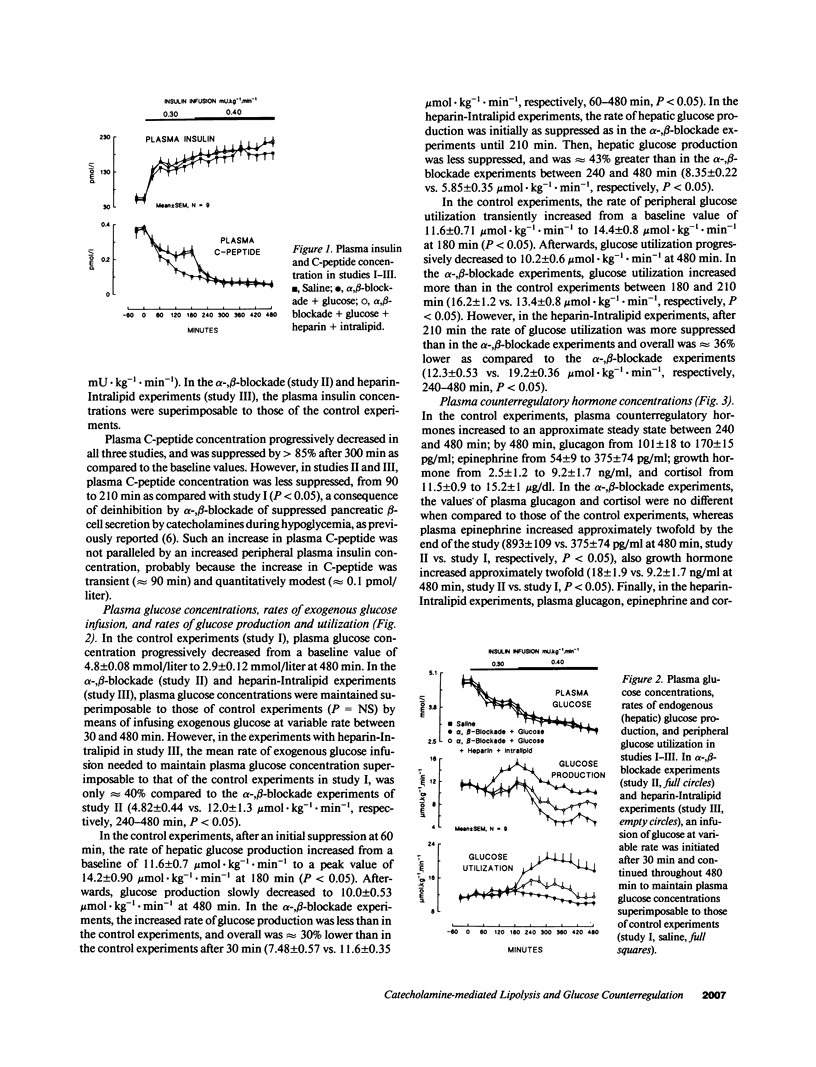
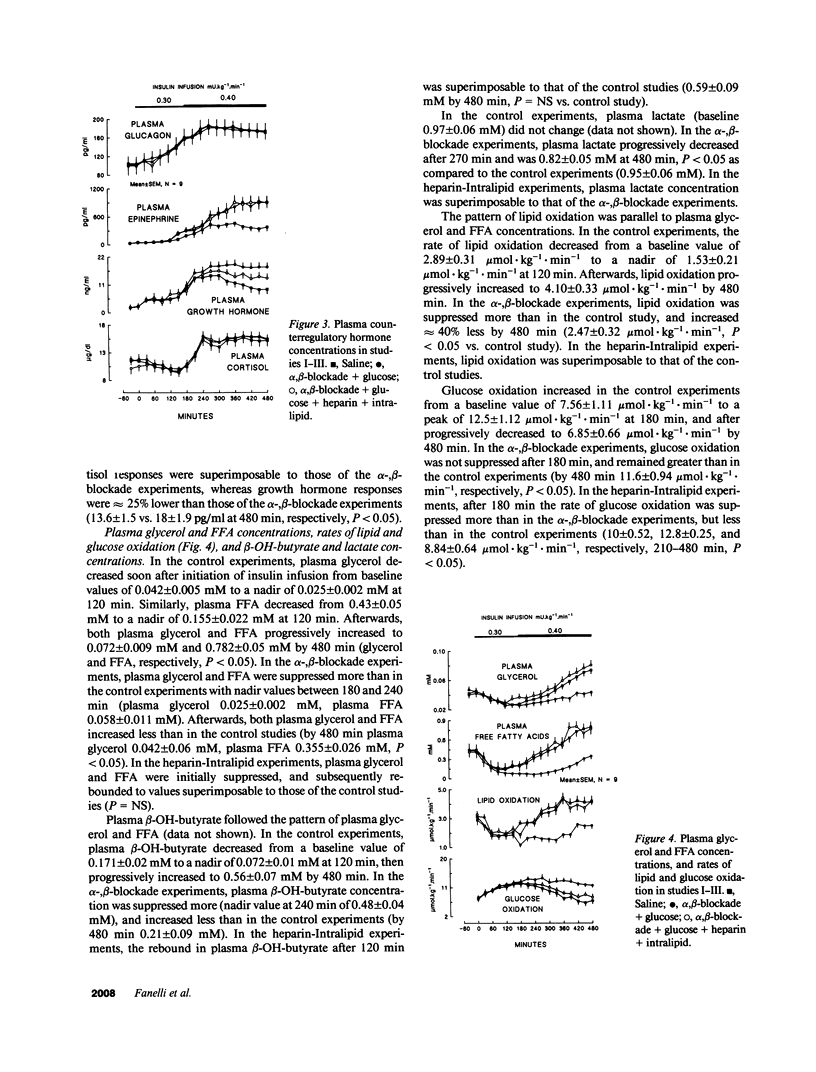
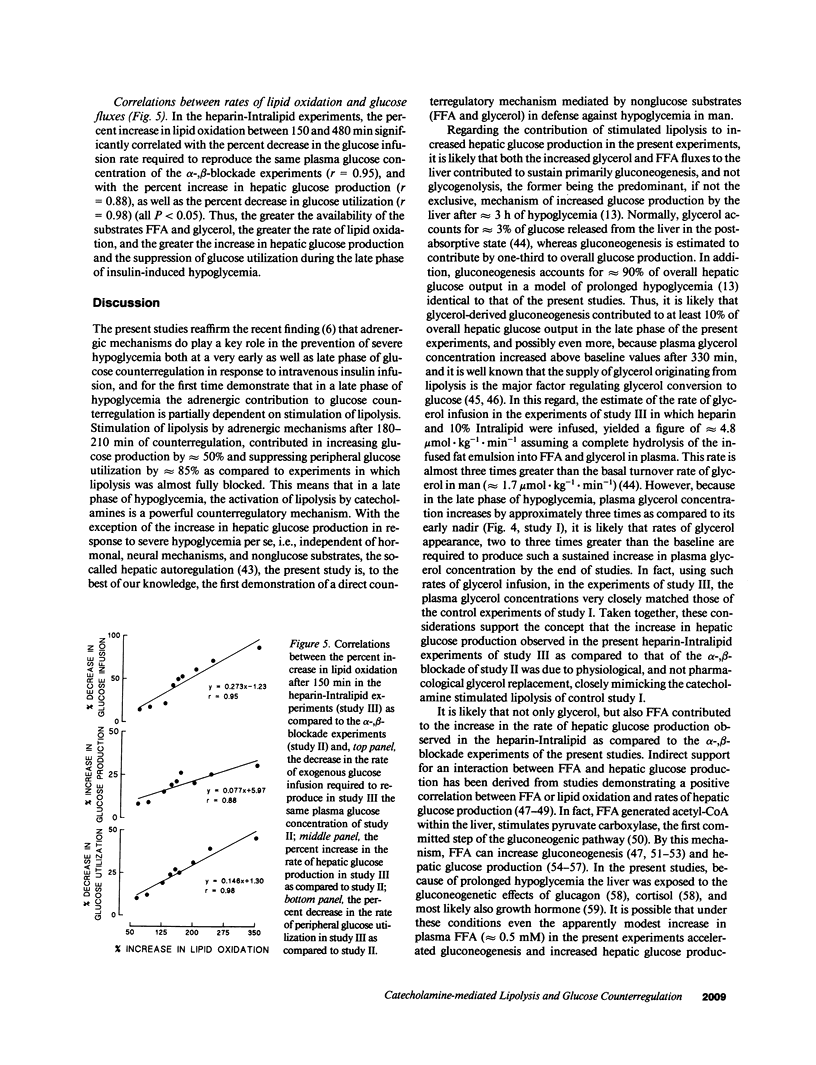
Abstract
Three studies were performed on nine normal volunteers to assess whether catecholamine-mediated lipolysis contributes to counterregulation to hypoglycemia. In these three studies, insulin was intravenously infused for 8 h (0.30 mU.kg-1.min-1 from 0 to 180 min, and 0.40 mU.kg-1.min-1 until 480 min). In study I (control study), only insulin was infused; in study II (direct + indirect effects of catecholamines), propranolol and phentolamine were superimposed to insulin and exogenous glucose was infused to reproduce the same plasma glucose (PG) concentration of study I. Study III (indirect effect of catecholamines) was the same as study II, except heparin (0.2 U.kg-1.min-1 after 80 min), 10% Intralipid (1 ml.min-1 after 160 min) and variable glucose to match PG of study II, were also infused. Glucose production (HGO), glucose utilization (Rd) [3-3H]glucose, and glucose oxidation and lipid oxidation (LO) (indirect calorimetry) were determined. In all three studies, PG decreased from approximately 4.8 to approximately 2.9 mmol/liter (P = NS between studies), and plasma glycerol and FFA decreased to a nadir at 120 min. Afterwards, in study I plasma glycerol and FFA increased by approximately 75% at 480 min, but in study II they remained approximately 40% lower than in study I, whereas in study III they rebounded as in study I (P = NS). In study II, LO was lower than in study I (1.69 +/- 0.13 vs. 3.53 +/- 0.19 mumol.kg-1.min-1, P less than 0.05); HGO was also lower between 60 and 480 min (7.48 +/- 0.57 vs. 11.6 +/- 0.35 mumol.kg-1.min-1, P less than 0.05), whereas Rd was greater between 210 and 480 min (19 +/- 0.38 vs. 11.4 +/- 0.34 mumol.kg-1.min-1, respectively, P less than 0.05). In study III, LO increased to the values of study I; between 4 and 8 h, HGO increased by approximately 2.5 mumol.kg-1.min-1, and Rd decreased by approximately 7 mumol.kg-1.min-1 vs. study II. We conclude that, in a late phase of hypoglycemia, the indirect effects of catecholamines (lipolysis mediated) account for at least approximately 50% of the adrenergic contribution to increased HGO, and approximately 85% of suppressed Rd.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amiel S. A., Archibald H. R., Chusney G., Williams A. J., Gale E. A. Ketone infusion lowers hormonal responses to hypoglycaemia: evidence for acute cerebral utilization of a non-glucose fuel. Clin Sci (Lond) 1991 Aug;81(2):189–194. doi: 10.1042/cs0810189. [DOI] [PubMed] [Google Scholar]
