Abstract
A fully developed, functional epididymis is important for male fertility. In particular, it is apparent that without the most proximal region, the initial segment (IS), infertility results. Therefore, it is important to understand the development and regulation of this crucial epididymal region. We have previously shown that many functions of the IS are regulated by luminal fluid factors/lumicrine factors from the testis. This study provides evidence that lumicrine factors activated the ERK pathway only in epithelial cells of the IS from Postnatal Day (P) 14 to P19 and sustained this activation into adulthood. The activated ERK pathway promoted cell proliferation and differentiation in the developing IS, although in the adult, its role was switched to maintain cell survival. To understand further the regulation of cell proliferation in the IS, we examined the role of DUSP6, an MAPK1/3 (ERK1/2) preferred phosphatase that is also regulated by lumicrine factors in the IS. Utilizing Dusp6−/− mice, our studies, surprisingly, revealed that Dusp6 was a major regulator of cell proliferation in the caput and corpus regions, whereas components of the ERK pathway, together with PTEN and SRC, were the major regulators of cell proliferation in the IS. We hypothesize that region-specific regulation of cell proliferation is caused by differences in the balance of activities between pro- and antiproliferation signaling pathway components for each epididymal region. An understanding of the mechanisms of cell proliferation may provide clues as to why the epididymis rarely succumbs to cancer.
Keywords: cell proliferation, DUSP6, epididymis, kinases, lumicrine regulation, male reproductive tract, MAPK1/3, phosphatases, signal transduction
DUSP6 is a major cell proliferation regulator in the caput and corpus epididymal regions, while the components of ERK pathway, together with SRC and PTEN, regulate cell proliferation in the initial segment.
INTRODUCTION
The epididymis plays a major role in the maturation, storage, and protection of spermatozoa; therefore, it is important for male fertility. Many studies have divided the epididymis into the traditional regions of initial segment (IS), caput, corpus, and cauda, according to their unique histology and function [1]. It is apparent that the most proximal region of the epididymis, the IS, plays an important role in sperm maturation, because without a fully developed IS, male infertility results [2, 3]. Therefore, it is important to examine the mechanisms by which the IS develops and functions normally and contributes to normal sperm maturation.
Androgens play a major role in the proper functioning of the IS, but testicular luminal fluid factors, so-called lumicrine factors, also appear to contribute [4]. In the rat, deprival of lumicrine factors by ligating the efferent ducts and, therefore, preventing testicular fluid from entering the epididymis results in apoptosis in the IS epithelium, which cannot be prevented by androgen replacement [5–9]. Thus, lumicrine factors regulate the cellular function of IS through an unknown process we have referred to as lumicrine regulation [4]. Early studies indicated that carefully performed efferent ductal ligation (EDL) would not affect blood supply, so this would exclude it as a cause of apoptosis [5, 6]. Extensive discussion on the putative lumicrine factors favors the fibroblast growth factors (FGFs) hypothesis [10–13], because several growth factors, especially multiple FGFs, were detected in the testicular luminal fluid [12, 13] whereas several growth factor receptors were localized to the apical membrane of epithelial cells of the IS [13]. It is interesting that under normal physiological conditions, the cells of the adult IS are constantly being stimulated by growth factors, the MAPK pathway is likely being activated constitutively, and yet these cells do not divide. Despite being regulated by androgens and growth factors, the IS (and the entire epididymis) rarely succumbs to cancer [14]; therefore, it is important to understand the regulation of cell proliferation in the epididymis.
We hypothesize that FGF signaling plays a role in cell proliferation of the developing epididymal duct but then switches to a protective role in the adult, especially within the IS. The present study focuses on examining downstream components of the FGF signaling pathway, specifically the role of p-MAPK1/3 (commonly known as p-ERK1/2) in cell proliferation during juvenile development and cell protection in the adult. To complement the study on p-MAPK1/3, we also examined Dusp6 (dual specificity phosphatase 6) null mice, because DUSP6 is a negative regulator of MAPK1/3 activities [15–17]. In the course of our investigation, we uncovered a potential novel mechanism whereby DUSP6 did not have a significant role in cell proliferation in the IS yet did in the more distal epididymal regions. Furthermore, we pursued the additional IS-specific factors that regulate cell proliferation in the IS, and we identified SRC and PTEN as potential regulators.
MATERIALS AND METHODS
Animals
Mice were handled according to approved protocols following the guidelines of the Institutional Animal Care and Use Committee of the University of Virginia. Dusp6−/− mice [17] were a generous gift from Dr. Suzanne L. Mansour (University of Utah).
Efferent Duct Ligation
To block lumicrine factors from reaching the epididymis, unilateral efferent ductal ligation (EDL) surgeries were performed using the protocol modified from the rat EDL procedure described previously in detail [18]. Great care was taken to avoid ligating nearby blood vessels. For the control, a sham operation was performed on the contralateral side within the same animal. Pentobarbital sodium injection (0.05 mg/g body wt) was used as the anesthesia during the surgery. Mice were killed by halothane gas overdose followed by cervical dislocation.
Real-Time PCR
Total RNA was isolated while RNase-free DNase was added to remove DNA contamination. The first-strand cDNA was synthesized using Superscript synthesis system (Invitrogene). Real-time PCR quantification of mRNA levels was performed using SensiMix dT kit (Quantace Ltd.). The Dusp6 primers were designed to span across an intron: P1 (5′-CGACTGGAATGAGAACACTGGTGG-3′) and P2 (5′-TCTAGATTGGTCTCGCAGTGCAGG-3′). The amplification of 18S rRNA subunit was served as the loading control. All samples were run in triplicate. Standard curves were generated with serial dilutions of plasmids containing Dusp6 or 18S rRNA gene fragments. A melting curve was adopted to analyze the specificity of PCR products. Data were analyzed using Microsoft Excel software. The Dusp6 mRNA expression was measured by the copy number of Dusp6 mRNA, then normalized by the copy number of 18S rRNA. In the comparison experiments, Dusp6/18S level in the control was set as one, and the relative values (mean ± SEM) of the test experiment were then calculated. A t-test was performed to identify significant changes.
In Situ Hybridization
Epididymal tissues were placed flat on a glass coverslip, covered with OCT compound (Sakura Finetek USA Inc., Torrance, CA), and dipped into dry ice-cooled isopentane for rapid freezing. The frozen tissues were stored at −80°C. A 901-bp of mouse Dusp6 cDNA fragment (770-1670 bp of Dusp6 mRNA, NM_026268.3) was subcloned into a TOPO vector (Invitrogen) and then used as the templates for in vitro transcription. Nonradioactive in situ hybridization was performed as described by Berger and Hediger [19].
Immunohistochemistry
For immunohistochemistry (IHC), the protocol was modified from that described previously [20]. Briefly, epididymal tissues were immersion-fixed in 4% paraformaldehyde with Tris-buffered saline (TBS) overnight at 4°C. After paraffin embedding and sectioning, slides were deparaffinized and rehydrated. Then, endogenous peroxidases were quenched by incubation in 0.5% H2O2 in methanol for 30 min. For antigen retrieval, slides were microwaved in antigen unmasking solution (Vector Laboratories) for 10 min on high in a 1300-W microwave and cooled for 1 h at room temperature. After blocking in the blocking solution containing 10% normal goat serum (Vector Laboratories), 0.5% (v/v) gelatin from cold-water fish skin (Sigma) and TBS for 1.5 h, slides were further blocked in avidin D block solution (Vector Laboratories) for 15 min and biotin block solution (Vector Laboratories) for 15 min. Slides were then incubated overnight at 4°C with p-MAPK1/3 antibody (T202/Y204; no. 9101, 1:200 working dilution; Cell Signaling Technology, Inc.) in the blocking solution. After washing in TBS, the slides were incubated with a 1:200 dilution of biotinylated secondary antibody (Vector Laboratories) in the blocking solution for 1.5 h at room temperature. All slides were washed in TBS and incubated with avidin-biotin complex (Vectastain Elite ABC kit; Vector Laboratories) for 30 min at room temperature, reacted with diaminobenzidine (Sigma), and counterstained in Harris hematoxylin. Finally, the slides were dehydrated, cleared with xylenes, and mounted with Vectamount permanent mounting media (Vector Laboratories).
