Abstract
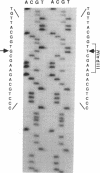
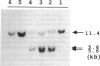
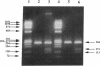
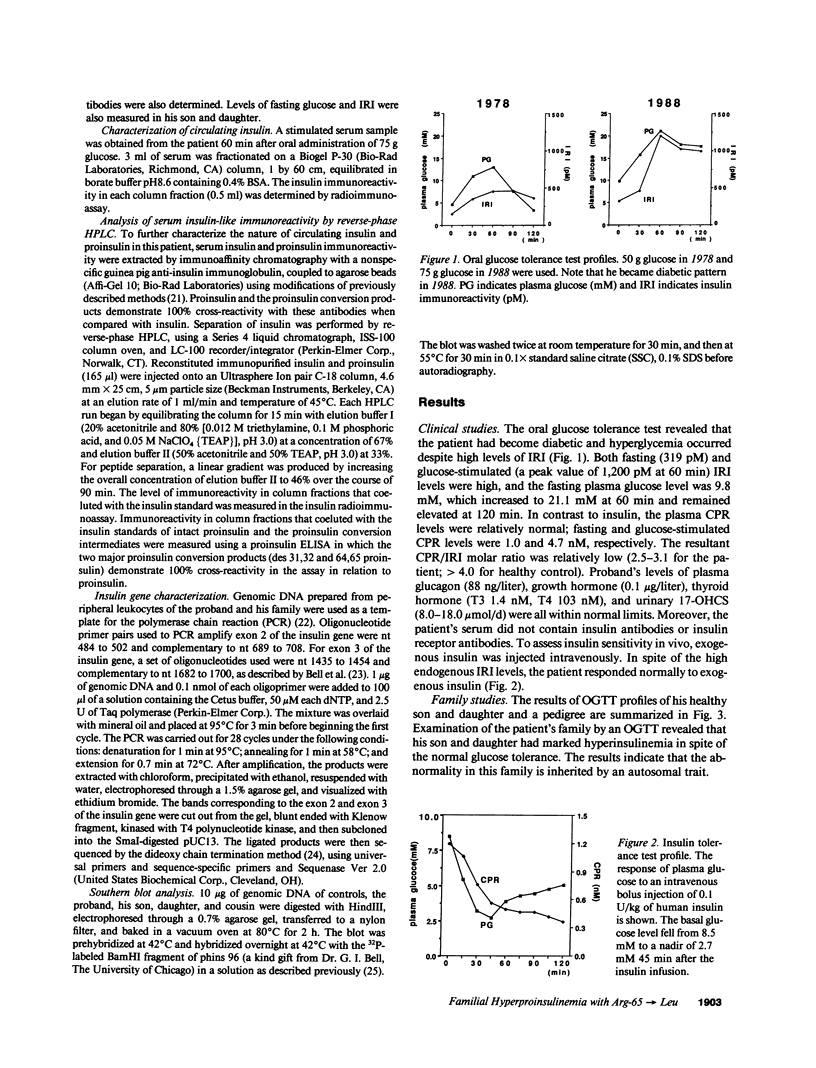
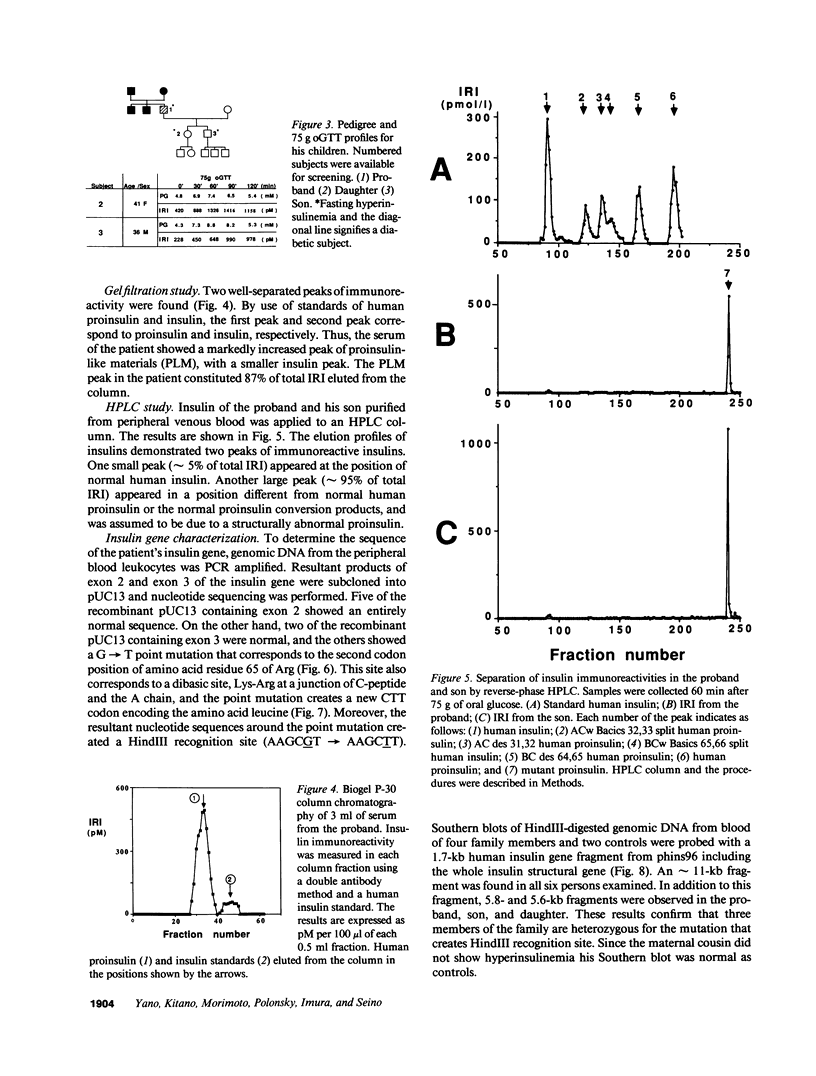
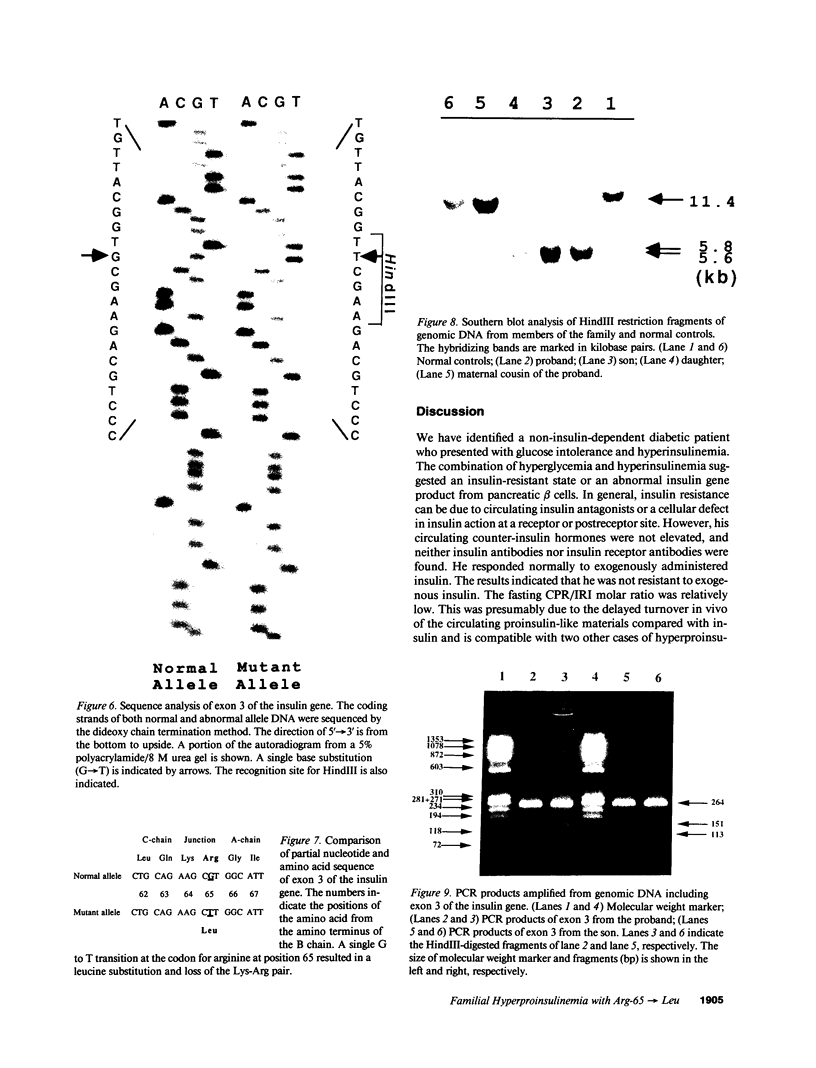
We have identified a 65-yr-old nonobese Japanese man with diabetes mellitus, fasting hyperinsulinemia (150-300 pM), and a reduced fasting C-peptide/insulin molar ratio of 2.5-3.0. Fasting hyperinsulinemia was also found in his son and daughter. Analysis of insulin isolated from the serum of the proband and his son by reverse-phase high performance liquid chromatography revealed a minor peak coeluting with human insulin and a major peak of proinsulin-like materials. The insulin gene of the patient was amplified by the polymerase chain reaction and the products were sequenced. A novel point mutation was identified in which guanine was replaced by thymine. The substitution gives rise to a new HindIII recognition site and results in the amino acid replacement of leucine for arginine at position 65. These results indicate that the amino-acid replacement prevents recognition of the C-peptide-A chain dibasic protease and results in an elevation of proinsulin-like materials in the circulation. Furthermore, in this family the proinsulin-like materials is due to a biosynthetic defect, inherited as an autosomal dominant trait. Rapid detection of this mutation can be accomplished by HindIII restriction enzyme mapping of polymerase chain reaction-generated DNA, which enables us to facilitate the diagnosis and screening.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbetti F., Raben N., Kadowaki T., Cama A., Accili D., Gabbay K. H., Merenich J. A., Taylor S. I., Roth J. Two unrelated patients with familial hyperproinsulinemia due to a mutation substituting histidine for arginine at position 65 in the proinsulin molecule: identification of the mutation by direct sequencing of genomic deoxyribonucleic acid amplified by polymerase chain reaction. J Clin Endocrinol Metab. 