- Arias P., Kerner W., Zier H., Navascués I., Pfeiffer E. F. Incidence of hypoglycemic episodes in diabetic patients under continuous subcutaneous insulin infusion and intensified conventional insulin treatment: assessment by means of semiambulatory 24-hour continuous blood glucose monitoring. Diabetes Care. 1985 Mar-Apr;8(2):134–140. doi: 10.2337/diacare.8.2.134. [DOI] [PubMed] [Google Scholar]
- Bending J. J., Pickup J. C., Collins A. C., Keen H. Rarity of a marked "dawn phenomenon" in diabetic subjects treated by continuous subcutaneous insulin infusion. Diabetes Care. 1985 Jan-Feb;8(1):28–33. doi: 10.2337/diacare.8.1.28. [DOI] [PubMed] [Google Scholar]
- Bendtson I., Kverneland A., Pramming S., Binder C. Incidence of nocturnal hypoglycaemia in insulin-dependent diabetic patients on intensive therapy. Acta Med Scand. 1988;223(6):543–548. doi: 10.1111/j.0954-6820.1988.tb17693.x. [DOI] [PubMed] [Google Scholar]
- Betley S., Alberti K. G., Agius L. Regulation of fatty acid and carbohydrate metabolism by insulin, growth hormone and tri-iodothyronine in hepatocyte cultures from normal and hypophysectomized rats. Biochem J. 1989 Mar 1;258(2):547–552. doi: 10.1042/bj2580547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackard W. G., Heidingsfelder S. A. Adrenergic receptor control mechanism for growth hormone secretion. J Clin Invest. 1968 Jun;47(6):1407–1414. doi: 10.1172/JCI105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogardus C., Lillioja S., Howard B. V., Reaven G., Mott D. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest. 1984 Oct;74(4):1238–1246. doi: 10.1172/JCI111533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli G. B., Dimitriadis G. D., Pehling G. B., Baker B. A., Haymond M. W., Cryer P. E., Gerich J. E. Abnormal glucose counterregulation after subcutaneous insulin in insulin-dependent diabetes mellitus. N Engl J Med. 1984 Jun 28;310(26):1706–1711. doi: 10.1056/NEJM198406283102605. [DOI] [PubMed] [Google Scholar]
