were mapped together with several non-serpentine sites (Fig. 5). Ni hyperaccumulators and non-hyperaccumulator accessions were basically mixed with each other, depending on the geography of serpentine outcrops. Due to the higher frequency and extent in the southern Balkans, hyperaccumulator populations mainly occur in Albania and Greece, and correspond to the ‘chalcidicum’ morphotype. The typical form of A. murale, instead, is restricted to non-serpentine soils and is mainly concentrated in the central Balkans.
Fig. 5.
Distribution of Alyssum murale in the Balkan peninsula. White and black circles indicate, respectively, non-Ni-hyperaccumulating and Ni-hyperaccumulating populations as resulting from DMG tests; black spots indicate the main serpentine areas in SE Europe.
Phylogenetics of Alysseae
The aligned ITS matrix for Alysseae (TreeBASE ID: 10525; Supplementary Data 1, available online) was 794 bp long, including coded gaps that were appended to the end of the matrix (120 positions). Variation within the ingroup (Alysseae) was relatively high, with 38·8 % of variable positions in at least one accession. The mean pairwise genetic distance (Kimura 2-parameters) between accessions was 0·079.
In the MP analysis, 318 characters were constant, 166 parsimony uninformative and 310 (39 %) potentially informative. The two most-parsimonious trees retrieved were 1251 steps long, with consistency index (CI) = 0·57 and retention index (RI) = 0·63; the first tree is shown in Fig. 1. Bayesian analysis (BI) resulted in a 50 % majority-rule consensus phylogram (Fig. 2) largely congruent with the MP trees.
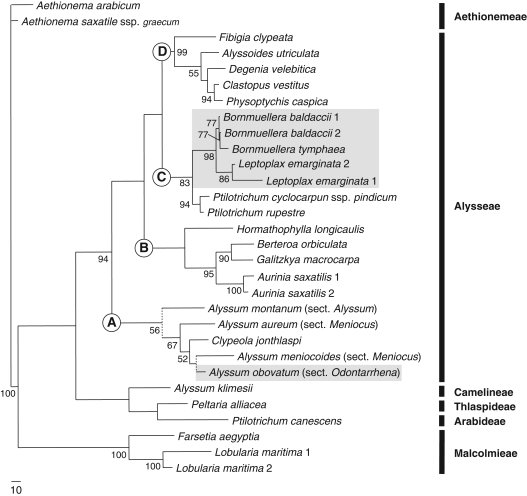
Fig. 1.
One of the two most-parsimonious trees of tribe Alysseae generated by ITS 5·8S sequences with branch lengths (L = 1251, CI = 0·57, RI = 0·63). The main clades discussed in the text are indicated by upper case letters (A–D). Bootstrap values >50 % are indicated at the nodes; nodes collapsing in the strict consensus are indicated by dotted lines. Grey boxes indicate clades including Ni hyperaccumulators.
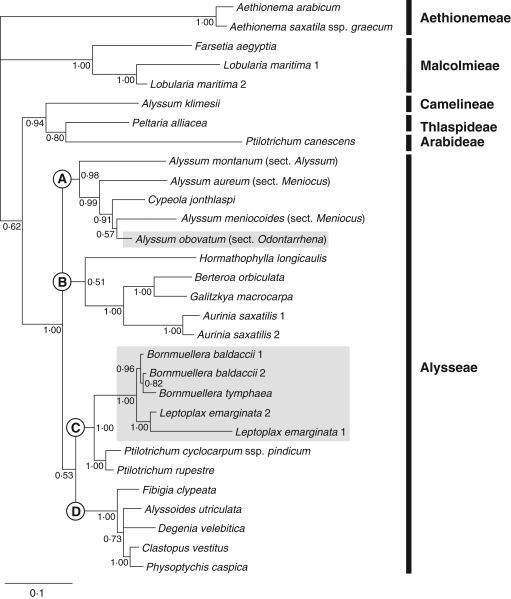
Fig. 2.
Bayesian phylogram (consensus) of tribe Alysseae generated by ITS 5·8S sequences. The main clades discussed in the text are indicated by upper case letters (A–D). Posterior probabilities are indicated at the nodes. Grey boxes indicate clades including Ni hyperaccumulators.
Monophyly of Alysseae, including Leptoplax, was strongly supported (94 % BS; 1·00 PP). Alyssum klimesii (Camelineae), Peltaria alliacea (Thlaspideae), Ptilotrichum canescens (Arabideae) and the Lobularia/Farsetia clade (Malcolmieae) were confirmed to fall outside this tribe. Four main clades were retrieved in Alysseae, here indicated as A, B, C and D. The deep nodes between these clades did not receive statistical support, leaving the relationships between these lineages basically unresolved. Clade A (56 % BS; 0·99 PP) included members of Alyssum sect. Alyssum, Alyssum sect. Odontarrhena and Alyssum sect. Meniocus, together with a clearly nested Clypeola. The relationship between Hormathophylla and the other genera of clade B did not receive bootstrap support and was only suggested in the BI analysis (0·51 PP) where Hormathophylla longicaulis was sister to a sub-clade of Aurinia, Berteroa and Galitzkya (95 % BS; 1·00 PP). In clade C (83 % BS; 1·00 PP), Ptilotrichum cyclocarpum and P. rupestre clustered together with 94 BS % and 1·00 PP; they were retrieved as sister to a strongly supported sub-clade (98 % BS; 1·00 PP) formed by the three accessions of Bornmuellera baldaccii and B. tymphaea (77 % BS; 0·79 PP) sister to Leptoplax emarginata (86 % BS; 1·00 PP). Leptoplax and Bornmuellera share 14 exclusive single nucleotide polymorphisms (SNPs), seven in ITS1 and seven in ITS2; the mean pairwise genetic distances (Kimura 2-parameters) between accessions of the two genera is 0·037. The two accessions of Leptoplax from Evia and Thessaly differed in 27 SNPs, accounting for their different branch lengths in the BI and MP analyses. Clade D (99 % BS; 1·00 PP) included Fibigia sister to a group formed by Alyssoides, Degenia, Clastopus and Physoptychis (55 % BS; 0·65 PP).
Phylogenetics of Alyssum sect. Odontarrhena
The aligned matrix was 651 bp long (TreeBASE ID: 10525; Supplementary Data 2, available online); nucleotide variation within the ingroup was low (89 variable positions = 13·7 %, of which 57 are in ITS1, eight in 5·8S and 24 in ITS2); as expected, the mean pairwise genetic distance was considerably lower (0·02) than that found among members of Alysseae.
In the MP analysis, 527 characters were constant, 46 were non-informative and 78 potentially informative. Heuristic search yielded 202 most-parsimonious trees with length (L) = 199, CI = 0·71, RI = 0·79; though poorly resolved, the resulting strict consensus (not shown) showed no conflict with the more resolved phylogram from the Bayesian analysis (Fig. 3), which is described here.