1990 Jul;71(1):164–169. doi: 10.1210/jcem-71-1-164. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Pictet R. L., Rutter W. J., Cordell B., Tischer E., Goodman H. M. Sequence of the human insulin gene. Nature. 1980 Mar 6;284(5751):26–32. doi: 10.1038/284026a0. [DOI] [PubMed] [Google Scholar]
- Burgess T. L., Kelly R. B. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- Carroll R. J., Hammer R. E., Chan S. J., Swift H. H., Rubenstein A. H., Steiner D. F. A mutant human proinsulin is secreted from islets of Langerhans in increased amounts via an unregulated pathway. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8943–8947. doi: 10.1073/pnas.85.23.8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay K. H., Bergenstal R. M., Wolff J., Mako M. E., Rubenstein A. H. Familial hyperproinsulinemia: partial characterization of circulating proinsulin-like material. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2881–2885. doi: 10.1073/pnas.76.6.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Given B. D., Cohen R. M., Shoelson S. E., Frank B. H., Rubenstein A. H., Tager H. S. Biochemical and clinical implications of proinsulin conversion intermediates. J Clin Invest. 1985 Oct;76(4):1398–1405. doi: 10.1172/JCI112116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruppuso P. A., Gorden P., Kahn C. R., Cornblath M., Zeller W. P., Schwartz R. Familial hyperproinsulinemia due to a proposed defect in conversion of proinsulin to insulin. N Engl J Med. 1984 Sep 6;311(10):629–634. doi: 10.1056/NEJM198409063111003. [DOI] [PubMed] [Google Scholar]