- Bolli G., De Feo P., Perriello G., De Cosmo S., Ventura M., Campbell P., Brunetti P., Gerich J. E. Role of hepatic autoregulation in defense against hypoglycemia in humans. J Clin Invest. 1985 May;75(5):1623–1631. doi: 10.1172/JCI111869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli G., de Feo P., Compagnucci P., Cartechini M. G., Angeletti G., Santeusanio F., Brunetti P. Important role of adrenergic mechanisms in acute glucose counterregulation following insulin-induced hypoglycemia in type I diabetes. Evidence for an effect mediated by beta-adrenoreceptors. Diabetes. 1982 Jul;31(7):641–647. doi: 10.2337/diab.31.7.641. [DOI] [PubMed] [Google Scholar]
- Bonadonna R. C., De Fronzo R. A. Glucose metabolism in obesity and type 2 diabetes. Diabete Metab. 1991 May;17(1 Pt 2):112–135. [PubMed] [Google Scholar]
- Bonadonna R. C., Zych K., Boni C., Ferrannini E., DeFronzo R. A. Time dependence of the interaction between lipid and glucose in humans. Am J Physiol. 1989 Jul;257(1 Pt 1):E49–E56. doi: 10.1152/ajpendo.1989.257.1.E49. [DOI] [PubMed] [Google Scholar]
- Boyle P. J., Cryer P. E. Growth hormone, cortisol, or both are involved in defense against, but are not critical to recovery from, hypoglycemia. Am J Physiol. 1991 Mar;260(3 Pt 1):E395–E402. doi: 10.1152/ajpendo.1991.260.3.E395. [DOI] [PubMed] [Google Scholar]
- Caprio S., Amiel S., Tamborlane W. V., Gelfand R. A., Sherwin R. S. Defective free-fatty acid and oxidative glucose metabolism in IDDM during hypoglycemia. Influence of glycemic control. Diabetes. 1990 Feb;39(2):134–141. doi: 10.2337/diab.39.2.134. [DOI] [PubMed] [Google Scholar]
- Caprio S., Gelfand R. A., Tamborlane W. V., Sherwin R. S. Oxidative fuel metabolism during mild hypoglycemia: critical role of free fatty acids. Am J Physiol. 1989 Mar;256(3 Pt 1):E413–E419. doi: 10.1152/ajpendo.1989.256.3.E413. [DOI] [PubMed] [Google Scholar]
- Clutter W. E., Rizza R. A., Gerich J. E., Cryer P. E. Regulation of glucose metabolism by sympathochromaffin catecholamines. Diabetes Metab Rev. 1988 Feb;4(1):1–15. doi: 10.1002/dmr.5610040104. [DOI] [PubMed] [Google Scholar]
- Correas I., Zulueta M. A., Marco J. Suppressor effect of morphine on the pancreatic polypeptide response to insulin-induced hypoglycemia in man. Metabolism. 1983 Oct;32(10):982–986. doi: 10.1016/0026-0495(83)90139-7. [DOI] [PubMed] [Google Scholar]
- DEBODO R. C., STEELE R., ALTSZULER N., DUNN A., BISHOP J. S. ON THE HORMONAL REGULATION OF CARBOHYDRATE METABOLISM; STUDIES WITH C14 GLUCOSE. Recent Prog Horm Res. 1963;19:445–488. [PubMed] [Google Scholar]