Immunofluorescence
For immunofluorescence (IF), tissue slides were deparaffinized and then rehydrated. After antigen retrieval, slides were blocked for 1.5 h at room temperature in the blocking solution and incubated overnight at 4°C in the blocking solution with the primary antibodies, including the antibody against p-Histone H3 (S10), a mitotic marker for dividing cells [21] (no. 06–570, 1:500 working dilution; Millipore), p-SRC antibody (Y529; no. 2105, 1:200 working dilution; Cell Signaling Technology, Inc.), or PTEN antibody (138G6; 9559, 1:200 working dilution; Cell Signaling Technology, Inc.). After being washed in TBS, the slides were incubated with a 1:200 dilution of Alexa Fluor secondary antibody (Molecular Probe) in the blocking solution for 1.5 h at room temperature. All slides were washed in TBS and mounted by Prolong anti-fade reagent (Molecular Probe) and viewed in a Zeiss microscope equipped with epifluorescence.
Western Blot Analysis
Tissue pieces from IS and caput were homogenized in ice-cold RIPA Buffer with protease and phosphatase inhibitors (Thermo Fisher Scientific). The homogenate was centrifuged (16 000 × g for 10 min) and analyzed using the Bradford protein assay (Bio-Rad, Inc.). Proteins were separated in SDS-PAGE gels and then transferred to nitrocellulose membranes. The membranes were blocked in 5% fat-free dry milk with TBS and incubated with the primary antibodies followed by the 1:2000 dilution of alkaline phosphatase-conjugated secondary antibody (Sigma), then reacted with 1 Step NBT/BCIP (Thermo Fisher Scientific). ImageQuant (GE Healthcare) was used to analyze signal density on the blots. A t-test was performed, and the levels of significance were set at P < 0.01 (**) and P < 0.05 (*). The following primary antibodies used for Western blot analysis were purchased from Cell Signaling Technology, Inc.: p-MAPK1/3 (T202/Y204; no. 9101, 1:1000 working dilution), MAPK1/3 (no. 9102, 1:1000 working dilution), MAPK1 (no. 9108, 1:1000 working dilution), p-MAP2K1/2 (S221; no. 2338, 1:2000 working dilution), MAP2K1/2 (no. 9122, 1:1000 working dilution), p-AKT (S473; no. 4058, 1:1000 working dilution), p-RPS6KB1 (T389; no. 9205, 1:1000 working dilution), and RPS6KB1 (no. 9202, 1:1000 working dilution). Other antibodies were purchased from Santa Cruz Biotechnology: p-MAPK8 (G-7; sc-6254, 1:200 working dilution), MAPK8 (D-2; sc-7345, 1:200 working dilution), p-MAPK14 (D-8; sc-7973, 1:200 working dilution), and ETV4 (16; sc-113, 1:200 working dilution). DUSP6 antibody (1:1000 working dilution) [22] was the generous gift from Dr. Johan Lennartsson (Uppsala University).
MAPK1-Directed Total Phosphatase Activities Assay
Measurement of MAPK1-directed total phosphatase activities relied on detecting dephosphorylation of a purified, dual-phosphorylated, His6-tagged MAPK1 (Stratagene) upon incubation with whole-cell lysate [23]. The negative control used buffer instead of cell lysate, and its activity was set as zero. The complete dephosphorylation of substrate His6-p-MAPK1 was set as one. The percentage of dephosphorylation of His6-p-MAPK1 by the cell lysate was calculated as the relative total phosphatase activities.
Protein Array
Protein array analysis was performed by Kinexus Bioinformatics Corporation. This service utilizes a proprietary technology based on multi-immunoblotting that uses panels of highly validated, pan-specific antibodies. It provided signal transduction protein profiling for each sample analyzed and information regarding the quantitative expression level for each protein detected. In the present study, we chose Kinetworks phospho-site cell-cycle status screen, which tracks 44 phosphorylation sites in cell cycle-related phosphoproteins to provide an overall picture of cell-cycle regulation. Four types of samples were collected in our experiments: IS from Dusp6−/−, IS from Dusp6+/+ littermate, the larger caput from Dusp6−/−, and caput from Dusp6+/+. Each sample was collected by pooling epididymal regions from three mice. This was repeated three times; thus, each type of sample had three replicates. A total of 12 samples of tissue lysates submitted for Kinetworks phospho-site cell-cycle status screen were prepared according to the manufacturer's instructions. The raw data was analyzed using t-test; any set that was significantly different (P < 0.01 or P < 0.05) was reported.
RESULTS
Lumicrine Regulation of FGF/MAPK Pathways in the Adult IS
Similar to the phenomenon observed in the rat, deprival of lumicrine factors from adult mouse epididymis caused a wave of cell death among the IS epithelial cells. As previously mentioned, we hypothesized that FGF signaling had a role in protection of IS from apoptosis. Therefore, we examined the regulation of lumicrine factors on FGF signaling in the mouse IS.
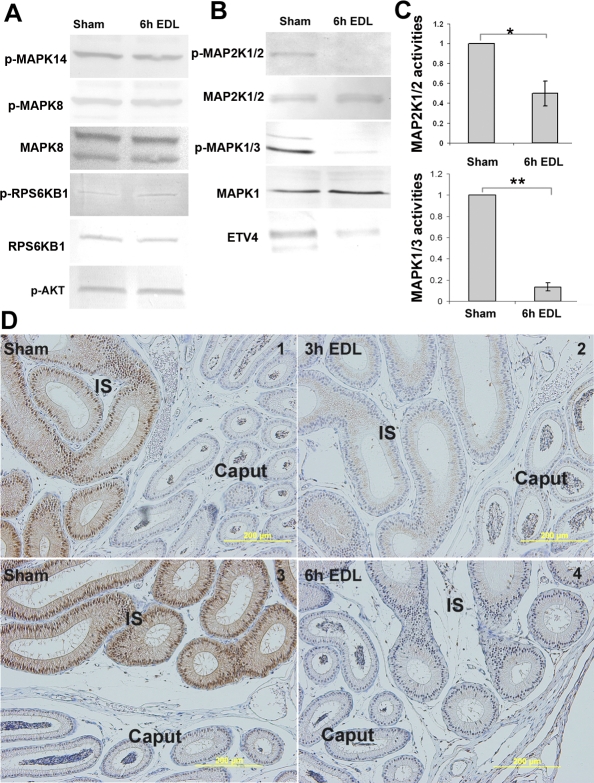
Figure 1, A and B, shows the presence of key molecules of ERK, JNK, p38, MTOR, and AKT pathways downstream of FGF signaling in the IS. To determine which pathway was regulated by lumicrine factors, an EDL was performed. It takes 2–4 h for sperm and fluid to be depleted from the IS after EDL; therefore, to ensure depletion of lumicrine factors, IS tissues were analyzed 6 h after EDL. At this early stage, cellular changes were not observed at the histological level. As shown in Figure 1A, the activities of MAPK8 (JNK), MAPK14 (p38), AKT, and RPS6KB1 (p70S6) showed no change following loss of lumicrine factors. In contrast, MAP2K1/2 (MEK1/2) and MAPK1/3 (ERK1/2) phosphorylation levels were reduced in the first 6 h following EDL (Fig. 1B). Quantitative measurements based on three to six replicates of Western blot analyses revealed a reduction of approximately 50% and approximately 79% in MAP2K1/2 and MAPK1/3 activities, respectively (Fig. 1C). Furthermore, within the first 6 h after EDL, protein expression of ETV4, a downstream target of p-MAPK1/3, was reduced (Fig. 1B).