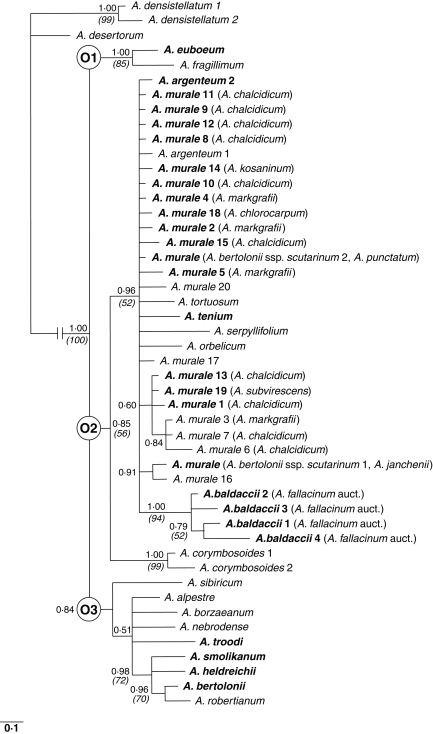
Fig. 3.
Bayesian phylogram of Alyssum section Odontarrhena generated by ITS 5·8S sequences. The main clades discussed in text are indicated as O1–O3. Posterior probabilities are shown at the nodes; corresponding bootstrap values >50 % of MP strict consensus tree (not shown) are reported in parentheses. Ni hyperaccumulator accessions as resulting from the DMG test are in bold; species names are based on evidence of the present study (see text) and taxonomic synonyms are given in parentheses.
Alyssum sect. Odontarrhena was retrieved as a monophyletic group (1·00 PP) including three major clades, here named O1, O2 and O3, with unresolved relationships. Clade O1 (1·00 PP) contained the Greek island endemics A. euboeum, an Ni hyperaccumulator from serpentine of Evia, and A. fragillimum, a non-hyperaccumulator from calcareous mountains on Crete. Clade O2 (0·85 PP) includes the non-hyperaccumulator A. corymbosoides as sister to a broad and largely unresolved group including all accessions of the predominantly Ni-hyperaccumulating taxa A. argenteum, A. baldaccii, A. murale, A. serpyllifolium and A. tenium mixed with the non-hyperaccumulators A. orbelicum and A. tortuosum (type species of section Odontarrhena). Clade O3 (0·84 PP) consists of four non-hyperaccumulator species with unresolved relationships and five mainly hyperaccumulator taxa. Of these, the four European species A. heldreichii, A. smolikanum, A. bertolonii and A. robertianum were retrieved as a monophyletic group (0·98 PP), with a clear affinity (0·96 PP) between the two latter taxa.
DISCUSSION
Double origin of Ni hyperaccumulation in tribe Alysseae
According to Warwick et al. (2008), Alysseae consist of 12 genera in addition to Straussiella which was not included in their molecular study. However, adding accessions of Leptoplax and two Mediterranean species of Ptilotrichum to the phylogenetic analysis provided results that shed new light on the number and relationships of Ni hyperaccumulator lineages in this group. Leptoplax emarginata has no affinity with Peltaria, in which it was included by some recent taxonomists (Ball, 1993; Jalas and Suominen, 1996; Appel and Al-Shehbaz, 2003; Warwick et al., 2008). Fruit characters shared by two taxa, such as the pendent, indehiscent, strongly compressed latiseptate silicles, are most probably homoplasious and due to parallel evolution. In contrast, our results support L. emarginata as a separate lineage, in agreement with the views of other taxonomists (Greuter et al., 1986; Hartvig, 1986, 2002b) who stressed its placement in a monotypic genus of Alysseae. Molecular data clearly demonstrate that Leptoplax is sister to the genus Bornmuellera (clade C, 96 % BS, 1·0 PP), as represented by the two Ni hyperaccumulator species B. tymphaea and B. baldaccii, endemic to the serpentine outcrops of continental Greece (Stefanović et al., 2003). The mean pairwise genetic distance within the Bornmuellera–Leptoplax clade is closer to that found among species of Alyssum sampled here than that among genera of Alysseae, corroborating the nearly congeneric status of the two taxa. In addition, L. emarginata and Bornmuellera are diploids with a similar genome size (Peer et al., 2006) and chromosome complements based on x = 8 (Constantinidis et al., 2002), unlike Peltaria with x = 7. Given their broadly sympatric distribution (Fig. 4) and identical specialization for serpentine soils, this condition may have contributed to the formation of the intergeneric hybrids and introgressive populations involving L. emarginata and Bornmuellera tymphaea reported from northern Greece (Hartvig, 1986, 2002b; Ball, 1993). Shoot concentrations of ≤34 000 and 31 000 µg g−1 (Chardot et al., 2005; see also Bani et al., 2009; Reeves et al., 2009; Redjala et al., 2010), respectively, show that Leptoplax and Bornmuellera have evolved a striking ability for Ni hyperaccumulation, representing an early synapomorphy due to common ancestry. Coupled with high biomass production (Chardot et al., 2005) and broad tolerance to different climatic constraints (from Mediterranean to sub-alpine habitats; Hartvig, 2002; pers. obs.), L. emarginata is an ideal model for phytoremediation and phytomining in different environments.
Fig. 4.
Chorological relationships among Bornmuellera (1), Leptoplax (2), Bornmuellera plus Leptoplax (overlapping area 3), Ptilotrichum cyclocarpum and P. rupestre (4); black spots indicate the main serpentine areas in SE Europe.
Removing Leptoplax from Peltaria, as advocated here, implies that the ability for Ni hyperaccumulation did not originate in Thlaspideae, since the approx. 15 hyperaccumulators formerly included in Thlaspi are firmly nested in tribe Noccaeae (Koch and Mummenhof, 2001; Koch and Al-Shehbaz, 2009). The recent phylogenetic scheme of metal hyperaccumulation in Brassicaceae (Krämer, 2010) should be amended by placing ‘Peltaria’ emarginata in Alysseae under Leptoplax, and removing Thlaspideae fom the list of tribes of Brassicaceae accumulating nickel.
Given their distant phylogenetic position, different mechanisms of hyperaccumulation and tolerance may have evolved in the Noccaea and Bornmuellera–Leptoplax clades, suggesting the latter as a model for further physiological and molecular investigations. Based on molecular evidence, the latter lineage is sister to the well supported group formed by Ptilotrichum rupestre and P. cyclocarpum, indicating a common origin from an ancestor distributed in montane areas of the central-eastern Mediterranean. In addition, ITS sequences reveal no affinity between these two species and the Asian type species of Ptilotrichum, P. canescens (tribe Arabideae), suggesting the transfer of the two Mediterranean taxa to a separate genus of Alysseae.
Ptilotrichum rupestre and P. cyclocarpum are strictly calcicolous species endemic to the central Apennines and the Balkan peninsula (Fig. 4), respectively, where they grow in rocky habitats such as cliffs and screes of calcareous and/or dolomitic nature (Pignatti, 1982; Hartvig, 2002b). The edaphic requirements of Bornmuellera–Leptoplax and Ptilotrichum corroborate the evidence that Brassicaceae are often a dominant group in the floras of Mg-rich substrates such as dolomites and serpentines (Proctor, 1999; Mota et al., 2008). From a phylogenetic viewpoint, the capacity for Ni hyperaccumulation in clade C may be seen as a symplesiomorphy secondarily lost in the Ptilotrichum rupestre–cyclocarpum lineage or, more probably, as a synapomorphy which originated in Bornmuellera and Leptoplax from a non-hyperaccumulator ancestor under the selective pressure of serpentine soils in the southern Balkans. The basophilous character of this ancestor may have favoured the evolution of new lineages with a stronger edaphic specialization, enhancing their competitive ability on ultrabasic serpentine outcrops. Recent phylogenetic analyses of Boraginaceae tribe Lithospermeae (Thomas et al., 2008; Cecchi and Selvi, 2009) revealed a similar relationship in the two serpentine endemics Halacsya sendtneri and Paramoltkia doerfleri, which are sister to a group of strictly basophilous species of Lithodora growing on limestone. This provides support that most serpentine endemics in the Balkan flora have as their closest relatives basophilous taxa linked to calcareous substrates (Stefanović et al., 2003). In addition, the ability to cope with high soil Mg concentration of many serpentine plants is often considered a key component of adaptation to ultramafics (Brady et al., 2005; Kazakou et al., 2008), suggesting that the possibly elevated amounts of this element in the substrates of Mediterranean Ptilotrichum may have been a prerequisite for the evolution of obligate serpentinophytism in Bornmuellera and Leptoplax.