- De Feo P., Gallai V., Mazzotta G., Crispino G., Torlone E., Perriello G., Ventura M. M., Santeusanio F., Brunetti P., Bolli G. B. Modest decrements in plasma glucose concentration cause early impairment in cognitive function and later activation of glucose counterregulation in the absence of hypoglycemic symptoms in normal man. J Clin Invest. 1988 Aug;82(2):436–444. doi: 10.1172/JCI113616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Feo P., Perriello G., De Cosmo S., Ventura M. M., Campbell P. J., Brunetti P., Gerich J. E., Bolli G. B. Comparison of glucose counterregulation during short-term and prolonged hypoglycemia in normal humans. Diabetes. 1986 May;35(5):563–569. [PubMed] [Google Scholar]
- De Feo P., Perriello G., Torlone E., Fanelli C., Ventura M. M., Santeusanio F., Brunetti P., Gerich J. E., Bolli G. B. Contribution of adrenergic mechanisms to glucose counterregulation in humans. Am J Physiol. 1991 Dec;261(6 Pt 1):E725–E736. doi: 10.1152/ajpendo.1991.261.6.E725. [DOI] [PubMed] [Google Scholar]
- De Feo P., Perriello G., Torlone E., Fanelli C., Ventura M. M., Santeusanio F., Brunetti P., Gerich J. E., Bolli G. B. Evidence against important catecholamine compensation for absent glucagon counterregulation. Am J Physiol. 1991 Feb;260(2 Pt 1):E203–E212. doi: 10.1152/ajpendo.1991.260.2.E203. [DOI] [PubMed] [Google Scholar]
- De Feo P., Perriello G., Torlone E., Ventura M. M., Fanelli C., Santeusanio F., Brunetti P., Gerich J. E., Bolli G. B. Contribution of cortisol to glucose counterregulation in humans. Am J Physiol. 1989 Jul;257(1 Pt 1):E35–E42. doi: 10.1152/ajpendo.1989.257.1.E35. [DOI] [PubMed] [Google Scholar]
- De Feo P., Perriello G., Torlone E., Ventura M. M., Santeusanio F., Brunetti P., Gerich J. E., Bolli G. B. Demonstration of a role for growth hormone in glucose counterregulation. Am J Physiol. 1989 Jun;256(6 Pt 1):E835–E843. doi: 10.1152/ajpendo.1989.256.6.E835. [DOI] [PubMed] [Google Scholar]
- De Feo P., Perriello G., Ventura M. M., Brunetti P., Santeusanio F., Gerich J. E., Bolli G. B. The pancreatic-adrenocortical-pituitary clamp technique for study of counterregulation in humans. Am J Physiol. 1987 Apr;252(4 Pt 1):E565–E570. doi: 10.1152/ajpendo.1987.252.4.E565. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988 Jun;37(6):667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Corbin J. G., Park C. R. Control of gluconeogenesis in liver. IV. Differential effects of fatty acids and glucagon on ketogenesis and gluconeogenesis in the perfused rat liver. J Biol Chem. 1969 Aug 10;244(15):4095–4102. [PubMed] [Google Scholar]
