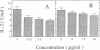Abstract
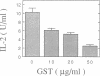
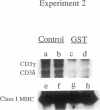
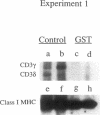
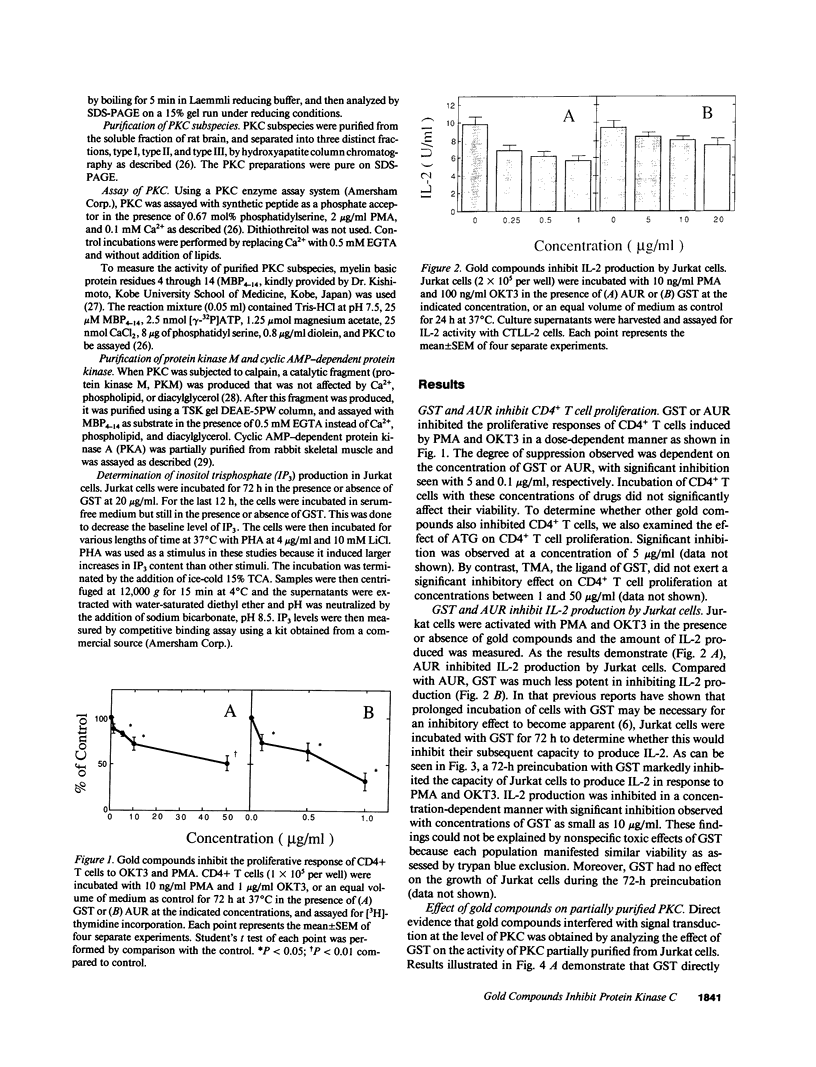
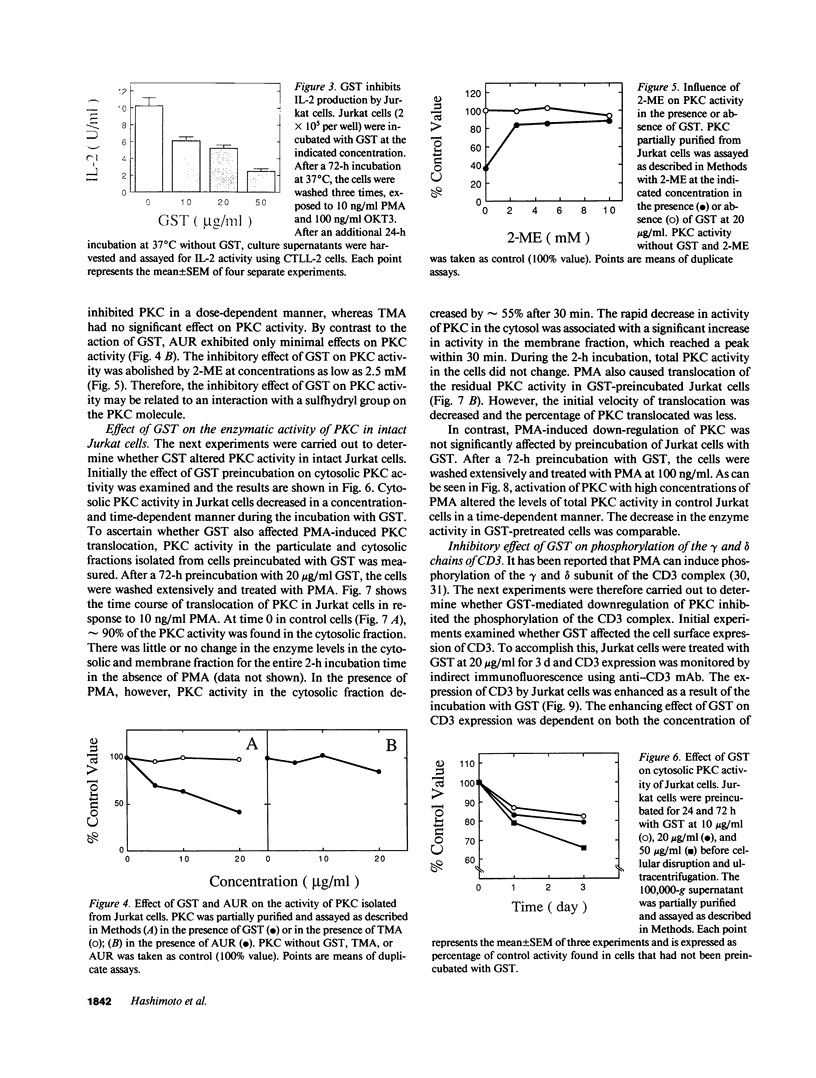
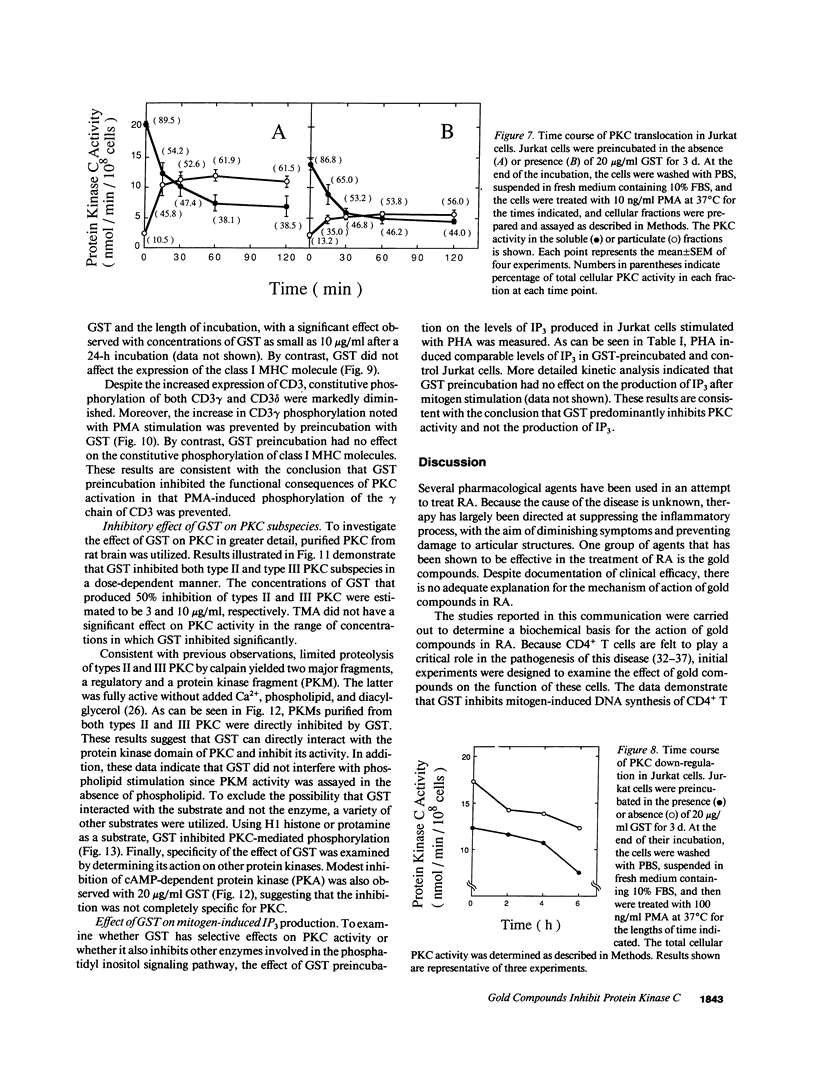
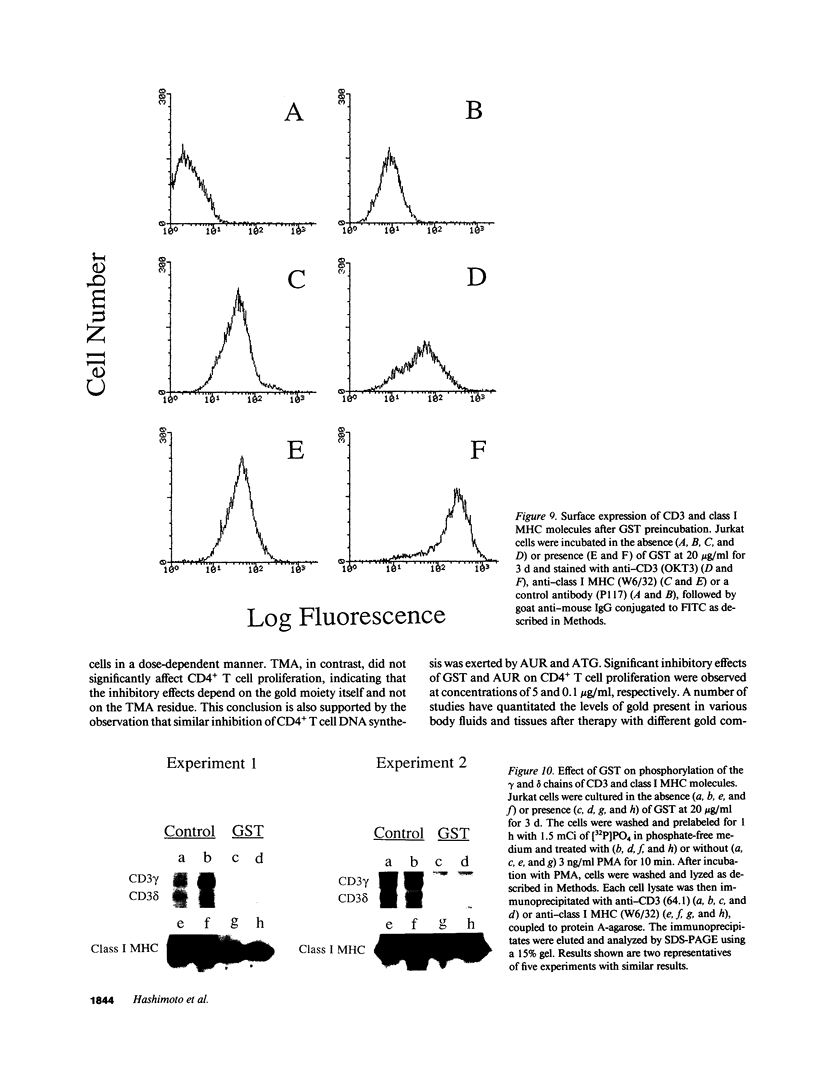
Previous studies have shown that the gold compounds, gold sodium thiomalate (GST) and auranofin (AUR), which are effective in the treatment of rheumatoid arthritis, inhibit functional activities of a variety of cells, but the biochemical basis of their effect is unknown. In the current studies, human T cell proliferation and interleukin 2 production by Jurkat cells were inhibited by GST or AUR at pharmacologically relevant concentrations. Because it has been documented that protein kinase C (PKC) is involved in T cell activation, the capacity of gold compounds to inhibit PKC partially purified from Jurkat cells was assayed in vitro. GST was found to inhibit PKC in a dose-dependent manner, but AUR caused no significant inhibition of PKC at pharmacologically relevant concentrations. The inhibitory effect of GST on PKC was abolished by 2-mercaptoethanol. To investigate the effect of GST on the regulation of PKC in vivo, the levels of PKC activity in Jurkat cells were examined. Cytosolic PKC activity decreased slowly in a concentration- and time-dependent manner as a result of incubation of Jurkat cells with GST. To ascertain whether GST inhibited PKC translocation and down-regulation, PKC activities associated with the membrane and cystosolic fractions were evaluated after phorbol myristate acetate (PMA) stimulation of GST incubated Jurkat cells. Translocation of PKC was markedly inhibited by pretreatment of Jurkat cells with GST for 3 d, but the capacity of PMA to down-regulate PKC activity in Jurkat cells was not altered by GST preincubation. The functional impact of GST-mediated downregulation of PKC in Jurkat cells was examined by analyzing PMA-stimulated phosphorylation of CD3. Although GST preincubated Jurkat cells exhibited an increased density of CD3, PMA-stimulated phosphorylation