Lack of geographical cohesion and polyphyly in Ni hyperaccumulators of Alyssum sect. Odontarrhena
Molecular data from our expanded taxonomic sample strongly support Alyssum section Odontarrhena as a monophyletic lineage characterized by a single ovule per locule, the two-seeded silicles and the usually branched inflorescence. Accordingly, this could also be treated as a genus separate from Alyssum s.s. (Warwick et al., 2008). Available data and DMG tests show that hyperaccumulator species of Alyssum are exclusively found in this section, corroborating its phylogenetic distinctiveness. Although other species from different Alyssum sections are also able to colonize serpentine soils (e.g. A. montanum, A. densistellatum, A. desertorum and A. mouradicum of section Alyssum), they cannot hyperaccumulate nickel, showing that this capacity did not evolve, or it was lost, outside section Odontarrhena. With the exception of A. sibiricum (Reeves and Adigüzel, 2004, 2008; pers. obs.), all serpentine taxa of this group hyperaccumulate Ni when growing on ultramafic soils, suggesting that this ability may itself be an adaptative strategy for metal tolerance. On the other hand, serpentinophytism is not an obligate condition in Odontarrhena, as shown by calcicolous species such as A. nebrodense, A. fragillimum and A. orbelicum.
Low ITS variation in this group does not allow a complete resolution of relationships among species and, coupled with weak phenotypic differentiation, suggests a relatively recent radiation in southern Europe. Our data show that the evolutionary distribution of hyperaccumulation is even more patchy than previously known (Mengoni et al., 2003), and that this capacity does not represent a synapomorphy for any of the three major clades that were retrieved. Hyperaccumulators and non-hyperaccumulators are mixed in each of these species groups, providing evidence for the multiple origins or losses of this ability from distinct ancestors. In addition, no clear correlation with geographical distribution can be found within the three clades, two of which include accessions from the western, central and eastern Mediterranean regions. A striking example is the western Alpine hyperaccumulator A. argenteum that is more closely related to the Balkan complex of A. murale than to the geographically closer A. bertolonii from central Italy. The latter, in turn, was closer to the Corso-Sardinian A. robertianum and, to a lesser extent, the Greek hyperaccumulators A. smolikanum and A. heldreichii. This corroborates the genetic continuity of members of section Odontarrhena and the likely polytopic origin of hyperaccumulators through repeated events over the different serpentine ‘islands’ of southern Europe, without involving strong genetic divergence events. A form of genetic pre-adaptation allowing the rapid development of this ability in populations that come into contact with ultramafic soils is therefore a remarkable synapomorphy for this group.
Our results are in line with other studies describing repeated evolution of metal accumulation ability and/or associated serpentine tolerance within single genera or species complexes. For example, Nyberg Berglund et al. (2001) showed that metal tolerance in serpentine populations of the Cerastium alpinum complex in Fennoscandia probably evolved two or more times independently. Enhanced Zn tolerance in metallicolous populations of Arabidopsis halleri were found to be of polyphyletic origin, resulting from independent local microevolutionary adaptation (Pauwels et al., 2006; Jiménez-Ambriz et al., 2007). In this species and Noccaea caerulescens, the contributions of colonization of metalliferous sites from local populations on non-metalliferous sites and from distant populations on other metalliferous sites are currently under investigation (Krämer, 2010). Rajakaruna et al. (2003) provided compelling evidence that a pair of cryptic species in the Lasthenia californica complex have undergone parallel evolution of serpentine tolerance based on differences in their ability to absorb various cations. Similar evidence was obtained for the genus Calochortus, in which DNA sequences revealed that ultramafic specialization evolved through seven events at least (Patterson and Givnish, 2004). All such findings suggest that metal tolerance and/or hyperaccumulation can be rapidly lost or gained within groups of closely related taxa through a selection process affecting the expression and regulation of genes that are not species specific or novel (Verbruggen et al., 2009).
Genetic variation within the A. murale clade is extremely low, with most geographically distinct accessions referred to A. murale or other related taxa having identical or similar ITS sequences. Considering the broadly overlapping ranges of these taxa and the high phenotypic plasticity that often obscures their weak morphological boundaries, we advocate a reduction in the number of species recognized. Molecular and morphological evidence from native populations (pers. obs.) indicate that A. bertolonii subsp. scutarinum, A. chlorocarpum, A. jankenii, A. markgrafii and A. chalcidicum, previously regarded as endemic hyperaccumulators (Brooks et al., 1979; Hasko and Çullaj, 2001; Vinterhalter and Vinterhalter, 2005; Bani et al., 2009), can hardly be recognized as distinct from A. murale. On the other hand, field observations and evidence from DMG tests show that most populations of this species growing in Albania and Greece have a clear preference for serpentine soils and an associated ability for Ni hyperaccumulation (Table 3, Fig. 5). The slight differentiation in trichome density and inflorescence morphology of these populations (Hartvig, 2002a) may support, at most, their recognition at the varietal rank. Accordingly, literature reports of hyperaccumulation in A. murale (Brooks and Radford, 1978; Reeves et al., 2001) are likely to be referred to this race or to closely related taxa from Anatolian serpentine areas. Finally, ITS sequences corroborate morphology in the recognition of Balkan endemics such as A. baldaccii (hyperaccumulator) and A. corymbosoides (non-hyperaccumulator), whereas there is no molecular support for the Alpine endemic A. argenteum, in spite of its distinctiveness in fruit characters. Summing up, the balance between molecular data, morphology, distribution and ecology should lead to the recognition of only 11 European Ni hyperaccumulator species of Alyssum, as summarized in Table 4.
Table 4.