- Exton J. H., Mallette L. E., Jefferson L. S., Wong E. H., Friedmann N., Miller T. B., Jr, Park C. R. The hormonal control of hepatic gluconeogenesis. Recent Prog Horm Res. 1970;26:411–461. doi: 10.1016/b978-0-12-571126-5.50014-5. [DOI] [PubMed] [Google Scholar]
- Faber O. K., Binder C., Markussen J., Heding L. G., Naithani V. K., Kuzuya H., Blix P., Horwitz D. L., Rubenstein A. H. Characterization of seven C-peptide antisera. Diabetes. 1978;27 (Suppl 1):170–177. doi: 10.2337/diab.27.1.s170. [DOI] [PubMed] [Google Scholar]
- Ferrannini E., Barrett E. J., Bevilacqua S., DeFronzo R. A. Effect of fatty acids on glucose production and utilization in man. J Clin Invest. 1983 Nov;72(5):1737–1747. doi: 10.1172/JCI111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism. 1988 Mar;37(3):287–301. doi: 10.1016/0026-0495(88)90110-2. [DOI] [PubMed] [Google Scholar]
- Friedmann B., Goodman E. H., Jr, Weinhouse S. Dietary and hormonal effects on gluconeogenesis and glycogenesis from pyrute-3-14C, fructose-U-14C and glycerol-2-14C in the rat. Endocrinology. 1970 Jun;86(6):1264–1271. doi: 10.1210/endo-86-6-1264. [DOI] [PubMed] [Google Scholar]
- Gerich J. E. Oral hypoglycemic agents. N Engl J Med. 1989 Nov 2;321(18):1231–1245. doi: 10.1056/NEJM198911023211805. [DOI] [PubMed] [Google Scholar]
- Golay A., Swislocki A. L., Chen Y. D., Reaven G. M. Relationships between plasma-free fatty acid concentration, endogenous glucose production, and fasting hyperglycemia in normal and non-insulin-dependent diabetic individuals. Metabolism. 1987 Jul;36(7):692–696. doi: 10.1016/0026-0495(87)90156-9. [DOI] [PubMed] [Google Scholar]
- Gottesman I., Mandarino L., Gerich J. Use of glucose uptake and glucose clearance for the evaluation of insulin action in vivo. Diabetes. 1984 Feb;33(2):184–191. doi: 10.2337/diab.33.2.184. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Keller U., Chiasson J. L., Liljenquist J. E., Cherrington A. D., Jennings A. S., Crofford O. S. The roles of insulin, glucagon, and free fatty acids in the regulation of ketogenesis in dogs. Diabetes. 1977 Nov;26(11):1040–1051. doi: 10.2337/diab.26.11.1040. [DOI] [PubMed] [Google Scholar]