of the gamma chain of CD3 was markedly inhibited. Specificity for the inhibitory effect of GST on PKC was suggested by the finding that GST did not alter the mitogen-induced increases in inositol trisphosphate levels in Jurkat cells. Finally, the mechanism of the GST-induced inhibition of PKC was examined in detail, using purified PKC subspecies from rat brain. GST inhibited type II PKC more effectively than type III PKC, and also inhibited the enzymatic activity of the isolated catalytic fragment of PKC. The inhibitory effect of GST on PKC activity could not be explained by competition with phospholipid or nonspecific interference with the substrate. These data suggest that the immunomodulatory effects of GST may result from its capacity to inhibit PKC activity.
Full text
PDF

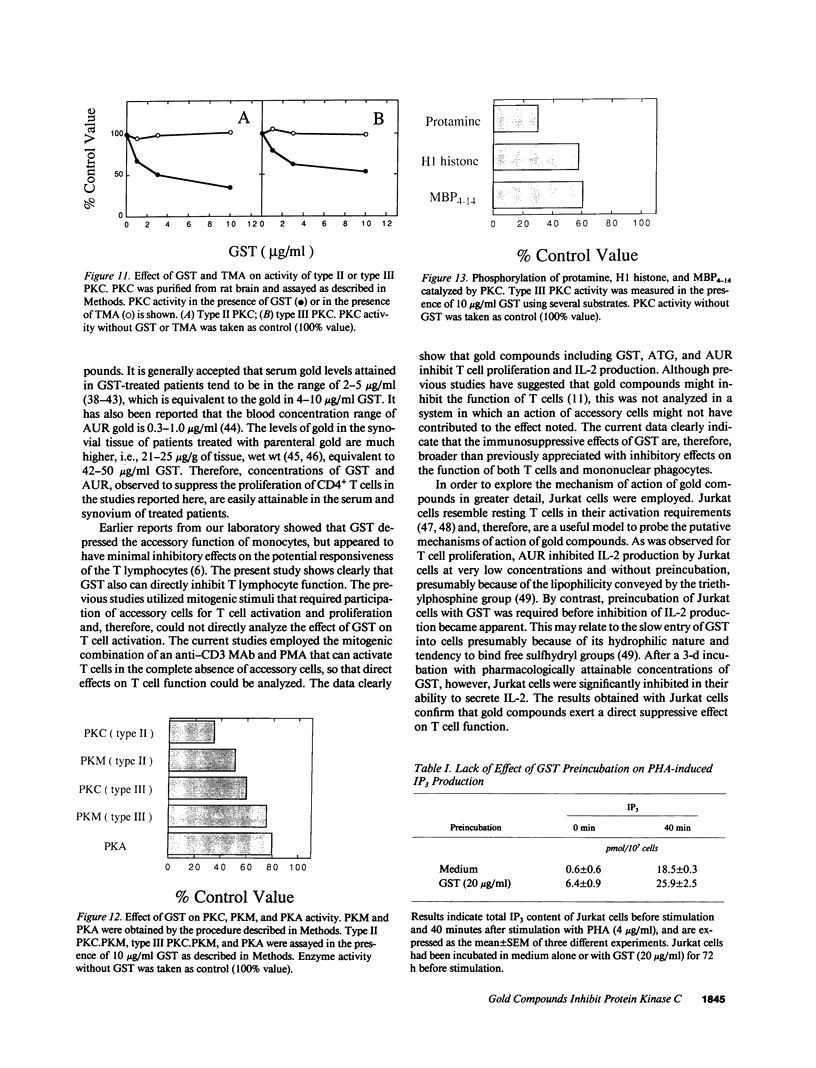



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander D. R., Hexham J. M., Lucas S. C., Graves J. D., Cantrell D. A., Crumpton M. J. A protein kinase C pseudosubstrate peptide inhibits phosphorylation of the CD3 antigen in streptolysin-O-permeabilized human T lymphocytes. Biochem J. 1989 Jun 15;260(3):893–901. doi: 10.1042/bj2600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Berry N., Nishizuka Y. Protein kinase C and T cell activation. Eur J Biochem. 1990 Apr 30;189(2):205–214. doi: 10.1111/j.1432-1033.1990.tb15478.x. [DOI] [PubMed] [Google Scholar]
- Billings R., Grahame R., Marks V., Wood P. J., Taylor A. Blood and urine gold levels during chrysotherapy for rheumatoid arthritis. Rheumatol Rehabil. 1975 Feb;14(1):13–18. doi: 10.1093/rheumatology/14.1.13. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P. Monomorphic anti-HLA-A,B,C monoclonal antibodies detecting molecular subunits and combinatorial determinants. J Immunol. 1982 Jan;128(1):129–135. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cantrell D. A., Davies A. A., Crumpton M. J. Activators of protein kinase C down-regulate and phosphorylate the T3/T-cell antigen receptor complex of human T lymphocytes. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8158–8162. doi: 10.1073/pnas.82.23.8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooke S. T., Snyder R. M., Butt T. R., Ecker D. J., Allaudeen H. S., Monia B., Mirabelli C. K. Cellular and molecular pharmacology of auranofin and related gold complexes. Biochem Pharmacol. 1986 Oct 15;35(20):3423–3431. doi: 10.1016/0006-2952(86)90608-8. [DOI] [PubMed] [Google Scholar]
- DeGré M., Mellbye O. J., Clarke-Jenssen O. Immune interferon in serum and synovial fluid in rheumatoid arthritis and related disorders. Ann Rheum Dis. 1983 Dec;42(6):672–676. doi: 10.1136/ard.42.6.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke O., Panayi G. S., Janossy G., Poulter L. W. An immunohistological analysis of lymphocyte subpopulations and their microenvironment in the synovial membranes of patients with rheumatoid arthritis using monoclonal antibodies. Clin Exp Immunol. 1982 Jul;49(1):22–30. [PMC free article] [PubMed] [Google Scholar]
- Froscio M., Murray A. W., Hurst N. P. Inhibition of protein kinase C activity by the antirheumatic drug auranofin. Biochem Pharmacol. 1989 Jul 1;38(13):2087–2089. doi: 10.1016/0006-2952(89)90061-0. [DOI] [PubMed] [Google Scholar]
- Galili U., Schlesinger M. The formation of stable E rosettes after neuraminidase treatment of either human peripheral blood lymphocytes or of sheep red blood cells. J Immunol. 1974 May;112(5):1628–1634. [PubMed] [Google Scholar]
- Gerber R. C., Paulus H. E., Bluestone R., Pearson C. M. Clinical response and serum gold levels in chrysotherapy. Lack of correlation. Ann Rheum Dis. 1972 Jul;31(4):308–310. doi: 10.1136/ard.31.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb N. L. Comparative pharmacokinetics of parenteral and oral gold compounds. J Rheumatol Suppl. 1982 Jul-Aug;8:99–109. [PubMed] [Google Scholar]
- Gottlieb N. L., Smith P. M., Smith E. M. Tissue gold concentration in a rheumatoid arthritic receiving chrysotherapy. Arthritis Rheum. 1972 Jan-Feb;15(1):16–22. doi: 10.1002/art.1780150103. [DOI] [PubMed] [Google Scholar]