List of European species of Alyssum sect. Odontarrhena with indication of range and edaphic habitat based on personal observations and literature data (Ball et al., 1993; Küpfer and Nieto Feliner, 1993; Hartvig, 2002a)
| Taxon | Geographic range | Edaphic preferences |
|---|---|---|
| Alyssum alpestre L. | W Alps | Limestone |
| Alyssum argenteum All. | W Alps | Mainly serpentine |
| Alyssum baldaccii Vierh. ex Nyár. | S and C Greece, Crete | Serpentine |
| = Alyssum fallacinum auct. non Hausskn. | ||
| Alyssum bertolonii Desv. | C Italy | Serpentine |
| Alyssum corymbosoides Form. | SE Balkans | Limestone or other basic soils |
| = Alyssum rechingeri Nyár. | ||
| = Alyssum rhodopense Form. | ||
| = Alyssum vranjanum Nyár. | ||
| Alyssum degenianum Nyár. | N Aegean sea (Samothraki) | Limestone or schist |
| Alyssum euboeum Halácsy | E Greece (Evia) | Serpentine |
| Alyssum fragillimum (Baldacci) Rech.f. | Crete | Limestone |
| Alyssum heldreichii Hausskn. | N Greece | Serpentine |
| Alyssum lesbiacum (P.Candargy) Rech.f. | Eastern Aegean (Lesbos) | Serpentine |
| Alyssum murale Waldst. & Kit. | C and S Balkans, Anatolia | All soil types |
| = Alyssum balkanicum Nyár. | ||
| = Alyssum bertolonii Desv. subsp. rigidum Nyár. | ||
| = Alyssum bertolonii Desv. subsp. scutarinum Nyár. | ||
| = Alyssum chalcidicum Janka | ||
| = Alyssum chlorocarpum Hausskn. | ||
| = Alyssum jancheni Nyár. ex Novák | ||
| = Alyssum kosaninum Nyár. | ||
| = Alyssum markgrafii O.E.Shulz* | ||
| = Alyssum orphanidis Nyár. | ||
| = Alyssum pichleri Velen. | ||
| = Alyssum punctatum Nyár. | ||
| = Alyssum subvirescens Form. | ||
| Alyssum nebrodense Tineo | Sicily and C Greece | Limestone |
| Alyssum orbelicum Ančev & Uzunov | SW Bulgaria | Limestone |
| Alyssum robertianum Bernard ex Gren. & Godr. | Corsica and Sardinia | Limestone and serpentine |
| = A. tavolarae Briq. | ||
| Alyssum samium T.R.Dudley & Christod. | Eastern Aegean (Samos) | Limestone |
| Alyssum serpyllifolium Desf. | Iberian peninsula | Limestone and serpentine |
| = A. malacitanum T.R.Dudley | ||
| = A. pintodasilvae T.R.Dudley | ||
| Alyssum sibiricum Willd. | E Europe, Anatolia and Siberia | All soil types |
| = A. borzaeanum Nyár. | ||
| = A. caliacrae Nyár. | ||
| = A. epirotum (Halácsy) Nyár. | ||
| = A. halacsyi Nyár. | ||
| = A. lepidulum Nyár. | ||
| = A. obtusifolium Steven ex DC. subsp. helioscopioides Nyár. | ||
| = A. suffrutescens (Boiss.) Halácsy | ||
| Alyssum smolikanum Nyár. | S Albania and N Greece | Serpentine |
| Alyssum tenium Halácsy | Cyclades (Tinos) | Serpentine |
| A. tortuosum Waldst. & Kit. | Continental Europe and CE Asia | Not serpentine |
| = A. grintescui Nyár. |
Species with European Ni-hyperaccumulating populations are in bold.
*No morphological or molecular evidence emerges from the present study for keeping A. markgrafii as distinct from A. murale as in Flora Europaea (Ball and Dudley, 1993).
CONCLUSIONS
To date, only a few studies have explored the phylogenetic patterns of metal accumulation at the macroevolutionary scale, i.e. the species level or above (Broadley et al., 2001; Jansen et al., 2002, 2004; Mengoni et al., 2003). Our data are basically consistent with these studies in suggesting that this ability and serpentine tolerance are usually associated with given plant groups but not monophyletic within them. Reassessing the evolutionary patterns of Ni hyperaccumulation in Alysseae demonstrated this ability to be confined to the phylogenetically distant clades of Bornmuellera–Leptoplax and Alyssum sect. Odontarrhena. Unquestionable lack of affinity between Leptoplax and Peltaria implies that it did not originate within Thlaspideae. Ni hyperaccumulation is not monophyletic for either Alyssum sect. Odontarrhena or any of its three European clades. Lack of geographical cohesion within these clades possibly results from a recent radiation and polytopic origin of Ni hyperaccumulation through local and independent events of microevolutionary adaptation. It appears that such an ability can be gained or lost multiple times in basically pre-adapted plant groups, through selection and adaptation processes involving structural or regulatory changes in the genome.
Elucidating relationships and affinities in Alysseae may have implications for further research on the molecular mechanisms and genetic bases of Ni hyperaccumulation. The latter have been investigated less with respect to other metals, especially Zn and Cd. Species of Bornmullera and Leptoplax and hyperaccumulators in Alyssum sect. Odontarrhena may be screened and compared with closely related non-hyperaccumulators through genome sequencing, microarray analysis or quantitative trait loci analysis to look for candidate genes involved in the uptake, transport and accumulation of nickel. The role of molecules such as histidine, organic acids, nicotianamine or glutathione (Montargé-Pelletier et al., 2008; Verbruggen et al., 2009) could also be compared in these species. Using closely related control species, new insights may be obtained on the genetic or epigenetic basis of Ni physiology. To date, lack of solid phylogenetic evidence has led to the use of model systems including only distantly related taxa, possibly reducing the information content of the results obtained. Examples are the systems A. lesbiacum/A. montanum used for the role of histidine (Krämer et al., 1996; Kerkeb and Krämer, 2003; Ingle et al. 2005) and the assimilation of Peltaria turkmena into Leptoplax emarginata when evaluating genetic similarity with Arabidopsis thaliana (Peer et al., 2006). The present data suggest new models at three evolutionary levels, such as Mediterranean Ptilotrichum vs. Bornmuellera–Leptoplax at the genus level (clade C), Alyssum euboeum vs. A. fragillimum (clade O1) or A. baldaccii vs. A. corymbosoides at the species level (clade O2) and A. robertianum or A. murale populations at the infraspecific level.
SUPPLEMENTARY DATA
Supplementary Material
ACKNOWLEDGEMENTS
We thank D. Uzunov (Cosenza), G. Giusso del Galdo (Catania) and C. Siniscalco (Torino) for providing material of A. orbelicum, A. troodi and A. argenteum. The curators of the mentioned herbaria allowed the study of exsiccata, and A. Mengoni helped with interpretation and discussion of the data. Two anonymous reviewers provided useful comments and suggestions on the first version of the manuscript. Research grants from the University of Firenze and the Italian Ministry for Scientific Research are acknowledged.
APPENDIX
List of 90 herbarium accessions of Alyssum section Odontarrhena, Bornmuellera and Leptoplax from European ultramafic outcrops tested for Ni hyperaccumulation.