FIG. 1.
The effects of loss of lumicrine factors in the adult IS. A) Western blot analysis of the levels of MAPK14, MAPK8, RPS6KB1, and AKT phosphorylation in the IS at 6 h after EDL compared to the sham controls. B) Western blot analysis showing the levels of MAP2K1/2 and MAPK1/3 phosphorylation and ETV4 protein expression in the IS at 6 h after EDL compared to the sham controls. C) Quantitative measurement of MAP2K1/2 and MAPK1/3 activities (phosphorylated MAP2K1/2 or MAPK1/3 versus total MAP2K1/2 or MAPK1) in the IS at 6 h after EDL (n ≥ 3 blots). In each comparison, the values of sham samples were set as one, and the relative values (mean ± SEM) of the EDL samples were then calculated. A t-test was performed. *P < 0.05 versus sham, **P < 0.01 versus sham. D) p-MAPK1/3 IHC staining of the IS at 3 h (2) and 6 h (4) after EDL compared to their sham controls (1 and 3). Signal was abolished by preincubation with synthesized p-MAPK1/3 peptide (data not shown). Bars = 200 μm.
In Figure 1D, IHC staining of p-MAPK1/3 revealed a high level of p-MAPK1/3 (brown color) located in the cytoplasm and nucleus of IS epithelial cells (Fig. 1D1 and 1D3), which was not observed in other cell types and not in the adjacent caput epithelium. Three hours after EDL, the staining of p-MAPK1/3 was reduced (Fig. 1D2), and staining was not observed 6 h after EDL (Fig. 1D4).
Lumicrine Regulation of DUSP6 Expression in the Adult IS
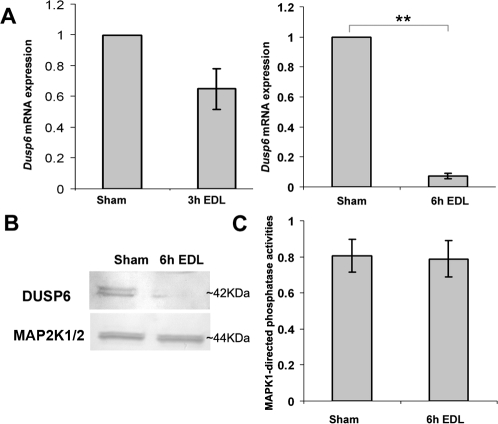
Quantitative real-time PCR revealed that Dusp6 mRNA levels began to decline 3 h after EDL and decreased to approximately 7% at 6 h after EDL (Fig. 2A). As shown in Figure 2B, DUSP6 antibody detected double bands of approximately 42 kDa of DUSP6 protein in the Western blot analysis. DUSP6 protein expression was reduced within the first 6 h after EDL. However, the activities of MAPK1-directed total phosphatases were not altered significantly at 6 h after EDL (Fig. 2C).
FIG. 2.
DUSP6 expression and MAPK1-directed total phosphatase activities in IS after loss of lumicrine factors. A) Quantification of Dusp6 mRNA expression level at 3 h (left) and 6 h (right) following EDL (n = 3). The mRNA level of Dusp6 expression from the sham side of IS was set as one. The relative values (mean ± SEM) of the EDL samples are shown. **P < 0.01 versus sham. B) Western blot analysis of the level of DUSP6 protein expression in the IS at 6 h after EDL compared to sham samples. The double bands of DUSP6 signal may represent phosphorylated and nonphosphorylated forms of DUSP6. Total MAP2K1/2 was used as a loading control. C) Quantitative measurement of MAPK1-directed total phosphatase activities in the IS at 6 h after EDL compared to sham samples (n = 3). The phosphatase activity of the negative control, which lacks any phosphatase, was set as zero. The complete dephosphorylation of substrate His6-p-MAPK1 was set as one. The relative MAPK1-directed total phosphatase activities of each sample were calculated as the percentage of dephosphorylation of His6-p-MAPK1.
Developmental Regulation of Dusp6 mRNA Expression and MAPK1/3 Activities
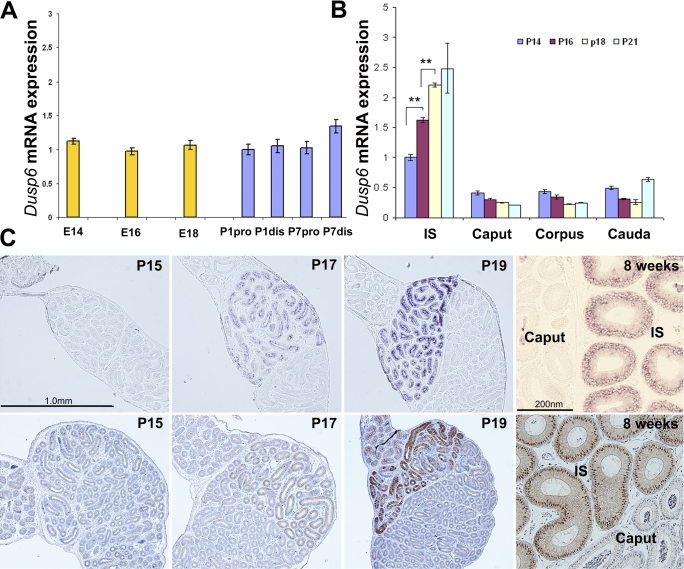
The expression of Dusp6 mRNA during embryonic development from Embryonic Day (E) 14 to Postnatal Day (P) 1 and early postnatal development from P1 to P7 was relatively stable and low (Fig. 3A). However, Dusp6 mRNA expression increased significantly from P14 to P18. The increase in expression of Dusp6 mRNA only occurred in the IS and not in other epididymal regions (Fig. 3B).
FIG. 3.
The developmental regulation of Dusp6 mRNA expression and MAPK1/3 phosphorylation in the mouse epididymis. A) Real-time PCR quantification of Dusp6 mRNA expression level in the mouse epididymis from E14 to P7. Pro and dis represent proximal and distal epididymis, respectively. The average level of Dusp6 mRNA expression at P1 proximal epididymis (P1pro) was set as one. B) Quantification of Dusp6 mRNA expression level in the four regions of the epididymides from P14 to P21 (n = 3). **P < 0.01. The average level of Dusp6 mRNA expression in the IS at P14 was set as one. C) In situ hybridization of Dusp6 (top) and IHC staining of p-MAPK1/3 (bottom) of the proximal epididymis of P15, P17, P19, and 8-wk-old mice. Sense probe hybridization only revealed background level signal (data not shown).
In situ hybridization of Dusp6 mRNA showed a similar finding. Figure 3C (top) shows that Dusp6 mRNA expression was low through the head of mouse epididymis at P15 and that expression increased only in the cytoplasm of the IS epithelium at P17. The higher Dusp6 expression was established within the epithelial cells of the IS at P19 and was sustained to adulthood. In situ hybridization of the control sections with Dusp6 sense probe only revealed background level signal (data not shown).