- Grahame R., Billings R., Laurence M., Marks V., Wood P. J. Tissue gold levels after chrysotherapy. Ann Rheum Dis. 1974 Nov;33(6):536–539. doi: 10.1136/ard.33.6.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanly J. G., Bresnihan B. Reduction of peripheral blood lymphocytes in patients receiving gold therapy for rheumatoid arthritis. Ann Rheum Dis. 1985 May;44(5):299–301. doi: 10.1136/ard.44.5.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husby G., Williams R. C., Jr Immunohistochemical studies of interleukin-2 and gamma-interferon in rheumatoid arthritis. Arthritis Rheum. 1985 Feb;28(2):174–181. doi: 10.1002/art.1780280212. [DOI] [PubMed] [Google Scholar]
- Huskisson E. C. Specific therapy for rheumatoid arthritis. Rheumatol Rehabil. 1976 Aug;15(3):133–135. [PubMed] [Google Scholar]
- Jessop J. D., Johns R. G. Serum gold determinations in patients with rheumatoid arthritis receiving sodium aurothiomalate. Ann Rheum Dis. 1973 May;32(3):228–232. doi: 10.1136/ard.32.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez J. S., Kupfer A., Gani V., Shaltiel S. Salt-induced conformational changes in the catalytic subunit of adenosine cyclic 3',5'-phosphate dependent protein kinase. Use for establishing a connection between one sulfhydryl group and the gamma-P subsite in the ATP site of this subunit. Biochemistry. 1982 Mar 30;21(7):1623–1630. doi: 10.1021/bi00536a024. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Kajikawa N., Shiota M., Nishizuka Y. Proteolytic activation of calcium-activated, phospholipid-dependent protein kinase by calcium-dependent neutral protease. J Biol Chem. 1983 Jan 25;258(2):1156–1164. [PubMed] [Google Scholar]
- Klareskog L., Forsum U., Wigren A., Wigzell H. Relationship between HLA-DR-expressing cells and T lymphocytes of different subsets in rheumatoid synovial tissue. Scand J Immunol. 1981 May;15(5):501–507. doi: 10.1111/j.1365-3083.1982.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Kotzin B. L., Strober S., Engleman E. G., Calin A., Hoppe R. T., Kansas G. S., Terrell C. P., Kaplan H. S. Treatment of intractable rheumatoid arthritis with total lymphoid irradiation. N Engl J Med. 1981 Oct 22;305(17):969–976. doi: 10.1056/NEJM198110223051702. [DOI] [PubMed] [Google Scholar]