Alyssum akamasicum Burtt.: Cyprus, Paphos, Akamas, 1941, Davis 3308 (type); 1999, Van Buggenhout Soc. éch. Eur. Méd. 18981, FI. A. argenteum All.: Italy, Aosta Valley, Chatillon, 1997, Baker; St. Vincent, 1902, Ferrari 274, FI; Valtournenche (type locality), 1900, Vaccari, FI; Emilia Romagna, Bobbio, 1977, Arrigoni et al., FI; 1978 (Mengoni et al., 2003); Mt. Prinzera, 1958, Minio, FI; Lombardia, Brallo pass, 1950, Viola, FI; Piedmont, Molette, 1996, Siniscalco, TO; Mt. Musiné, 1860, Chabert, FI; Piossasco, 1998, Siniscalco, TO; Sacra di S. Michele, 1905, Ferrari, FI. A. baldaccii Vierh. ex Nyár.: Greece, Crete, Gonies, 1995, Baker; Mylopotamos, 1899, Baldacci It. Cret. Alt. 52, FI (type); Sterea Ellas, Fourka pass, 2001, Bigazzi & Selvi 01·02, FI; Mt. Kallidhromon, 2008, Cecchi & Selvi 08·12, FI; Thessaly, Kedrhos, 2008, Cecchi & Selvi 08·08, FI. A. bertolonii Desv.: Italy, Emilia Romagna, Mt. Prinzera, 1858, Parlatore, FI; Ligury, Sarzana, 1992, Ferrarini, FI; Tuscany, Impruneta, 1842, Scaffai, FI; Mt. Gabbro, 1864, Beccari, FI; Mt. Massi, 1843, Ricasoli, FI; Monterufoli, 2006, Cecchi 06·06, H.Cecchi; Mt. Ferrato, 2008, 1884, Sommier, FI; L.Cecchi & M.Cecchi 08·35, H.Cecchi; Pomarance, 1987, Gabbrielli et al., FI; Italy, Tuscany, Riparbella, 1868, Amidei, FI; Upper Tibery Valley, 1979, Pignatti, FI. A. cypricum Nyár.: Cyprus, Limassol, Mt. Troodos, 1991, Alziar et al., FI. A. euboeum Halácsy: Greece, Evi, (Mengoni et al., 2003); Limni, 2008, Cecchi & Selvi 08·20, FI. A. heldreichii Hausskn.: Greece, Thessaly, Doliana, 2008, Cecchi & Selvi 08·06, FI; Malakasi, 1896, Sintenis It. Thess. 574, FI; Witomo, 1896, Sintenis It. Thess. 218, FI; Western Macedonia, Mt. Vourinos, 2008, Cecchi & Selvi 08·26, FI. A. lesbiacum (Candargy) Rech.f.: Greece, North Aegean Islands, Lesbos, 1992, Baker. A. murale Waldst. & Kit. (= A. chalcidicum Janka): Albania, sine loco, Hasko 07·24, FI; Berat, Perisnake, 1892, Baldacci It. Alb. 181, FI; Devoli, Bitincka, Hasko 04·01, FI; Has, Mt. Paštrik (= A. kosaninum Nyár.), Cecchi et al. 06·17, FI; Librazhd, Librazhd (= A. markgrafii O.E.Schulz), Cecchi et al. 07·21, FI; Hasko 05·05, FI; Hasko 05·06, FI; Hasko 06·19, FI; Perrënjas, Cecchi et al. 07·22, FI; Hasko 05·09, FI; Hasko 06·20, FI; Mirditë, Rubik, Hasko 05·08, FI; Pogradeč, Piskupat, Cecchi et al. 07·23, FI; Hasko 04·02, FI; Hasko 05·10, FI; Hasko 06·21, FI; Pukë, Mt. Prenkollit, Cecchi et al. 06·18, FI; Shkodër, Mt. Bardanjolt (= A. janchenii Nyár., = A. bertolonii Desv. subsp. scutarinum Nyár.), Cecchi et al. 06·13, FI; Bosnia Erzegovina, Srpska Republic, Višegrad, 1897, Fiala, FI; Greece, Central Macedonia, Drosia (= A. subvirescens Form.), 2001, Bigazzi & Selvi 01·01, FI; Ierissos-Gomation, 1871, Janka, FI (type of A. chalcidicum Janka); Thessaloniki, 1857, Orphanides Fl. Graec. Exs. 644, FI (type of A. orphanidis); 1997, Baker; Evia, Mt. Dyrphys, 1876, Heldreich, FI; Steni, 1901, Leonis, FI; Evia, Limni, 2008, Cecchi & Selvi 08·19, FI; Epirus, Mt. Smolikas, 1896, Baldacci It. Alb. IV 206, FI; Sterea Ellas, Mt. Parnes, ?, Heldreich, FI; Thessaly, Kalambaka (= A. chlorocarpum Janka), 2008, Cecchi & Selvi 08·05, FI; Kedrhos, 2008, Cecchi & Selvi 08·09, FI; Western Mecedonia, Palaiokastron, 2008, Cecchi & Selvi 08·23, FI; Macedonia, Pindus Mts., 1896, Sintenis It. Thessal. 488, FI; Samarina, 2008, Cecchi & Selvi 08·22, FI; Kosovo, Prizren (= A. punctatum Nyár.), 1962, Metlesics; Macedonia, Raduˇšc (= A. chlorocarpum), 1936, O.Behr & E.Behr, FI. A. robertianum Bernard ex Gren. & Godr.: France, Corsica, Mt. Fosco, 1881, Chabert, FI; Mt. S. Leonardo, 1881, Chabert, FI. A. serpyllifolium Desf.: Portugal, Bragança, Bragança (= A. pintodasilvae T.R.Dudley), 1995, ?; Campo Redondo (= A. pintodasilvae T.R.Dudley), 1884, Moller; Spain, Andalusia, Sierra Bermeja [= A. malacitanum (Rivas Goday) T.R.Dudley], 1994, Baker; Galicia, Puente Basandre, 1996, Baker; Malaga, Sierra de Carratraca, 1982, Losa Quintana Soc. éch. Eur. Méd. 11242. A. smolikanum Nyár.: Greece, Epirus, Mt. Smolikas, 2008, Cecchi & Selvi 08·28, FI. A. tenium Halácsy: Greece, Cyclades, Tinos, 1901, Heldreich Heldr. Herb. Gr. Norm. 1608, FI; 1978 (Mengoni et al., 2003); 1997, Baker. A. troodi Boiss.: Cyprus, Limassol, Mt. Troodos, 1880, Sintenis & Rigo 844, FI (type); Cyprus, Limassol, Mt. Troodos, 1948, Mavromoustakis. Bornmuellera baldaccii (Degen) Heywood: Greece, Epirus, Mt. Smolikas, 1896, Baldacci It. Alb. IV 211, FI; 2008, Cecchi & Selvi 08·29, FI. B. tymphaea (Hausskn.) Hausskn.: Greece, Western Macedonia, Mt. Vourinos, 2008, Cecchi & Selvi 08·27, H.Cecchi. Leptoplax emarginata (Boiss.) O.E.Schulz: Greece, Evia, Mt. Dirphys, 2008, Cecchi & Selvi 08·18, FI; Thessaly, Kedrhos, 2008, Cecchi & Selvi 08·10, FI.