- Haneda M., Chan S. J., Kwok S. C., Rubenstein A. H., Steiner D. F. Studies on mutant human insulin genes: identification and sequence analysis of a gene encoding [SerB24]insulin. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6366–6370. doi: 10.1073/pnas.80.20.6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneda M., Polonsky K. S., Bergenstal R. M., Jaspan J. B., Shoelson S. E., Blix P. M., Chan S. J., Kwok S. C., Wishner W. B., Zeidler A. Familial hyperinsulinemia due to a structurally abnormal insulin. Definition of an emerging new clinical syndrome. N Engl J Med. 1984 May 17;310(20):1288–1294. doi: 10.1056/NEJM198405173102004. [DOI] [PubMed] [Google Scholar]
- Heding L. G. Specific and direct radioimmunoassay for human proinsulin in serum. Diabetologia. 1977 Sep;13(5):467–474. doi: 10.1007/BF01234498. [DOI] [PubMed] [Google Scholar]
- Horwitz D. L., Starr J. I., Mako M. E., Blackard W. G., Rubenstein A. H. Proinsulin, insulin, and C-peptide concentrations in human portal and peripheral blood. J Clin Invest. 1975 Jun;55(6):1278–1283. doi: 10.1172/JCI108047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto Y., Sakura H., Ishii Y., Yamamoto R., Kumakura S., Sakamoto Y., Matsuda A., Kuzuya T. A new case of abnormal insulinemia with diabetes. Reduced insulin values determined by radioreceptor assay. Diabetes. 1986 Nov;35(11):1237–1242. doi: 10.2337/diab.35.11.1237. [DOI] [PubMed] [Google Scholar]
- Kwok S. C., Steiner D. F., Rubenstein A. H., Tager H. S. Identification of a point mutation in the human insulin gene giving rise to a structurally abnormal insulin (insulin Chicago). Diabetes. 1983 Sep;32(9):872–875. doi: 10.2337/diab.32.9.872. [DOI] [PubMed] [Google Scholar]
- Nanjo K., Miyano M., Kondo M., Sanke T., Nishimura S., Miyamura K., Inouye K., Given B. D., Chan S. J., Polonsky K. S. Insulin Wakayama: familial mutant insulin syndrome in Japan. Diabetologia. 1987 Feb;30(2):87–92. doi: 10.1007/BF00274577. [DOI] [PubMed] [Google Scholar]
- Nanjo K., Sanke T., Kondo M., Nishimura S., Miyano M., Linuma J., Miyamura K., Inouye K., Given B. D., Polonsky K. S. Mutant insulin syndrome: identification of two families with [LeuA3]insulin and determination of its biological activity. Trans Assoc Am Physicians. 1986;99:132–142. [PubMed] [Google Scholar]