When p-MAPK1/3 staining was performed on the contralateral epididymis (Fig. 3C, bottom), a similar pattern was observed; p-MAPK1/3 level was undetectable at P15, began to show in the epithelial cells of the IS at P17, and was higher at P19. This higher phosphorylation level of MAPK1/3 was sustained to adulthood.
Role of ERK/DUSP6 Pathway During IS Development in Juvenile Mice
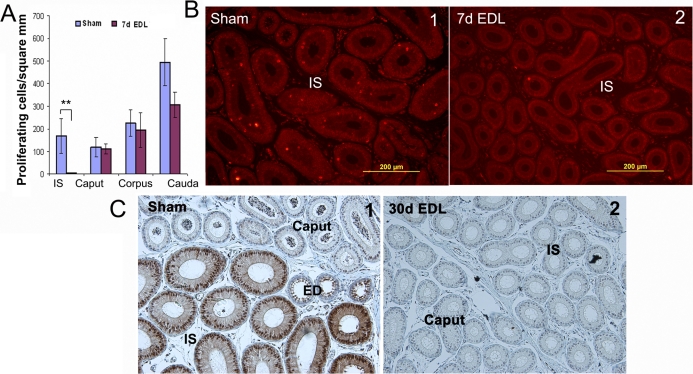
Unlike adult mice, apoptosis in the IS of juvenile mice was not observed following loss of lumicrine factors. No immediate cytological effect was noticed during the first 24 h following EDL. However, the rapid cell proliferation that was observed during puberty (Fig. 4B1) almost completely ceased in the IS after 7 days of EDL (Fig. 4, A and B2). At the same time point, no significant effect on other regions of epididymis was observed (Fig. 4A).
FIG. 4.
The effects of loss of lumicrine factors on cell proliferation and differentiation of juvenile (P21) mice epididymides. A) Measurement of cell proliferative activity in the four regions of the epididymis at 7 days after EDL (n = 3). Proliferative activity was measured by counting the p-Histone H3 positive cells per epithelial area. **P < 0.01 versus sham. B) Representative figures of p-Histone H3 staining of sham (1) and at 7 days after EDL (2). C) Comparison of underdeveloped IS at the EDL side (2) with the mature IS at the sham control side (1) from juvenile mice, which underwent an EDL on P21 and were analyzed 30 days later. Note p-MAPK1/3 staining is a marker of a mature IS (1).
After 14 and 30 days of EDL, cell proliferative activity was kept to a low level in the IS (data not shown). In addition, the loss of lumicrine factors also prevented the principal cells in the IS from differentiating, as measured by the increase of cell height. Therefore, the IS was not fully developed in the 30-day EDL side even when the mouse was 7 wk old (Fig. 4C,2). The control sham side had a fully developed IS. Note that p-MAPK1/3 staining was a marker of mature IS (Fig. 4C1).
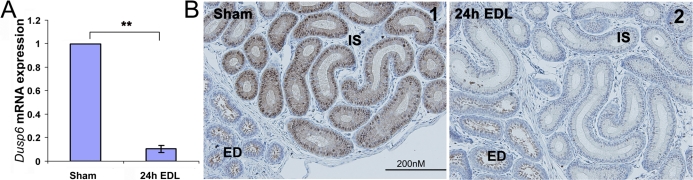
Similar to that observed in adult mice, the loss of lumicrine factors from juvenile mice epididymis caused a decrease of Dusp6 mRNA expression to approximately 10% of the sham control level during the first 24 h (Fig. 5A). Consistently, p-MAPK1/3 staining in the IS epithelial cells completely diminished 24 h after EDL (Fig. 5B2).
FIG. 5.
The effects of loss of lumicrine factors on Dusp6 expression and MAPK1/3 activities of juvenile (P21) mouse IS. A) Quantification of Dusp6 mRNA expression level at 24 h after EDL (n = 3). The mRNA level of Dusp6 expression from the sham side of IS was set as one. The relative values (mean ± SEM) of the EDL samples are shown. **P < 0.01 versus sham. B) IHC staining of p-MAPK1/3 in both sham (1) and at 24 h after EDL (2).
Epididymal Phenotype of Dusp6−/− Mice
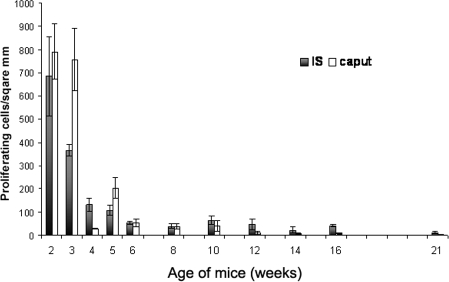
Before examination of Dusp6−/− mice, we first gathered information on cell proliferative activity during postnatal development of the epididymis in wild-type mice. Using IF staining of p-Histone H3, we assessed the proliferative activity in the IS and caput from Postnatal Week 2 to Postnatal Week 21. Caput was selected as a control. High proliferative activity of the epithelial cells of the IS and the caput was observed at Weeks 2 and 3; this activity declined at Weeks 4 to 5 and remained a low level after Week 6 (about P40) (Fig. 6).
FIG. 6.
Measurement of the proliferative activity of the proximal mouse epididymis during postnatal development. Proliferative activity was measured by counting the number of p-Histone H3-positive cells per epithelial area using ImagePlus software. Data shown represent mean ± SEM.
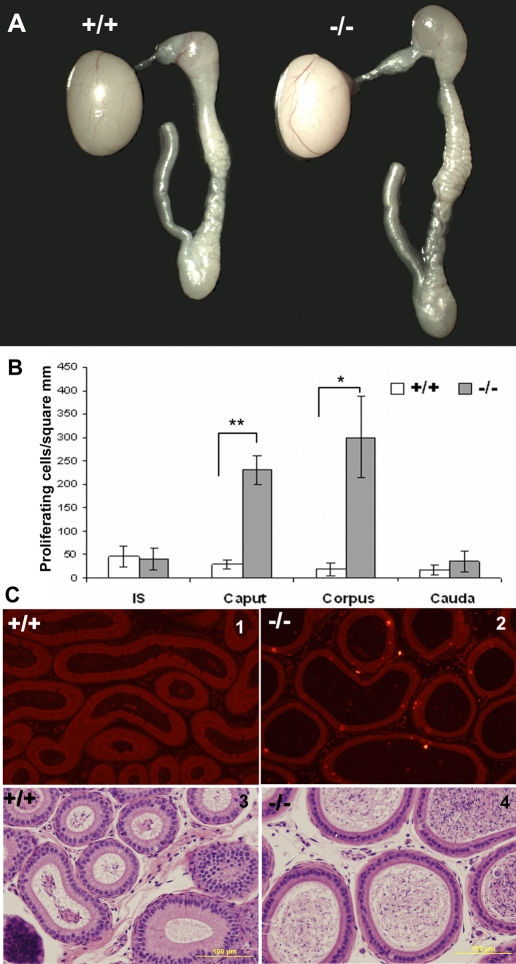
The Dusp6−/− mouse line was established by Dr. Suzanne L. Mansour's group [17]. It has been reported previously that loss of Dusp6 caused skeletal dwarfism in an incompletely penetrant manner; approximately 40% of P5 to P16 Dusp6−/− mice had body weights less than 75% of the littermate average (so-called small mice) [17]. Consistent with the original report, small Dusp6−/− mice were observed. Unfortunately, these small mice rarely survived to 4 wk after birth, making it difficult to examine their epididymides during development. The normal-body-weight Dusp6−/− mice were healthy and fertile. However, when we examined the adult epididymides of 46 normal-body-weight Dusp6−/− mice, 15 had a larger epididymis on one or both sides. Figure 7A is a representative figure showing the epididymis from a 10-wk-old Dusp6−/− mouse compared to a Dusp6+/+ littermate. We randomly chose five Dusp6−/− mice (age, 10–14 wk) with the visible larger epididymides and compared their epididymal weights with their Dusp6+/+ littermates or the age-matched Dusp6+/+ mice. The weights (mean ± SEM) of the epididymides from Dusp6−/− and Dusp6+/+ mice were 48.93 ± 4.08 mg (n = 5) and 33.92 ± 3.14 mg (n = 5), respectively, and were significantly different (P < 0.05). The larger epididymis was rarely observed in Dusp6+/+ (2/51) and Dusp6+/− (1/68) littermates, suggesting this phenotype resulted from the loss of Dusp6.