- Kumon A., Nishiyama K., Yamamura H., Nishizuka Y. Multiplicity of adenosine 3',5'-monophosphate-dependent protein kinases from rat liver and mode of action of nucleoside 3',5'-monophosphate. J Biol Chem. 1972 Jun 25;247(12):3726–3735. [PubMed] [Google Scholar]
- Kung P., Goldstein G., Reinherz E. L., Schlossman S. F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979 Oct 19;206(4416):347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- Lampson L. A., Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980 Jul;125(1):293–299. [PubMed] [Google Scholar]
- Lewis A. J., Walz D. T. Immunopharmacology of gold. Prog Med Chem. 1982;19:1–58. doi: 10.1016/s0079-6468(08)70327-8. [DOI] [PubMed] [Google Scholar]
- Lipsky P. E., Ziff M. Inhibition of antigen- and mitogen-induced human lymphocyte proliferation by gold compounds. J Clin Invest. 1977 Mar;59(3):455–466. doi: 10.1172/JCI108660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman B. H., Carlson P. L., Loose L. D., Sanders K. M. Effects of gold sodium thiomalate and tenidap sodium (CP-66,248-2) on a model of macrophage differentiation using HL-60 cells. Arthritis Rheum. 1990 Jan;33(1):29–36. doi: 10.1002/art.1780330104. [DOI] [PubMed] [Google Scholar]
- Littman B. H., Schwartz P. Gold inhibition of the production of the second complement component by lymphokine-stimulated human monocytes. Arthritis Rheum. 1982 Mar;25(3):288–296. doi: 10.1002/art.1780250306. [DOI] [PubMed] [Google Scholar]
- Lorber A., Cohen R. L., Chang C. C., Anderson H. E. Gold determination in biological fluids by atomic absorption spectrophotometry: application to chrysotherapy in rheumatoid arthritis patients. Arthritis Rheum. 1968 Apr;11(2):170–177. doi: 10.1002/art.1780110207. [DOI] [PubMed] [Google Scholar]
- Lorber A., Simon T., Leeb J., Peter A., Wilcox S. Chrysotherapy. Suppression of immunoglobulin synthesis. Arthritis Rheum. 1978 Sep-Oct;21(7):785–791. doi: 10.1002/art.1780210708. [DOI] [PubMed] [Google Scholar]
- Lucas S., Marais R., Graves J. D., Alexander D., Parker P., Cantrell D. A. Heterogeneity of protein kinase C expression and regulation in T lymphocytes. FEBS Lett. 1990 Jan 15;260(1):53–56. doi: 10.1016/0014-5793(90)80064-p. [DOI] [PubMed] [Google Scholar]