LITERATURE CITED
- Adigüzel N, Reeves RD. A new nickel-accumulating species of Alyssum (Cruciferae) from western Turkey. Edinburgh Journal of Botany. 2002;59:215–219. [Google Scholar]
- Al-Shehbaz IA, Beilstein MA, Kellogg EA. Systematics and phylogeny of the Brassicaceae (Cruciferae): an overview. Plant Systematics and Evolution. 2006;259:89–120. [Google Scholar]
- Appel O, Al-Shehbaz IA. Cruciferae. In: Kubitzki K, Bayer C, editors. The families and genera of vascular plants: V. Flowering plants, dicotyledons: Malvales, Capparales, and non-betalain Caryophyllales. Berlin: Springer; 2003. pp. 75–174. [Google Scholar]
- APG III (Angiosperm Phylogeny Group III) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society. 2009;161:105–121. [Google Scholar]
- Assunção AGL, Schat H, Aarts MGM. Thlaspi caerulescens, an attractive model species to study heavy metal hyperaccumulation in plants. New Phytologist. 2003;159:351–360. doi: 10.1046/j.1469-8137.2003.00820.x. [DOI] [PubMed] [Google Scholar]
- Bailey CD, Koch MA, Mayer M, et al. Toward a global phylogeny of the Brassicaceae. Molecular Biology and Evolution. 2006;23:2142–2160. doi: 10.1093/molbev/msl087. [DOI] [PubMed] [Google Scholar]
- Ball PW. Peltaria Jacq. In: Tutin TG, Heywood VH, Burges NA, et al., editors. 2nd edn. Cambridge: Cambridge University Press; 1993. p. 358. Flora Europaea1. [Google Scholar]
- Ball PW, Dudley TR. Alyssum L. In: Tutin TG, Heywood VH, Burges NA, et al., editors. 2nd edn. Cambridge: Cambridge University Press; 1993. pp. 359–369. Flora Europaea1. [Google Scholar]
- Bani A, Echevarria G, Mullaj A, Reeves R, Morel JL, Sulçe S. Nickel hyperaccumulation by Brassicaceae in serpentine soils of Albania and northwestern Greece. Northeastern Naturalist. 2009;16:385–404. [Google Scholar]
- Beilstein MA, Al-Shehbaz IA, Kellogg EA. Brassicaceae phylogeny and trichome evolution. American Journal of Botany. 2006;93:607–619. doi: 10.3732/ajb.93.4.607. [DOI] [PubMed] [Google Scholar]
- Borchsenius F. FastGap 1·1. 2009 http://192.38.46.42/aubot/fb/FastGap_home.htm. (21 May 2009) [Google Scholar]
- Boyd RS. Ecology of metal hyperaccumulation. New Phytologist. 2004;162:563–567. doi: 10.1111/j.1469-8137.2004.01079.x. [DOI] [PubMed] [Google Scholar]
- Boyd RS, Martens SN. The significance of metal hyperaccumulation for biotic interactions. Chemoecology. 1998;8:1–7. [Google Scholar]
- Brady KU, Kruckeberg AR, Bradshaw HD., Jr Evolutionary ecology of plant adaptation to serpentine soils. Annual Review of Ecology, Evolution and Systematics. 2005;36:243–266. [Google Scholar]
- Broadley MR, Willey NJ, Wilkins JC, Baker AJM, Mead A, White PJ. Phylogenetic variation in heavy metal accumulation in angiosperms. New Phytologist. 2001;152:9–27. doi: 10.1046/j.0028-646x.2001.00238.x. [DOI] [PubMed] [Google Scholar]
- Broadley MR, White PJ, Hammond JP, Zelko I, Lux A. Zinc in plants. New Phytologist. 2007;173:677–702. doi: 10.1111/j.1469-8137.2007.01996.x. [DOI] [PubMed] [Google Scholar]
- Brooks RR. Serpentine and its vegetation. A multidisciplinary approach. Portland: Dioscorides Press; 1987. [Google Scholar]
- Brooks RR, editor. Plants that hyperaccumulate heavy metals. Wallingford, Oxon: CAB International; 1998. [Google Scholar]
- Brooks RR, Radford CC. Nickel accumulation by European species of the genus Alyssum. Proceedings of the Royal Society B: Biological Science. 1978;200:217–224. [Google Scholar]
- Brooks RR, Lee J, Reeves RD, Jaffré T. Detection of nickeliferous rocks by analysis of herbarium specimens of indicator plants. Journal of Geochemical Exploration. 1977;7:49–77. [Google Scholar]
- Brooks RR, Morrison RS, Reeves RD, Dudley TR, Akman Y. Hyperaccumulation of nickel by Alyssum Linnaeus (Cruciferae) Proceedings of the Royal Society B: Biological Science. 1979;203:387–403. doi: 10.1098/rspb.1979.0005. [DOI] [PubMed] [Google Scholar]
- Cecchi L, Selvi F. Phylogenetic relationships of the monotypic genera Halacsya and Paramoltkia and the origins of serpentine adaptation in circummediterranean Lithospermeae (Boraginaceae): insights from ITS and matK DNA sequences. Taxon. 2009;58:700–714. [Google Scholar]
- Chaney RL, Angle JS, McIntosh MS, et al. Using hyperaccumulator plants to phytoextract soil Ni and Cd. Zeitschrift Naturforschung. 2005;60:190–198. [PubMed] [Google Scholar]
- Chardot V, Massoura ST, Echevarria G, Reeves RD, Morel JL. Phytoextraction potential of the nickel hyperaccumulators Leptoplax emarginata and Bornmuellera tymphaea. International Journal of Phytoremediation. 2005;7:323–335. doi: 10.1080/16226510500327186. [DOI] [PubMed] [Google Scholar]
- Constantinidis T, Bareka E-P, Kamari G. Karyotaxonomy of Greek serpentine angiosperms. Botanical Journal of the Linnean Society. 2002;139:109–124. [Google Scholar]
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Research. 1988;16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doksopulo EP. Nickel in rocks, soils, water and plants adjacent to the talc deposits of the Chorchanskaya Group. Tbilisi: Izdatel vo Tbiliskovo Universitet; 1961. [Google Scholar]
- Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- Dudley TR. Studies in Alyssum: near Eastern representatives and their allies, I. Journal of the Arnold Arboretum. 1964a;45:57–100. [Google Scholar]
- Dudley TR. Synopsis of the genus Alyssum. Journal of the Arnold Arboretum. 1964b;45:359–373. [Google Scholar]
- Ghaderian SM, Mohtadi A, Rahiminejad MR, Baker AJM. Nickel and other metal uptake and accumulation by species of Alyssum (Brassicaceae) from the ultramafics of Iran. Environmental Pollution. 2007;145:293–298. doi: 10.1016/j.envpol.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Greuter W, Raus T, Long G. Geneva: Conservatoire et Jardin botaniques de la Ville de Genève; 1986. Med-Checklist3. [Google Scholar]
- Hartvig P. Leptoplax O. E. Schulz. In: Strid A, editor. New York: Cambridge University Press; 1986. pp. 275–276. Mountain flora of Greece1. [Google Scholar]
- Hartvig P. In: Alyssum. Strid A, Tan K, editors. Ruggell: Gantner Verlag; 2002a. pp. 199–224. Flora Hellenica2. [Google Scholar]
- Hartvig P. In: Ptilotrichum; Leptoplax; Bornmuellera. Strid A, Tan K, editors. Ruggell: Gantner Verlag; 2002b. pp. 230–234. Flora Hellenica2. [Google Scholar]
- Hasko A, Çullaj A. Nickel hyper-accumulating species and their potential use for the phyto-remediation of polluted areas. Options Méditerranée, sér. A. 2001;47:137–150. [Google Scholar]