FIG. 7.
Epididymal phenotype of Dusp6−/− versus Dusp6+/+. A) A representative figure of a larger epididymis from 10-wk-old Dusp6−/− mice compared to Dusp6+/+ litter mate. B) Measurement of cell proliferative activity in the four regions of large Dusp6−/− epididymides (n = 3). *P < 0.05 versus Dusp6+/+, **P < 0.01 versus Dusp6+/+. Proliferative activity was measured by counting the p-Histone H3-positive cells per epithelial area. C) Representative p-Histone H3 staining of Dusp6+/+ caput (1) and large Dusp6−/− caput (2) and representative histological sections of Dusp6+/+ caput (3) and large Dusp6−/− caput (4). Bars = 100 μm.
Among the larger epididymides, the caput and corpus were the regions that were larger. In Dusp6+/+ mice, all four epididymal regions stopped proliferating 6 wk after birth (about P40). Very few proliferating cells were detected along the duct in these mice (Fig. 7C1). However, in the Dusp6−/− mice with the large epididymides, epithelial cells in the caput and/or corpus continued to undergo proliferation (Fig. 7, B and C2) beyond P40, resulting in an increased size of the lumen (Fig. 7C4).
Differences of Cell-Cycle Components in the IS and Caput
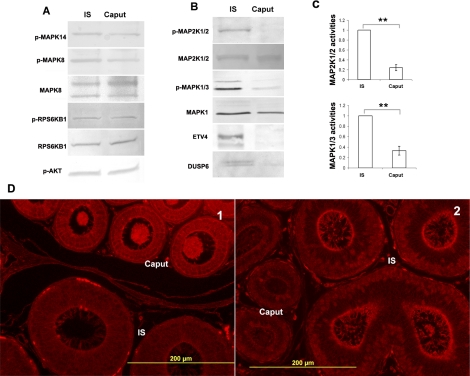
To understand the epididymal phenotype of Dusp6−/− mice, we first investigated the differences in cell-cycle regulation in the IS and caput. In Figure 8A, the activities of several kinases and the expression of their downstream targets were compared between the IS and caput. The phosphorylation levels of AKT, MAPK8, MAPK14, and RPS6KB1 showed no difference between the IS and caput. However, p-MAP2K1/2 and p-MAPK1/3 levels were higher in the IS (Fig. 8B). Quantitative measurements indicated that MAP2K1/2 and MAPK1/3 activities were approximately fourfold higher and approximately threefold higher, respectively, in the IS than in the caput (Fig. 8C). Consistent with MAP2K1/2 and MAPK1/3 kinase activities, ETV4 and DUSP6 protein expression were also higher in the IS compared to the caput (Fig. 8B).
FIG. 8.
Comparisons of activity and expression of cell proliferation-related proteins between the adult IS and caput. A) Western blot analysis of the levels of MAPK14, MAPK8, RPS6KB1, and AKT phosphorylation in the IS and the caput. B) Western blot analysis of the levels of MAP2K1/2 and MAPK1/3 phosphorylation and ETV4 and DUSP6 protein expression in the IS and the caput. C) Quantitative measurements of MAP2K1/2 and MAPK1/3 activities (phosphorylated MAP2K1/2 or MAPK1/3 vs. total MAP2K1/2 or MAPK1) in the IS and caput (n = 3). In each comparison, the values of IS samples were set as one; the relative values (mean ± SEM) of caput samples are shown. **P < 0.01 versus caput. D) IF staining of p-SRC Y529 and total PTEN in the mouse IS and caput. Note the lower intensity of p-SRC Y529 staining in the epithelial cells of the IS compared to the caput (1) and the higher intensity of PTEN staining in the apical area of epithelial cells in the IS compared to the caput (2). Bars = 200 μm.
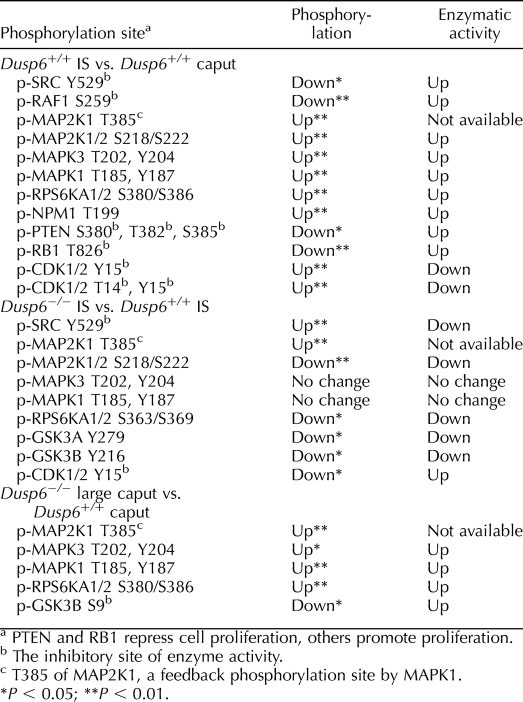
A protein array analysis was performed to examine the global differences of cell-cycle components between the IS and caput. In this screen, 44 phosphorylation sites in cell cycle-related phosphoproteins were tracked. The phosphoproteins for which phosphorylation levels were different between the IS and the caput are listed in Table 1.
TABLE 1.
Comparison of cell cycle-related phosphoproteins in the IS and caput, prior to and after Dusp6 disruption.
The components of the proproliferative ERK pathway had higher activities in the IS compared to caput. MAP2K1/2, MAPK1/3, and RPS6KA1/2 (RSK1/2) had higher levels of phosphorylation at the sites corresponding to the higher kinase activities in the IS (Table 1). Lower phosphorylation of RAF1 at S259, an inhibitory site, showed higher RAF1 activity in the IS (Table 1). Besides the components in the ERK pathway, the oncogene SRC had lower phosphorylation at the inhibitory site Y529, and the oncogene NPM1 had higher phosphorylation at the activation site T199 in the IS. Thus, both oncogenes had higher activities in the IS than in the caput (Table 1).
At least three cell-cycle components were in a more inhibitory or inactive state in the IS compared to the caput. First, phosphorylation of PTEN at the inhibitor sites S380, T382, and S385 was lower in the IS than in the caput, showing that PTEN phosphatase activity and its antiproliferation ability were higher in the IS (Table 1). Second, phosphorylation of retinoblastoma-associated protein 1 (RB1) at inhibitory site T826 was lower in the IS compared to the caput; thus, RB1 was presumably more able to repress cell proliferation in the IS (Table 1). Third, the inhibition of CDK1/2 activity by phosphorylation at inhibitory sites T14 and Y15 was higher in the IS than in the caput, indicating that CDK1/2 was in an inactive state in the IS (Table 1).