- Manger B., Weiss A., Weyand C., Goronzy J., Stobo J. D. T cell activation: differences in the signals required for IL 2 production by nonactivated and activated T cells. J Immunol. 1985 Dec;135(6):3669–3673. [PubMed] [Google Scholar]
- Mascarenhas B. R., Granda J. L., Freyberg R. H. Gold metabolism in patients with rheumatoid arthritis treated with gold compounds--reinvestigated. Arthritis Rheum. 1972 Jul-Aug;15(4):391–402. doi: 10.1002/art.1780150410. [DOI] [PubMed] [Google Scholar]
- Matsubara T., Ziff M. Inhibition of human endothelial cell proliferation by gold compounds. J Clin Invest. 1987 May;79(5):1440–1446. doi: 10.1172/JCI112972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabelli C. K., Sung C. P., Picker D. H., Barnard C., Hydes P., Badger A. M. Effect of metal containing compounds on superoxide release from phorbol myristate acetate stimulated murine peritoneal macrophages: inhibition by auranofin and spirogermanium. J Rheumatol. 1988 Jul;15(7):1064–1069. [PubMed] [Google Scholar]
- Mori T., Takai Y., Minakuchi R., Yu B., Nishizuka Y. Inhibitory action of chlorpromazine, dibucaine, and other phospholipid-interacting drugs on calcium-activated, phospholipid-dependent protein kinase. J Biol Chem. 1980 Sep 25;255(18):8378–8380. [PubMed] [Google Scholar]
- Nel A. E., Wooten M. W., Galbraith R. M. Molecular signaling mechanisms in T-lymphocyte activation pathways: a review and future prospects. Clin Immunol Immunopathol. 1987 Aug;44(2):167–186. doi: 10.1016/0090-1229(87)90064-x. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Oliver D., Sommer K. R., Panyim S., Spiker S., Chalkley R. A modified procedure for fractionating histones. Biochem J. 1972 Sep;129(2):349–353. doi: 10.1042/bj1290349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parente J. E., Walsh M. P., Girard P. R., Kuo J. F., Ng D. S., Wong K. Effects of gold coordination complexes on neutrophil function are mediated via inhibition of protein kinase C. Mol Pharmacol. 1989 Jan;35(1):26–33. [PubMed] [Google Scholar]
