Abstract
Signaling through the EGF receptor is regulated by endocytosis. ARAP1 is a protein with Arf guanosine triphosphatase-activating protein (GAP) and Rho GAP domains. We investigated the role of ARAP1 in EGF receptor endocytic trafficking. Following EGF treatment of cells, ARAP1 rapidly and transiently associated with the edge of the cell and punctate structures containing Rab5, rabaptin 5 and EGFR but not early embryonic antigen 1 (EEA1). EGF associated with the ARAP1-positive punctate structures prior to EEA1-positive early endosomes. Recruitment of ARAP1 to the punctate structures required active Rab5 and an additional signal from EGFR. Decreasing ARAP1 levels with small interfering RNA accelerated association of EGF with EEA1 endosomes and degradation of EGFR. Phosphorylation of extracellular-signal-regulated kinase (ERK) and c-Jun-amino-terminal kinase (JNK) was diminished and more transient in cells with reduced levels of ARAP1 than in controls. Based on these findings, we propose that ARAP1 regulates the endocytic traffic of EGFR and, consequently, the rate of EGFR signal attenuation.
Keywords: ADP ribosylation factor, ARAP1, GTPase-activating protein, EGF, endocytosis, Rab5
Receptor tyrosine kinases (RTKs) are transmembrane proteins that mediate responses to extracellular signals that control differentiation, proliferation and migration (1–4). Unchecked activity of RTKs may be part of malignant transformation. Endocytosis is integral to regulation of signaling from the receptors (1,5–7).
EGF receptor (EGFR) family proteins have been used as a model to study RTKs that contribute to the development of some forms of breast cancer (6,8,9). Ligands such as EGF or transforming growth factor bind to EGFR, which stimulates the tyrosine kinase activity of the receptor. The activated receptor phosphorylates itself and signals through nonreceptor tyrosine kinases, phospholipase C, the Ras pathway and phosphatidyl inositol (PI) 3-kinase. The signals from the EGFR are regulated by the endocytic pathway. EGFR is internalized by clathrin-dependent endocytosis (10–14). The receptor is concentrated in clathrin-coated pits by binding to the clathrin adaptor AP-2. The pits form buds that separate from the planar membrane to become clathrin-coated vesicles. The vesicles shed the clathrin coat to form intermediate vesicles. The intermediate vesicles fuse with early endosomes, identified by the protein early embryonic antigen 1 (EEA1). Clathrin-independent endocytosis of EGFR also delivers receptors to early endosomes. From the EEA1-positive compartment, the receptor is sorted towards recycling or degradative pathways.
Receptor internalization and targeting to the EEA1-positive compartment are regulated by several mechanisms (7,11,12,15–19). One mechanism involves the small GTP-binding protein Rab5, which controls the transfer of receptor from the plasma membrane (PM) to an intermediate compartment and from the intermediate compartment to EEA1-positive early endosomes (20–28). Rab5 functions through a number of effectors including rabaptin5, rabankyrin5 and EEA1. Rab5 is activated by the exchange factor Rin1 functioning downstream of EGFR (29). Other small GTP-binding proteins, Rho and Rac, inhibit receptor endocytosis (30–32). The effects of Rho and Rac are mediated by serine/threonine kinases.
Arf family GTP-binding proteins are regulators of membrane traffic and actin remodeling (for reviews, see 33–35). The six mammalian Arfs are divided into three classes based on primary structure. Arf1, a class 1 Arf, regulates intra-Golgi and endosomal membrane trafficking by controlling the function of coat proteins including coatomer, clathrin adaptor protein-1 (AP-1) and AP-3 and Golgi-localized, gamma ear homology domain, Arf binding protein (GGA). Arf1 has also been implicated in the endocytosis of EGFR through a clathrin-and caveolin-independent pathway (36). Arf6, a class 3 Arf, regulates endocytosis of receptors at the PM by a pathway that is independent of clathrin and caveolin. Arfs have slow nucleotide exchange rates and undetectable guanosine triphosphatase (GTPase) activity. Function of Arfs is dependent on accessory proteins called guanine nucleotide exchange factors and GTPase-activating proteins (GAPs) (37–39).
Thirty-one genes in humans encode for proteins with Arf GAP domains (39–42). Five subtypes of Arf GAPs have been implicated in the regulation of the actin cytoskeleton associated with cell movement (43). These include the ARAPs, Arf GAPs that also contain a Rho GAP, five PH, a SAM and a Ras association domain (44–49). Two PH domains contain the consensus sequence for phosphatidyl inositol 3 phosphate (PIP3) binding, and the ARAPs have been found to be targets for tyrosine kinases and Rap1 (44,46,47).
ARAP1 is a target of PIP3 and phosphotyrosine signaling and regulates Arf and Rho proteins. Based on these observations, we hypothesized that ARAP1 is part of the EGFR signaling pathway regulating endocytic traffic of EGFR. EGF caused a translocation of ARAP1 to an endocytic compartment containing Rab5, rabaptin5, EGF and EGFR. The compartment was rapidly formed and transient. EGF associated with the compartment prior to being delivered to the EEA1-positive early endosome. Reduction of ARAP1 expression accelerated EGFR internalization and degradation and diminished EGF-dependent phosphorylation of ERK and JNK. Taken together, the data support a model in which ARAP1 regulates a pre-early endosome compartment that controls the rate of EGFR signal attenuation.