- Nanjo K., Sanke T., Miyano M., Okai K., Sowa R., Kondo M., Nishimura S., Iwo K., Miyamura K., Given B. D. Diabetes due to secretion of a structurally abnormal insulin (insulin Wakayama). Clinical and functional characteristics of [LeuA3] insulin. J Clin Invest. 1986 Feb;77(2):514–519. doi: 10.1172/JCI112331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbow S. J., Woodhead J. S., Yue D. K., Luzio S. D., Hales C. N. Measurement of human proinsulin by an indirect two-site immunoradiometric assay. Diabetologia. 1979 Oct;17(4):229–234. doi: 10.1007/BF01235859. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki Y., Kawakami T., Kanazawa Y., Akanuma Y., Takaku F. Posttranslational cleavage of proinsulin is blocked by a point mutation in familial hyperproinsulinemia. J Clin Invest. 1985 Jul;76(1):378–380. doi: 10.1172/JCI111973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson S., Fickova M., Haneda M., Nahum A., Musso G., Kaiser E. T., Rubenstein A. H., Tager H. Identification of a mutant human insulin predicted to contain a serine-for-phenylalanine substitution. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7390–7394. doi: 10.1073/pnas.80.24.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. F., Oyer P. E. The biosynthesis of insulin and a probable precursor of insulin by a human islet cell adenoma. Proc Natl Acad Sci U S A. 1967 Feb;57(2):473–480. doi: 10.1073/pnas.57.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H., Seino Y., Inagaki N., Hinokio Y., Yamamoto T., Yasuda K., Masuda K., Someya Y., Imura H. Tissue distribution and species difference of the brain type glucose transporter (GLUT3). Biochem Biophys Res Commun. 1991 Jan 31;174(2):470–477. doi: 10.1016/0006-291x(91)91440-n. [DOI] [PubMed] [Google Scholar]