- Parker P. J., Coussens L., Totty N., Rhee L., Young S., Chen E., Stabel S., Waterfield M. D., Ullrich A. The complete primary structure of protein kinase C--the major phorbol ester receptor. Science. 1986 Aug 22;233(4766):853–859. doi: 10.1126/science.3755547. [DOI] [PubMed] [Google Scholar]
- Paulus H. E., Machleder H. I., Levine S., Yu D. T., MacDonald N. S. Lymphocyte involvement in rheumatoid arthritis. Studies during thoracic duct drainage. Arthritis Rheum. 1977 Jul-Aug;20(6):1249–1262. doi: 10.1002/art.1780200614. [DOI] [PubMed] [Google Scholar]
- Rubinstein H. M., Dietz A. A. Serum gold. II. Levels in rheumatoid arthritis. Ann Rheum Dis. 1973 Mar;32(2):128–132. doi: 10.1136/ard.32.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi K., Tsukuda M., Ase K., Kikkawa U., Nishizuka Y. Mode of activation and kinetic properties of three distinct forms of protein kinase C from rat brain. J Biochem. 1988 May;103(5):759–765. doi: 10.1093/oxfordjournals.jbchem.a122343. [DOI] [PubMed] [Google Scholar]
- Shearman M. S., Berry N., Oda T., Ase K., Kikkawa U., Nishizuka Y. Isolation of protein kinase C subspecies from a preparation of human T lymphocytes. FEBS Lett. 1988 Jul 18;234(2):387–391. doi: 10.1016/0014-5793(88)80122-4. [DOI] [PubMed] [Google Scholar]
- Stohl W., Linker-Israeli M. Inhibitory effects of anti-CD2 monoclonal antibodies on interleukin 2 production and interleukin 2 receptor expression in anti-CD3-induced T cell activation. Cell Immunol. 1989 May;120(2):351–365. doi: 10.1016/0008-8749(89)90203-7. [DOI] [PubMed] [Google Scholar]
- Sutton B. M., McGusty E., Walz D. T., DiMartino M. J. Oral gold. Antiarthritic properties of alkylphosphinegold coordination complexes. J Med Chem. 1972 Nov;15(11):1095–1098. doi: 10.1021/jm00281a001. [DOI] [PubMed] [Google Scholar]
- Thiele D. L., Kurosaka M., Lipsky P. E. Phenotype of the accessory cell necessary for mitogen-stimulated T and B cell responses in human peripheral blood: delineation by its sensitivity to the lysosomotropic agent, L-leucine methyl ester. J Immunol. 1983 Nov;131(5):2282–2290. [PubMed] [Google Scholar]