Results
We compared the localization of ARAP1 in HeLa cells with the localization in HeLa cells treated with EGF. Two isoforms of ARAP1 have been reported. The larger isoform contains a SAM domain. In these experiments, we used an antiserum that recognized ARAP1 that comigrates with recombinant ARAP1 that has the SAM domain (Figure S1). As we have previously reported, ARAP1 has a perinuclear distribution in untreated cells, colocalizing with a number of markers of the Golgi apparatus (50–53)including Golgi 58K, GM130 and AP-1 [(44)and Figure 1A]. Following treatment with EGF, the distribution of ARAP1 was partially changed, with some protein in puncta near and at the cell edge (Figure 1A–C). ARAP1 did not colocalize with other organellar markers (52,54–58)including AP-2, Lamp1, mannose-6-phosphate receptor (M6PR) or EEA1 with or without EGF (Figure 1A). After treatment with EGF, ARAP1 colocalized with EGFR and EGF (Figure 1A,B,D). Rabaptin 5 also moved to the edge of the cell with ARAP1 after EGF treatment (Figure 1A).
Figure 1.
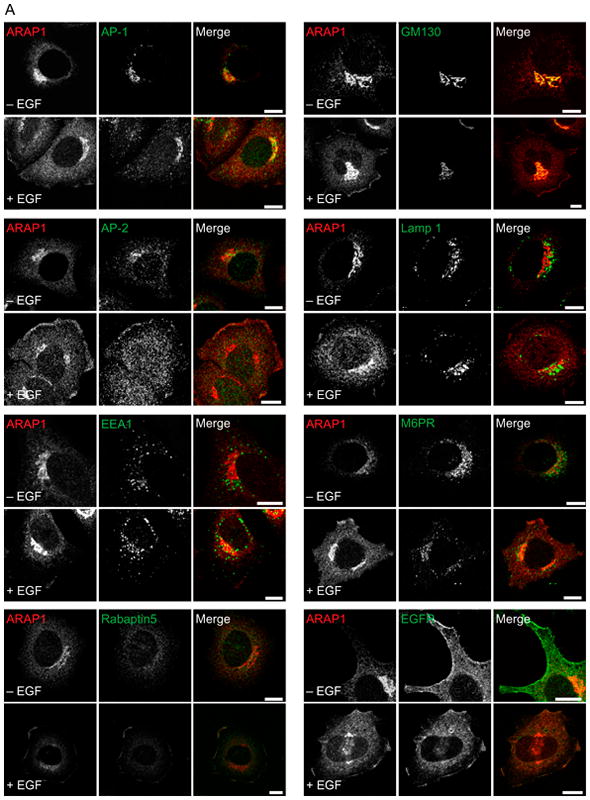
Effect of EGF on the localization of ARAP1 in HeLa cells. A) Localization of endogenous ARAP1. After 6 h of serum deprivation, HeLa cells were treated with 100 ng/mL of EGF for 5 min at 37°C. Cells were fixed, and the localization of endogenous ARAP1 and AP-1, AP-2, EEA1, EGFR, GM130, Lamp 1, M6PR or Rabaptin5 was analyzed by immunofluorescence microscopy. Scale bar, 10 μm. B) Relative localization of endogenous ARAP1 and EGF. Serum-starved HeLa cells were treated with fluorescent EGF for 5 min before fixing and staining for ARAP1. ARAP1 and EGF were visualized using confocal microscopy. Scale bar, 10 μm. Arrows indicate colocalization of ARAP1 and EGF. C) ARAP1 at the cell edge. HeLa cells were serum-starved for 6 h and then treated with 100 ng/mL EGF for 5 min. The cells were fixed and stained for ARAP1. The intensity of ARAP1 in a masked area that included the cell edge was quantified using IPLab image processing software. The data shown are for 25 cells under each condition. The error bars are the SEM. D) Colocalization of ARAP1 with EGFR. HeLa cells, expressing recombinant Flag-ARAP1, where indicated and treated with or without 100 ng/mL EGF for 5 min. The cells were fixed and stained for ARAP1 and EGFR. Images of the cells were captured using confocal microscopy. Pearson coefficients were determined for 25 cells under each condition using Zeiss lsm5 image program. The error bars are the SEM.