- Lager I., Lönnroth P., von Schenck H., Smith U. Reversal of insulin resistance in type I diabetes after treatment with continuous subcutaneous insulin infusion. Br Med J (Clin Res Ed) 1983 Dec 3;287(6406):1661–1664. doi: 10.1136/bmj.287.6406.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecavalier L., Bolli G., Cryer P., Gerich J. Contributions of gluconeogenesis and glycogenolysis during glucose counterregulation in normal humans. Am J Physiol. 1989 Jun;256(6 Pt 1):E844–E851. doi: 10.1152/ajpendo.1989.256.6.E844. [DOI] [PubMed] [Google Scholar]
- Lecavalier L., Bolli G., Gerich J. Glucagon-cortisol interactions on glucose turnover and lactate gluconeogenesis in normal humans. Am J Physiol. 1990 Apr;258(4 Pt 1):E569–E575. doi: 10.1152/ajpendo.1990.258.4.E569. [DOI] [PubMed] [Google Scholar]
- Lillioja S., Bogardus C., Mott D. M., Kennedy A. L., Knowler W. C., Howard B. V. Relationship between insulin-mediated glucose disposal and lipid metabolism in man. J Clin Invest. 1985 Apr;75(4):1106–1115. doi: 10.1172/JCI111804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire E. A., Helderman J. H., Tobin J. D., Andres R., Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol. 1976 Oct;41(4):565–573. doi: 10.1152/jappl.1976.41.4.565. [DOI] [PubMed] [Google Scholar]
- Meriläinen P. T. Metabolic monitor. Int J Clin Monit Comput. 1987;4(3):167–177. doi: 10.1007/BF02915904. [DOI] [PubMed] [Google Scholar]
- Miles J. M., Haymond M. W., Gerich J. E. Effects of free fatty acids, insulin, glucagon and adrenaline on ketone body production in humans. Ciba Found Symp. 1982;87:192–213. doi: 10.1002/9780470720691.ch11. [DOI] [PubMed] [Google Scholar]
- Miles J., Glasscock R., Aikens J., Gerich J., Haymond M. A microfluorometric method for the determination of free fatty acids in plasma. J Lipid Res. 1983 Jan;24(1):96–99. [PubMed] [Google Scholar]
- Nurjhan N., Campbell P. J., Kennedy F. P., Miles J. M., Gerich J. E. Insulin dose-response characteristics for suppression of glycerol release and conversion to glucose in humans. Diabetes. 1986 Dec;35(12):1326–1331. doi: 10.2337/diab.35.12.1326. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M. Brain metabolism: a perspective from the blood-brain barrier. Physiol Rev. 1983 Oct;63(4):1481–1535. doi: 10.1152/physrev.1983.63.4.1481. [DOI] [PubMed] [Google Scholar]