To validate the protein array data, IF staining of the IS and adjacent caput regions with p-SRC Y529 antibody was performed. Consistent with the protein array data, the intensity of p-SRC Y529 staining was weaker in the epithelial cells of the IS compared to the caput, confirming the higher SRC activity in the IS (Fig. 8D1). In addition, IF staining of PTEN indicated that the expression of PTEN was concentrated at a region near the apical membrane of epithelial cells and was higher in the IS than in the caput (Fig. 8D2).
Differential Responses of the IS and Caput to Loss of Dusp6
The loss of Dusp6 often resulted in a larger caput and/or corpus. We selectively compared the larger Dusp6−/− epididymides with Dusp6+/+ epididymides. Two pairs of protein array analysis (Dusp6−/− IS vs. Dusp6+/+ IS, Dusp6−/− large caput vs. Dusp6+/+ caput) were conducted to analyze the changes that occurred in the IS and caput, respectively, following loss of Dusp6.
In the comparison of Dusp6−/− IS versus Dusp6+/+ IS, phosphorylation of the activation sites of MAP2K1/2, RPS6KA1/2, and GSK3A/B was lower in Dusp6−/− IS, and phosphorylation of the inhibitory site of SRC was higher in Dusp6−/− IS. Thus, MAP2K1/2, RPS6KA1/2, GSK3A/B, and SRC were less active in Dusp6−/− IS compared to Dusp6+/+ IS (Table 1). In addition, the inhibition of CDK1/2 activity by phosphorylation on Y15 was reduced in the Dusp6−/− IS (Table 1), and MAP2K1 T385 phosphorylation level was higher in Dusp6−/− IS compared to Dusp6+/+ IS (Table 1). Notably, MAPK1/3 activities in the IS of Dusp6−/− mice did not show any changes compared to Dusp6+/+ mice (Table 1).
In the comparison of Dusp6−/− large caput versus Dusp6+/+ caput, the activities of MAPK1/3 and downstream targets RPS6KA1/2 were higher in the large caput of Dusp6−/− mice compared to Dusp6+/+ caput (Table 1). Phosphorylation of GSK3B on inhibitory site S9 was reduced; thus, GSK3B activity was higher in the large Dusp6−/− caput (Table 1).
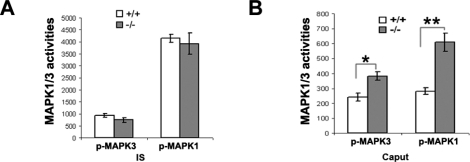
To confirm the protein array data, we conducted Western blot analysis of MAPK1/3 activities in Dusp6−/− mice with the larger epididymides. Compared to Dusp6+/+, the loss of Dusp6 resulted in a significant increase in the MAPK1/3 activities in the large caput (Fig. 9B) but not in the normal size IS (Fig. 9A), although the basal level of MAPK1/3 activity in the caput was much lower than that in the IS (Fig. 9).
FIG. 9.
Quantitative measurements of MAPK1/3 activities in Dusp6−/− mice with large epididymides compared to Dusp6+/+mice. A) MAPK3 and MAPK1 activities in Dusp6−/− IS compared to Dusp6+/+ IS (n = 3). B) MAPK3 and MAPK1 activities in Dusp6−/− large caput compared to Dusp6+/+ caput (n = 3). The arbitrary units in the figures represent the intensity of Western blot bands of phosphorylated MAPK3 or MAPK1, which were normalized to the loading control of total MAPK1. *P < 0.05 versus Dusp6+/+, **P < 0.01 versus Dusp6+/+.
DISCUSSION
Previous studies using the rat indicated that loss of protection of lumicrine factors would lead to the apoptosis of subset cells in the IS and that this cell death is TUNEL-positive and p53-independent [5]. In the present study report, we confirmed a similar phenomenon in the mouse.
Roles of High Activation of ERK Pathway in IS Development and Survival
Before the present study, it was unclear which downstream signal transduction pathways contributed to the protection of the IS from apoptosis. Here, we report high activities of MAPK1/3 are present specifically in the epithelium cells of IS (Fig. 1D), which are regulated by lumicrine factors. Presumably, lumicrine factors activated MAPK1/3 from P14 to P19 and sustained this activation into adulthood in the epithelial cells of the IS. The loss of lumicrine factors did not affect activities of MAPK14, MAPK8, RPS6KB1, and AKT but reduced the high activities of MAPK1/3 in the IS of adult mice. In fact, the activity of entire MAP2K1/2/MAPK1/3/ETV4/DUSP6 pathway was reduced within the first 6 h after EDL (Fig. 1). Without the support of a highly active ERK pathway, the epithelial cells of adult mice IS underwent apoptosis. In juvenile mice, MAPK1/3 activities in the IS were also reduced following loss of lumicrine factors (Fig. 5), which led to the cessation of cell proliferation and differentiation (Fig. 4). This resulted in the failure of the IS to develop. Therefore, activation of the ERK pathway by lumicrine factors is correlated with IS development in juvenile mice and with IS survival in adult mice.
To provide direct evidence that FGF signaling, upstream of the ERK pathway, is involved in cell proliferation and survival, two experiments are currently being undertaken: 1) in vivo electroporation of gene-silencing reagents of the FGF receptors to determine if it would lead to down-regulation of the ERK pathway and apoptosis, and 2) perfusion of FGFs into the lumen of the IS following EDL to determine if we can prevent apoptosis and restore cell proliferation.
Lumicrine Regulation of Dusp6 Expression in the IS
This study supports the previous finding that Dusp6 expression level is determined by the level of MAPK1/3 phosphorylation [24–26]. In the IS, Dusp6 mRNA expression paralleled MAPK1/3 activities. From P14 to P19, a simultaneous increase of mRNA expression of Dusp6 and phosphorylation of MAPK1/3 was observed (Fig. 3). Following EDL, both mRNA expression of Dusp6 and phosphorylation of MAPK1/3 declined simultaneously (Figs. 1C, 2A, and 5), and protein expression of DUSP6 also declined within the first 6 h after EDL (Fig. 2B). Because lumicrine factors regulate MAPK1/3 activities in the IS and MAPK1/3 activities regulate Dusp6 expression, lumicrine factors thus regulate a cascade of events involving MAPK1/3 and Dusp6.
Limited Ability of DUSP6 to Inactivate MAPK1/3 in the IS
Although DUSP6 could attenuate MAPK1/3 activities by dephosphorylation of these kinases [15], several lines of evidence from our experiments suggest that DUSP6 has only a limited role in inactivating MAPK1/3 in the IS. First, Dusp6 mRNA and protein levels were constantly high in the IS, but MAPK1/3 activities were not suppressed and were sustained following puberty (Fig. 3C). Second, DUSP6 expression declined sharply after EDL, but no increase of MAPK1/3 activities was observed at any time point after EDL (data not shown). Third, technical reasons did not allow us to examine directly the ability of DUSP6 to inactivate MAPK1/3; therefore, MAPK1-directed total phosphatase activities were examined. As shown in Figure 2C, MAPK1-directed total phosphatase activities did not alter significantly after EDL, suggesting that the phosphatases, including DUSP6, may not be the major contributors that inactivate MAPK1/3 after loss of lumicrine factors. Fourth, and most important, targeting deletion of Dusp6 did not increase MAPK1/3 activities in the IS (Fig. 9A).