- Thiele D. L., Lipsky P. E. Regulation of cellular function by products of lysosomal enzyme activity: elimination of human natural killer cells by a dipeptide methyl ester generated from L-leucine methyl ester by monocytes or polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2468–2472. doi: 10.1073/pnas.82.8.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukuda M., Asaoka Y., Sekiguchi K., Kikkawa U., Nishizuka Y. Properties of protein kinase C subspecies in human platelets. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1387–1395. doi: 10.1016/s0006-291x(88)81295-6. [DOI] [PubMed] [Google Scholar]
- Walz D. T., DiMartino M. J., Chakrin L. W., Sutton B. M., MISHER A. Antiarthritic properties and unique pharmacologic profile of a potential chrysotherapeutic agent: S K & F D-30162. J Pharmacol Exp Ther. 1976 Apr;197(1):142–152. [PubMed] [Google Scholar]
- Walz D. T., DiMartino M. J., Griswold D. E. Comparative pharmacology and biological effects of different gold compounds. J Rheumatol Suppl. 1982 Jul-Aug;8:54–60. [PubMed] [Google Scholar]
- Wolf R. E., Hall V. C. Inhibition of in vitro proliferative response of cultured T lymphocytes to interleukin-2 by gold sodium thiomalate. Arthritis Rheum. 1988 Feb;31(2):176–181. doi: 10.1002/art.1780310204. [DOI] [PubMed] [Google Scholar]
- Wright V., Amos R. Do drugs change the course of rheumatoid arthritis? Br Med J. 1980 Apr 5;280(6219):964–966. doi: 10.1136/bmj.280.6219.964-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Criss W. E., Takai Y., Yamamura H., Nishizuka Y. A hepatic soluble cyclic nucleotide-independent protein kinase. Stimulation by basic polypeptides. J Biol Chem. 1979 Jun 25;254(12):5049–5052. [PubMed] [Google Scholar]
- Yasuda I., Kishimoto A., Tanaka S., Tominaga M., Sakurai A., Nishizuka Y. A synthetic peptide substrate for selective assay of protein kinase C. Biochem Biophys Res Commun. 1990 Feb 14;166(3):1220–1227. doi: 10.1016/0006-291x(90)90996-z. [DOI] [PubMed] [Google Scholar]
- Yoshimura N., Kikuchi T., Sasaki T., Kitahara A., Hatanaka M., Murachi T. Two distinct Ca2+ proteases (calpain I and calpain II) purified concurrently by the same method from rat kidney. J Biol Chem. 1983 Jul 25;258(14):8883–8889. [PubMed] [Google Scholar]