- Perriello G., De Feo P., Torlone E., Calcinaro F., Ventura M. M., Basta G., Santeusanio F., Brunetti P., Gerich J. E., Bolli G. B. The effect of asymptomatic nocturnal hypoglycemia on glycemic control in diabetes mellitus. N Engl J Med. 1988 Nov 10;319(19):1233–1239. doi: 10.1056/NEJM198811103191901. [DOI] [PubMed] [Google Scholar]
- Pontiroli A. E., Lanzi R., Monti L. D., Pozza G. Effect of acipimox, a lipid lowering drug, on growth hormone (GH) response to GH-releasing hormone in normal subjects. J Endocrinol Invest. 1990 Jun;13(6):539–542. doi: 10.1007/BF03348621. [DOI] [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Newsholme E. A., Garland P. B. Regulation of glucose uptake by muscle. 8. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):652–665. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Cryer P. E., Gerich J. E. Role of glucagon, catecholamines, and growth hormone in human glucose counterregulation. Effects of somatostatin and combined alpha- and beta-adrenergic blockade on plasma glucose recovery and glucose flux rates after insulin-induced hypoglycemia. J Clin Invest. 1979 Jul;64(1):62–71. doi: 10.1172/JCI109464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Cryer P. E., Haymond M. W., Gerich J. E. Adrenergic mechanisms for the effects of epinephrine on glucose production and clearance in man. J Clin Invest. 1980 Mar;65(3):682–689. doi: 10.1172/JCI109714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Cryer P. E., Haymond M. W., Gerich J. E. Adrenergic mechanisms of catecholamine action on glucose homeostasis in man. Metabolism. 1980 Nov;29(11 Suppl 1):1155–1163. doi: 10.1016/0026-0495(80)90025-6. [DOI] [PubMed] [Google Scholar]
- Rizza R. A., Haymond M. W., Verdonk C. A., Mandarino L. J., Miles J. M., Service F. J., Gerich J. E. Pathogenesis of hypoglycemia in insulinoma patients: suppression of hepatic glucose production by insulin. Diabetes. 1981 May;30(5):377–381. doi: 10.2337/diab.30.5.377. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Toews C. J., Shafrir E. Role of free fatty acids in glucose homeostasis. Arch Intern Med. 1969 Mar;123(3):299–313. [PubMed] [Google Scholar]
- Saccà L., Vigorito C., Cicala M., Corso G., Sherwin R. S. Role of gluconeogenesis in epinephrine-stimulated hepatic glucose production in humans. Am J Physiol. 1983 Sep;245(3):E294–E302. doi: 10.1152/ajpendo.1983.245.3.E294. [DOI] [PubMed] [Google Scholar]