Previous studies indicated that phosphorylation of DUSP6 at S159 and S197 subjected this enzyme to degradation through the proteasome pathway [27]. Our Western blot analysis of DUSP6 revealed a pattern of double bands (Fig. 2B), and as suggested by previous studies, the top band was probably the phosphorylated form of DUSP6, which is presumably the target for degradation [18, 27]. It is possible that DUSP6 has less phosphatase activity in the IS, which is the result of an increase in phosphorylation that leads to a higher level of degradation.
Targeting deletion of Dusp6 did not increase the already high MAPK1/3 activities in the IS (Fig. 9A). An interesting and similar phenomenon was also observed when another independently established Dusp6−/− mouse line was employed to study the physiological roles of DUSP6 in the heart. Although loss of Dusp6 increased basal MAPK1/3 phosphorylation in the heart, loss of Dusp6 did not increase or prolong the already-stimulated MAPK1/3 activation, which was induced by the hypotrophy-inducing stimuli [16], suggesting that DUSP6 activity is differentially regulated in certain situations.
Dusp6, a Key Regulator of Cell Proliferation in the Caput and Corpus
The loss of Dusp6 often produced a larger epididymis, especially in the caput and corpus regions, as a result of higher cell proliferation (Fig. 7). The higher cell proliferation was associated with increased MAPK1/3 activities (Fig. 9B), which likely resulted from reduced dephosphorylation of MAPK1/3 in Dusp6−/− mice. These results demonstrated that Dusp6 is a key negative regulator of cell proliferation in the caput and corpus.
In an independently established Dusp6−/− mouse line, basal MAPK1/3 phosphorylation in the heart, spleen, kidney, brain, and fibroblasts was increased [16]. Consistent with the increased MAPK1/3 activity, the hearts were larger because of an increase in myocyte proliferation [16]. Furthermore, mouse embryonic fibroblasts derived from that Dusp6−/− mice had a reduced apoptosis rate compared with those derived from Dusp6+/+ mice [16]. These data provide further support for our hypothesis that DUSP6 plays roles in cell proliferation and survival in the epididymis.
Differential Cell Proliferation Regulation Between the IS and Caput
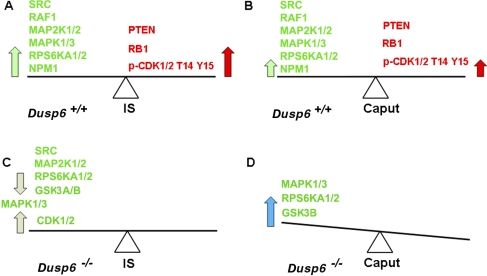
Our Western blot analysis and protein array analysis illustrated differences in the regulation of cell proliferation between the IS and caput (Fig. 8, A–C, and Table 1). In the IS, ERK pathway components and the oncogenes SRC and NPM1 had high proproliferation activities (Fig. 10A, big green arrow), whereas PTEN and RB1 had high activities that acted to repress cell proliferation and CDK1/2 was inactivated by phosphorylation at T14 and Y15 (Fig. 10A, big red arrow). These two opposite forces may balance out in the IS (Fig. 10A), resulting in cessation of cell proliferation. In the caput, forces for proproliferation and for antiproliferation were reduced (Fig. 10B, small arrows) compared to the IS, which may also reach a balance and result in cessation of cell proliferation.
FIG. 10.
Diagram illustrating our working hypothesis of regulation of cell proliferation in the IS and caput before and after Dusp6 disruption. A) In the Dusp6+/+ IS, strong proproliferation activities of SRC, RAF1, MAP2K1/2, MAPK1/3, RPS6KA1/2, and NPM1 (large green arrow) balance strong antiproliferation activities of PTEN, RB1, and phosphorylation of CDK1/2 at T14 Y15 (large red arrow). B) In the Dusp6+/+ caput, the lesser proproliferation activities of SRC, RAF1, MAP2K1/2, MAPK1/3, RPS6KA1/2, and NPM1 (small green arrow) balance the lesser inhibitory force from PTEN, RB1, and phosphorylation of CDK1/2 at T14 Y15 (small red arrow). C) After loss of Dusp6 in the IS, the decreased proproliferation responses resulting from decreased SRC, MAP2K1/2, RPS6KA1/2, and GSK3A/B activities balance the increased proproliferation responses resulting from increased CDK1/2 activity (light gray arrows). MAPK1/3 activities were not affected by the loss of Dusp6. In the end result, cell proliferation regulation did not altered significantly in the Dusp6−/− IS, and the tissue size of Dusp6−/− IS was unchanged. D) Increased MAPK1/3, RPS6KA1/2, and GSK3B activities resulting from Dusp6 disruption (blue arrow) tipped the original balance to a proproliferation state in the Dusp6−/− caput, which resulted in a larger caput.
In the search for IS-specific cell proliferation regulators, SRC and PTEN drew special attention. Both protein array data and IF staining revealed higher SRC activity in the IS than in the caput (Table 1 and Fig. 8D1). Higher SRC activity is probably related to the higher activation of the ERK pathway in the IS, because SRC could activate multiple components upstream of the MAPK1/3 cascade [28]. Moreover, IF staining and protein array analysis also revealed that PTEN had higher levels of expression and activity in the IS than in the caput (Table 1 and Fig. 8D2). High PTEN activity may cause a reduction in the activities of components of the PI3K/AKT pathway [29] in the IS. It may also explain why the components of AKT pathway do not respond to lumicrine factor stimulation. Furthermore, the inhibition of cell proliferation by PTEN [29] may be one of the reasons that even with a constantly activated ERK pathway, IS epithelial cells stop proliferating after P40.
Different Effects on Loss of Dusp6 in the IS and Caput
In the IS, targeting deletion of Dusp6 increased CDK1/2 activity but decreased SRC, MAP2K1/2, RPS6KA1/2, and GSK3A/B activities. These two opposite responses may balance each other (Fig. 10C, light gray arrows). The loss of Dusp6 did not alter MAPK1/3 activities in the IS; however, T385 MAP2K1, a feedback phosphorylation site by MAPK1 [30], showed increased phosphorylation (Table 1), suggesting MAPK1 activities may be increased by the loss of Dusp6 at some point but return to original levels, presumably by some feedback regulation. Eventually, unchanged MAPK1/3 activities and the balance of the increased and decreased proproliferation activities resulted in no obvious changes in cell division in the IS of Dusp6−/− mice (Fig. 10C), and the size of Dusp6−/− IS was normal. However, the caput was often larger in Dusp6−/− mice. As shown in Table 1, in the larger caput, MAPK1/3, RPS6KA1/2, and GSK3B activities were higher, which could tip the balance to a proproliferation state (Fig. 10D); thus. cell proliferation continued, eventually leading to a larger epididymis.
In the present report, we demonstrated that the regulation of MAPK1/3 and DUSP6 is correlated with epididymal cell proliferation and survival in a region-specific manner. We also identified additional components, such as SRC and PTEN, which appear to play a role in this region-specific regulation. Our current working hypothesis is that region-specific regulation of cell proliferation results from differences in the balance of activities between pro- and antiproliferation signaling pathway components for each epididymal region. Understanding signal transduction pathways in the epididymis may help us to understand both the potential mechanisms of epididymal development and function and why the epididymis, which is under the constant stimulation of androgen and growth factors, rarely succumbs to cancer.