- Ingle RA, Mugford ST, Rees JD, Campbell MM, Smith JAC. Constitutively high expression of the histidine biosynthetic pathway contributes to nickel tolerance in hyperaccumulator plants. The Plant Cell. 2005;17:2089–2106. doi: 10.1105/tpc.104.030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalas J, Suominen J. Helsinki: The Committee for Mapping the Flora of Europe and Societas Biologica Fennica Vanamo; 1996. Atlas florae Europeae11. [Google Scholar]
- Jansen S, Broadley M, Robbrecht E, Smets E. Aluminium hyperaccumulation in angiosperms: a review of its phylogenetic significance. Botanical Review. 2002;68:235–269. [Google Scholar]
- Jansen S, Watanabe T, Caris P, et al. The distribution and phylogeny of aluminium accumulating plants in the Ericales. Plant Biology. 2004;6:498–505. doi: 10.1055/s-2004-820980. [DOI] [PubMed] [Google Scholar]
- Jiménez-Ambriz G, Petit C, Bourrié I, Dubois S, Olivieri I, Ronce O. Life history variation in the heavy metal tolerant plant Thlaspi caerulescens growing in a network of contaminated and noncontaminated sites in southern France: role of gene flow, selection and phenotypic plasticity. New Phytologist. 2007;173:199–215. doi: 10.1111/j.1469-8137.2006.01923.x. [DOI] [PubMed] [Google Scholar]
- Kazakou E, Dimitrakopoulos PG, Baker AJM, Reeves RD, Troumbis AY. Hypotheses, mechanisms and trade-offs of tolerance and adaptation to serpentine soils: from species to ecosystem level. Biological Reviews. 2008;83:495–508. doi: 10.1111/j.1469-185X.2008.00051.x. [DOI] [PubMed] [Google Scholar]
- Kerkeb L, Krämer U. The role of free histidine in xylem loading of nickel in Alyssum lesbiacum and Brassica juncea. Plant Physiology. 2003;131:716–24. doi: 10.1104/pp102.010686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Al-Shehbaz IA. Phylogeny of Brassica and wild relatives. In: Gupta SK, editor. Biology and breeding of crucifers. Boca Raton: Taylor and Francis Group; 2009. pp. 1–19. [Google Scholar]
- Koch M, Mummenhof K. Thlaspi s. str. (Brassicaceae) versus Thlaspi s. l.: morphological and anatomical characters in the light of ITS nrDNA sequence data. Plant Systematics and Evolution. 2001;227:209–225. [Google Scholar]
- Krämer U. Metal hyperaccumulation in plants. Annual Review of Plant Biology. 2010;61:517–534. doi: 10.1146/annurev-arplant-042809-112156. [DOI] [PubMed] [Google Scholar]
- Krämer U, Cotter-Howells JD, Charnock JM, Baker AJM, Smith JAC. Free histidine as a metal chelator in plants that accumulate nickel. Nature. 1996;379:635–638. [Google Scholar]
- Küpfer P, Nieto Feliner G. Alyssum L. In: Castroviejo S, Aedo C, Gómez Campo C, et al., editors. Flora Iberica. Vol. 4. Madrid: Real Jardín Botánico, CSIC; 1993. pp. 167–184. [Google Scholar]
- Li YM, Chaney R, Brewer E, et al. Development of a technology for commercial phytoextraction of nickel: economic and technical considerations. Plant and Soil. 2003;249:107–115. [Google Scholar]
- Macnair MR. The hyperaccumulation of metals by plants. Advances in Botanical Research. 2003;40:63–105. [Google Scholar]
- Menezes de Sequeira E. Toxicity and movement of heavy metals in serpentinic rocks (northeastern Portugal) Agronomia Lusitanica. 1969;30:115–154. [Google Scholar]
- Mengoni A, Baker AJM, Bazzicalupo M, et al. Evolutionary dynamics of nickel hyperaccumulation in Alyssum revealed by ITS nrDNA analysis. New Phytologist. 2003;159:691–699. doi: 10.1046/j.1469-8137.2003.00837.x. [DOI] [PubMed] [Google Scholar]
- Minguzzi C, Vergnano O. Il contenuto di nichel nelle ceneri di Alyssum bertolonii Desv. Memorie della Società Toscana di Scienze Naturali, seria A. 1948;55:49–74. [Google Scholar]
- Mizuno N, Horie K, Mizuno T, Nosaka S. Chemical composition and Ni compound crystals in Ni-hyperaccumulator Thlaspi japonicum. Japanese Journal of Soil Science and Plant Nutrition. 2001;72:529–534. [Google Scholar]
- Montargés-Pelletier E, Chardot V, Echevarria G, Michot LJ, Bauer A, Morel JL. Identification of nickel chelators in three hyperaccumulating plants: an X-ray spectroscopic study. Phytochemistry. 2008;69:1695–1709. doi: 10.1016/j.phytochem.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Mota JF, Medina-Cazorla JM, Navarro FB, et al. Dolomite flora of the Baetic ranges glades (south Spain) Flora. 2008;203:359–375. [Google Scholar]
- Nyárády EJ. Studiu preliminar asupra unor specii de Alyssum din sectia Odontarrhena. Buletinul Grădinii Botanice si al Muzeului Botanic de la Universitatea din Cluj. 1928;7:3–160. [Google Scholar]
- Nyárády EJ. Studiu preliminar asupra unor specii de Alyssum din Sectia Odontarrhena. Buletinul Grădinii Botanice si al Muzeului Botanic de la Universitatea din Cluj. 1929a;8:152–156. [Google Scholar]
- Nyárády EJ. Studiu preliminar asupra unor specii de Alyssum din Sectia Odontarrhena. Buletinul Grădinii Botanice si al Muzeului Botanic de la Universitatea din Cluj. 1929b;9:1–69. [Google Scholar]
- Nyárády EJ. Synopsis specierum, variationum et formatum sectionis Odontarrhenae generis Alyssum. Analele Academiei Republicii Populare Române, seria: Geologie, Geografie, Biologie, Ştiinţe tehnice şi agricole. 1949;3:1–130. [Google Scholar]
- Nyberg Berglund AB, Dahlgren S, Westerbergh A. Evidence for parallel evolution and site-specific selection of serpentine tolerance in Cerastium alpinum during the colonization of Scandinavia. New Phytologist. 2004;161:199–209. [Google Scholar]
- Page RDM. TreeView: an application to display phylogenetic trees on personal computers. Bioinformatics. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- Palmer EF, Warwick F, Keller W. Brassicaceae (Cruciferae) family, plant biotechnology and phytoremediation. International Journal of Phytoremediation. 2001;3:245–287. [Google Scholar]
- Palmgren MG, Clemens S, Williams LE, et al. Zinc biofortification of cereals: problems and solutions. Trends in Plant Science. 2008;13:464–73. doi: 10.1016/j.tplants.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Patterson TB, Givnish TJ. Geographic cohesion, chromosomal evolution, parallel adaptive radiations, and consequent floral adaptations in Calochortus (Calochortaceae): evidence from a cpDNA phylogeny. New Phytologist. 2004;161:253–264. [Google Scholar]
- Pauwels M, Frerot H, Bonnin I, Saumitou-Laprade P. A broad-scale analysis of population differentiation for Zn tolerance in an emerging model species for tolerance study: Arabidopsis halleri (Brassicaceae) European Society for Evolutionary Biology. 2006;19:1838–1850. doi: 10.1111/j.1420-9101.2006.01178.x. [DOI] [PubMed] [Google Scholar]
- Peer WA, Mahmoudian M, Lahner B, Reeves RD, Murphy AS, Salt DE. Identifying model metal hyperaccumulating plants: germplasm analysis of 20 Brassicaceae accessions from a wide geographical area. New Phytologist. 2003;159:421–430. doi: 10.1046/j.1469-8137.2003.00822.x. [DOI] [PubMed] [Google Scholar]