- Seth J., Brown L. M. A simple radioimmunoassay for plasma cortisol. Clin Chim Acta. 1978 May 16;86(1):109–120. doi: 10.1016/0009-8981(78)90465-5. [DOI] [PubMed] [Google Scholar]
- Shaw W. A., Issekutz T. B., Issekutz B., Jr Gluconeogenesis from glycerol at rest and during exercise in normal, diabetic, and methylprednisolone-treated dogs. Metabolism. 1976 Mar;25(3):329–339. doi: 10.1016/0026-0495(76)90091-3. [DOI] [PubMed] [Google Scholar]
- Struck E., Ashmore J., Wieland O. Effects of glucagon and long chain fatty acids on glucose production by isolated perfused rat liver. Adv Enzyme Regul. 1966;4:219–224. doi: 10.1016/0065-2571(66)90016-1. [DOI] [PubMed] [Google Scholar]
- Taskinen M. R., Bogardus C., Kennedy A., Howard B. V. Multiple disturbances of free fatty acid metabolism in noninsulin-dependent diabetes. Effect of oral hypoglycemic therapy. J Clin Invest. 1985 Aug;76(2):637–644. doi: 10.1172/JCI112016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taskinen M. R., Sane T., Helve E., Karonen S. L., Nikkilä E. A., Yki-Järvinen H. Bedtime insulin for suppression of overnight free-fatty acid, blood glucose, and glucose production in NIDDM. Diabetes. 1989 May;38(5):580–588. doi: 10.2337/diab.38.5.580. [DOI] [PubMed] [Google Scholar]
- Thiébaud D., DeFronzo R. A., Jacot E., Golay A., Acheson K., Maeder E., Jéquier E., Felber J. P. Effect of long chain triglyceride infusion on glucose metabolism in man. Metabolism. 1982 Nov;31(11):1128–1136. doi: 10.1016/0026-0495(82)90163-9. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Kreisberg R. A., Felts P. W. Mechanism for the stimulation of gluconeogenesis by fatty acids in perfused rat liver. Proc Natl Acad Sci U S A. 1966 Jul;56(1):247–254. doi: 10.1073/pnas.56.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe R. R., Shaw J. H. Effect of epinephrine infusion and adrenergic blockade on glucose oxidation in conscious dogs. Metabolism. 1986 Jul;35(7):673–678. doi: 10.1016/0026-0495(86)90177-0. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H., Helve E., Sane T., Nurjhan N., Taskinen M. R. Insulin inhibition of overnight glucose production and gluconeogenesis from lactate in NIDDM. Am J Physiol. 1989 Jun;256(6 Pt 1):E732–E739. doi: 10.1152/ajpendo.1989.256.6.E732. [DOI] [PubMed] [Google Scholar]