Acknowledgment
We thank Dr. Suzanne L. Mansour (University of Utah) for providing Dusp6−/− mice.
Footnotes
Supported by NIH-NICHD grant HD052035.
REFERENCES
- Robaire B, Hinton BT, Orgebin-Crist M-C.The epididymis. Neill JD.Knobil and Neill's Physiology of Reproduction, 3rd ed. New York:Elsevier;2006: 1071–1148. [Google Scholar]
- Sonnenberg-Riethmacher E, Walter B, Riethmacher D, Godecke S, Birchmeier C.The c-ros tyrosine kinase receptor controls regionalization and differentiation of epithelial cells in the epididymis. Genes Dev 1996; 10: 1184–1193. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Sonnenberg-Riethmacher E, Cooper TG.Receptor tyrosine kinase c-ros knockout mice as a model for the study of epididymal regulation of sperm function. J Reprod Fertil Suppl 1998; 53: 137–147. [PubMed] [Google Scholar]
- Hinton BT, Lan ZJ, Lye RJ, Labus JC.Regulation of epididymal function by testicular factors: The lumicrine hypothesis. Goldberg E.The Testis. New York:Springer-Verlag;2000: 163–173. [Google Scholar]
- Turner TT, Riley TA.p53-Independent, region-specific epithelial apoptosis is induced in the rat epididymis by deprivation of luminal factors. Mol Reprod Dev 1999; 53: 188–197. [DOI] [PubMed] [Google Scholar]
- Abe K, Takano H.Early degeneration of the epithelial cells in the initial segment of the epididymal duct in mice after efferent duct cutting. Arch Histol Cytol 1989; 52: 299–310. [DOI] [PubMed] [Google Scholar]
- Nicander L, Osman DI, Ploen L, Bugge HP, Kvisgaard KN.Early effects of efferent ductule ligation on the proximal segment of the rat epididymis. Int J Androl 1983; 6: 91–102. [DOI] [PubMed] [Google Scholar]
- Robaire B, Fan X.Regulation of apoptotic cell death in the rat epididymis. J Reprod Fertil Suppl 1998; 53: 211–214. [PubMed] [Google Scholar]
- Fawcett DW, Hoffer AP.Failure of exogenous androgen to prevent regression of the initial segments of the rat epididymis after efferent duct ligation or orchidectomy. Biol Reprod 1979; 20: 162–181. [DOI] [PubMed] [Google Scholar]
- Hinton BT, Kirby JL, Rodriguez CM, Lye RJ, Troan BV, Yang L.Signal transduction pathways to gene expression. Hinton BT, Terry TT.The third international conference on the epididymis. Charlottesville, VA:The Van Doren Company;2003: 103–113. [Google Scholar]
- Cotton LM, O'Bryan MK, Hinton BT.Cellular signaling by fibroblast growth factors (FGFs) and their receptors (FGFRs) in male reproduction. Endocr Rev 2008; 29: 193–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan ZJ, Labus JC, Hinton BT.Regulation of gamma-glutamyl transpeptidase catalytic activity and protein level in the initial segment of the rat epididymis by testicular factors: role of basic fibroblast growth factor. Biol Reprod 1998; 58: 197–206. [DOI] [PubMed] [Google Scholar]
- Kirby JL, Yang L, Labus JC, Hinton BT.Characterization of fibroblast growth factor receptors expressed in principal cells in the initial segment of the rat epididymis. Biol Reprod 2003; 68: 2314–2321. [DOI] [PubMed] [Google Scholar]
- Okazaki IJ, Pryor JL.Cancer of the epididymis. Robaire B, Hinton BT.The epididymis from molecules to clinic practice. New York:Kluwar Academic/Plenum Publishers;2002: 555–561. [Google Scholar]
- Ramos JW.The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. Int J Biochem Cell Biol 2008; 40: 2707–2719. [DOI] [PubMed] [Google Scholar]
- Maillet M, Purcell NH, Sargent MA, York AJ, Bueno OF, Molkentin JD.DUSP6 (MKP3) null mice show enhanced ERK1/2 phosphorylation at baseline and increased myocyte proliferation in the heart affecting disease susceptibility. J Biol Chem 2008; 283: 31246–31255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Scott DA, Hatch E, Tian X, Mansour SL.Dusp6 (Mkp3) is a negative feedback regulator of FGF-stimulated ERK signaling during mouse development. Development 2007; 134: 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino MA, Hinton BT.Expression of multiple gamma-glutamyl transpeptidase messenger ribonucleic acid transcripts in the adult rat epididymis is differentially regulated by androgens and testicular factors in a region-specific manner. Endocrinology 1994; 135: 1146–1156. [DOI] [PubMed] [Google Scholar]
- Berger UV, Hediger MA.Differential distribution of the glutamate transporters GLT-1 and GLAST in tanycytes of the third ventricle. J Comp Neurol 2001; 433: 101–114. [DOI] [PubMed] [Google Scholar]
- Yang L, Fox SA, Kirby JL, Troan BV, Hinton BT.Putative regulation of expression of members of the Ets variant 4 transcription factor family and their downstream targets in the rat epididymis. Biol Reprod 2006; 74: 714–720. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Norris RD, Eisch AJ.Chronic morphine induces premature mitosis of proliferating cells in the adult mouse subgranular zone. J Neurosci Res 2004; 76: 783–794. [DOI] [PubMed] [Google Scholar]
- Jurek A, Amagasaki K, Gembarska A, Heldin CH, Lennartsson J.Negative and positive regulation of MAPK phosphatase 3 controls platelet-derived growth factor-induced Erk activation. J Biol Chem 2009; 284: 4626–4634. [DOI] [PubMed] [Google Scholar]
- Levinthal DJ, Defranco DB.Reversible oxidation of ERK-directed protein phosphatases drives oxidative toxicity in neurons. J Biol Chem 2005; 280: 5875–5883. [DOI] [PubMed] [Google Scholar]
- Owens DM, Keyse SM.Differential regulation of MAP kinase signaling by dual-specificity protein phosphatases. Oncogene 2007; 26: 3203–3213. [DOI] [PubMed] [Google Scholar]
- Keyse SM.Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev 2008; 27: 253–261. [DOI] [PubMed] [Google Scholar]
- Ekerot M, Stavridis MP, Delavaine L, Mitchell MP, Staples C, Owens DM, Keenan ID, Dickinson RJ, Storey KG, Keyse SM.Negative-feedback regulation of FGF signaling by DUSP6/MKP-3 is driven by ERK1/2 and mediated by Ets factor binding to a conserved site within the DUSP6/MKP-3 gene promoter. Biochem J 2008; 412: 287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti S, Gimond C, Chambard JC, Touboul T, Roux D, Pouyssegur J, Pages G.Extracellular signal-regulated kinases phosphorylate mitogen-activated protein kinase phosphatase 3/DUSP6 at serines 159 and 197, two sites critical for its proteasomal degradation. Mol Cell Biol 2005; 25: 854–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Watkins SC, Walker WH.Testosterone activates mitogen-activated protein kinase via Src kinase and the epidermal growth factor receptor in Sertoli cells. Endocrinology 2007; 148: 2066–2074. [DOI] [PubMed] [Google Scholar]
- Cantley LC, Neel BG.New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci U S A 1999; 96: 4240–4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Wilsbacher JL, Collisson T, Cobb MH.The N-terminal ERK-binding site of MEK1 is required for efficient feedback phosphorylation by ERK2 in vitro and ERK activation in vivo. J Biol Chem 1999; 274: 34029–34035. [DOI] [PubMed] [Google Scholar]