- Peer WA, Mahmoudian M, Freeman JL, et al. Assessment of plants from the Brassicaceae family as genetic models for the study of nickel and zinc hyperaccumulation. New Phytologist. 2006;172:248–260. doi: 10.1111/j.1469-8137.2006.01820.x. [DOI] [PubMed] [Google Scholar]
- Pignatti S. Bologna: Edagricole; 1982. Flora d'Italia1. [Google Scholar]
- Pilon-Smits E. Phytoremediation. Annual Review of Plant Biology. 2005;56:15–39. doi: 10.1146/annurev.arplant.56.032604.144214. [DOI] [PubMed] [Google Scholar]
- Proctor J. Toxins, nutrient shortage and droughts: the serpentine challenge. Trends in Ecology and Evolution. 1999;14:334–335. [Google Scholar]
- Psaras GK, Constantinidis T, Cotsopoulos B, Manetas Y. Relative abundance of nickel in the leaf epidermis of eight hyperaccumulators: evidence that the metal is excluded from both guard cells and trichomes. Annals of Botany. 2000;86:73–78. [Google Scholar]
- Rajakaruna N, Baldwin BG, Chan R, Desrochers AM, Bohm BA, Whitton J. Edaphic races and phylogenetic taxa in the Lasthenia californica complex (Asteraceae: Heliantheae): an hypothesis of parallel evolution. Molecular Ecology. 2003;12:1675–1679. doi: 10.1046/j.1365-294x.2003.01843.x. [DOI] [PubMed] [Google Scholar]
- Redjala T, Sterckeman T, Skiker S, Echevarria G. Contribution of apoplast and symplast to short term nickel uptake by maize and Leptoplax emarginata roots. Environmental and Experimental Botany. 2010;68:99–106. [Google Scholar]
- Reeves RD. Nickel and zinc accumulation by species of Thlaspi L., Cochlearia L., and other genera of the Brassicaceae. Taxon. 1988;37:309–318. [Google Scholar]
- Reeves RD. Tropical hyperaccumulators of metals and their potential for phytoextraction. Plant and Soil. 2003;249:57–65. [Google Scholar]
- Reeves RD, Adigüzel N. Rare plants and nickel accumulators from Turkish serpentine soils, with special reference to Centaurea species. Turkish Journal of Botany. 2004;28:147–153. [Google Scholar]
- Reeves RD, Adigüzel N. The nickel hyperaccumulating plants of the serpentines of Turkey and adjacent areas: a review with new data. Turkish Journal of Biology. 2008;32:143–153. [Google Scholar]
- Reeves RD, Baker AJM. Studies on metal uptake by plants from serpentine and non-serpentine populations of Thlaspi geosingense Hálácsy (Cruciferae) New Phytologist. 1984;98:191–204. doi: 10.1111/j.1469-8137.1984.tb06108.x. [DOI] [PubMed] [Google Scholar]
- Reeves RD, Baker AJM. Metal-accumulating plants. In: Raskin I, Ensley BD, editors. Phytoremediation of toxic metals: using plants to clean up the environment. Hoboken: Wiley-Intersciences; 2000. pp. 193–229. [Google Scholar]
- Reeves RD, Brooks RR. European species of Thlaspi L. (Cruciferae) as indicators of nickel and zinc. Journal of Geochemical Exploration. 1983;18:275–283. [Google Scholar]
- Reeves RD, Brooks RR, Press JR. Nickel accumulation by species of Peltaria Jacq. (Cruciferae) Taxon. 1980;29:629–633. [Google Scholar]
- Reeves RD, Brooks RR, Macfarlane RM. Nickel uptake by Californian Streptanthus and Caulanthus with particular reference to the hyperaccumulator S. polygaloides Gray (Brassicaceae) American Journal of Botany. 1981;68:708–712. [Google Scholar]
- Reeves RD, Brooks RR, Dudley TR. Uptake of nickel by species of Alyssum, Bornmuellera and other genera of Old World tribus Alysseae. Taxon. 1983;32:184–192. [Google Scholar]
- Reeves RD, Baker AJM, Bohridi A, Berazaín R. Nickel hyperaccumulation in the serpentine flora of Cuba. Annals of Botany. 1999;83:29–38. [Google Scholar]
- Reeves RD, Kruckeberg AR, Adigüzel N, Krämer U. Studies on the flora of serpentine and other metalliferous areas of western Turkey. South African Journal of Science. 2001;97:513–517. [Google Scholar]
- Reeves RD, Baker AJM, Becquer T, Echevarria G, Miranda ZJG. The flora and biogeochemistry of the ultramafic soils of Goiàs State, Brazil. Plant and Soil. 2007;293:107–119. [Google Scholar]
- Reeves RD, Adigüzel N, Baker AJM. Nickel hyperaccumulation in Bornmuellera kiyakii Aytaç & Aksoy and associated plants of the Brassicaceae from Kizildağ (Derebucak, Konya-Turkey) Turkish Journal of Biology. 2009;33:33–40. [Google Scholar]
- Ronquist F, Huelsenbeck LP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Simmons MP, Ochoterena H. Gaps as characters in sequence-based phylogenetic analyses. Systematic Biology. 2000;49:369–381. [PubMed] [Google Scholar]
- Stefanović V, Tan K, Iatrou G. Distribution of the endemic Balkan flora on serpentine I. – Obligate serpentine endemics. Plant Systematics and Evolution. 2003;242:149–170. [Google Scholar]
- Swofford DL. PAUP* 4·0. Phylogenetic analysis using parsimony (and other methods) vers. 4·0. Sunderland: Sinauer Associates; 2000. [Google Scholar]
- Thomas DC, Weigend M, Hilger HH. Phylogeny and systematics of Lithodora (Boraginaceae-Lithospermeae) and its affinities to the monotypic genera Mairetis, Halacsya and Paramoltkia based on ITS1 and trnLUAA-sequence data and morphology. Taxon. 2008;57:79–97. [Google Scholar]
- Tschugaeff L. Ueber ein neues, empfindliches Reagens auf Nickel. Berichte der deutschen chemischen Gesellschaft. 1905;38:2520–2522. [Google Scholar]
- Verbruggen N, Hermans C, Schat H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytologist. 2009;181:759–776. doi: 10.1111/j.1469-8137.2008.02748.x. [DOI] [PubMed] [Google Scholar]
- Vergnano Gambi O, Brooks RR, Radford CC. L'accumulo di nichel nelle specie italiane del genere Alyssum. Webbia. 1979;33:269–277. [Google Scholar]
- Vergnano Gambi O, Gabbrielli R. Ecophysiological and geochemical aspects of nickel, chromium and cobalt accumulation in the vegetation of some Italian ophiolitic outcrops. Ofioliti. 1979;4:199–208. [Google Scholar]
- Vinterhalter B, Vinterhalter D. Nickel hyperaccumulation in shoot cultures of Alyssum markgrafii. Biologia Plantarum. 2005;49:121–124. [Google Scholar]
- Warwick SI, Sauder CA, Al-Shehbaz IA. Phylogenetic relationships in the tribe Alysseae (Brassicaceae) based on nuclear ribosomal ITS DNA sequences. Botany. 2008;86:315–336. [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White JW, editors. PCR protocols. A guide to methods and applications. New York: Academic Press; 1990. pp. 315–322. [Google Scholar]
- Whiting SN, Reeves RD, Richards D, et al. Research priorities for conservation of metallophyte biodiversity and their potential for restoration and site remediation. Restoration Ecology. 2004;12:106–116. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.