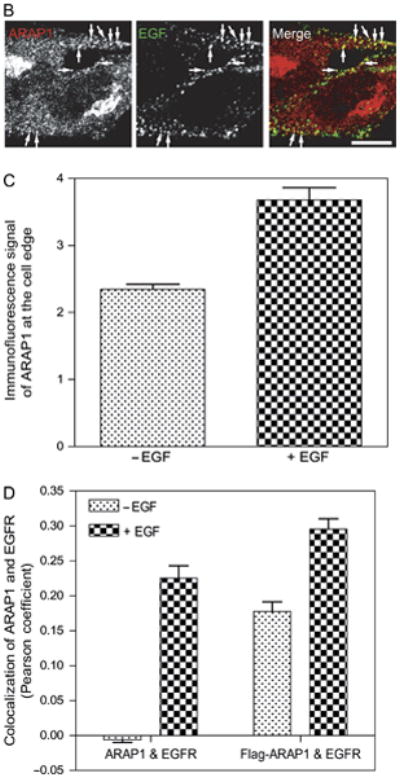
We next examined the distribution of recombinant Flag epitope-tagged ARAP1 (Flag-ARAP1) (Figure 2). In this paper, we examined the form of ARAP1 with the SAM domain. Different than endogenous ARAP1, Flag-ARAP1 did not associate with the Golgi apparatus. However, similar to endogenous ARAP1, Flag-ARAP1 associated with EGFR and rabaptin 5 after treatment of cells with EGF. Flag-ARAP1 also colocalized with fluorescent EGF. As with endogenous protein, we did not observe colocalization of Flag-ARAP1 with other markers of organelles, including M6PR, AP-1, AP-2 and EEA1 (Figure S2). In addition, Flag-ARAP1 did not colocalize with endogenous APPL1 (59)(data not shown).
Figure 2.
EGF affects the distribution of recombinant ARAP1. HeLa cells expressing Flag-ARAP1 were serum-starved for 6 h and then treated with EGF (100 ng/mL) for 5 min at 37°C. Fixed cells were immunostained with antibodies against the Flag epitope for ARAP1 and the indicated proteins. Arrows indicate colocalization with Flag-ARAP1 and fluorescent EGF. A higher magnification is shown in the inset. Scale bar, 10 μm.
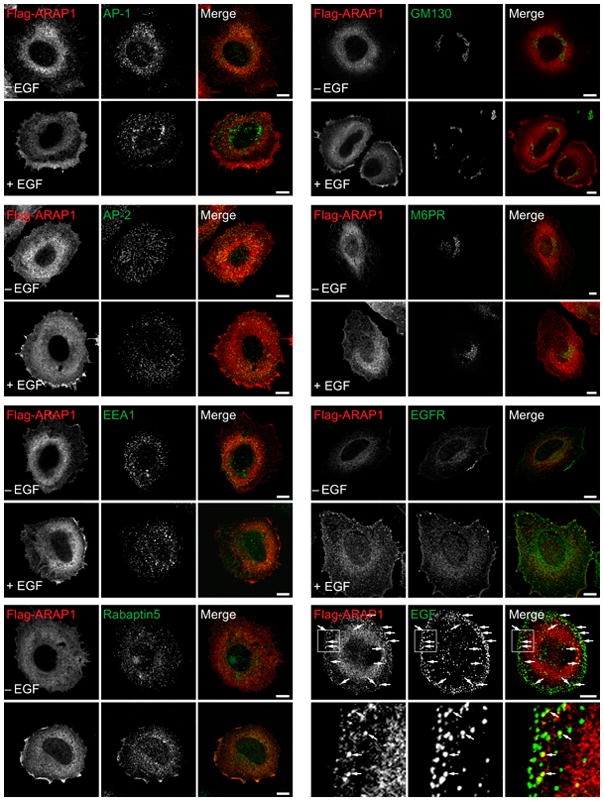
Because of the association of ARAP1 with rabaptin 5, the relative localization of ARAP1 and markers of organelles with Rab5 was examined. We were not able to find an antibody that was reliable for visualizing endogenous Rab5. Therefore, green fluorescent protein (GFP)–Rab5 was used (Figure 3). Expression of recombinant Rab5 can influence the distribution of proteins associated with membrane-bound organelles. There were two notable differences in protein distribution between cells expressing Flag-ARAP1 with GFP–Rab5 and those expressing Flag-ARAP1 alone. First, Flag-ARAP1 associated with M6PR, APPL1 and AP-1 after EGF treatment. Second, the Flag-ARAP1-positive puncta were larger than those observed with endogenous proteins. However, the key finding that EGF-induced association of ARAP1 with a compartment containing EGF and EGFR was the same. The compartment also contained GFP–Rab5 and rabaptin5 (Figures 3 and 4A,B). Also, similar to experiments examining endogenous ARAP1 or Flag-ARAP1 expressed by itself, recombinant Flag-ARAP1 expressed with GFP–Rab5 did not colocalize with EEA1, AP-2 or Lamp1 (Figure S2).
Figure 3.
Effect of EGF on the distribution of recombinant ARAP1 in cells that also express GFP–Rab5. Serum-starved HeLa cells expressing Flag-ARAP1 and GFP–Rab5 were treated with 100 ng/mL EGF for 5 min where indicated. The cells were fixed and prepared for immunofluorescence staining to visualize the indicated proteins. Some merged images contain insets, which are enlarged images of the indicated boxed areas. Scale bar, 10 μm.
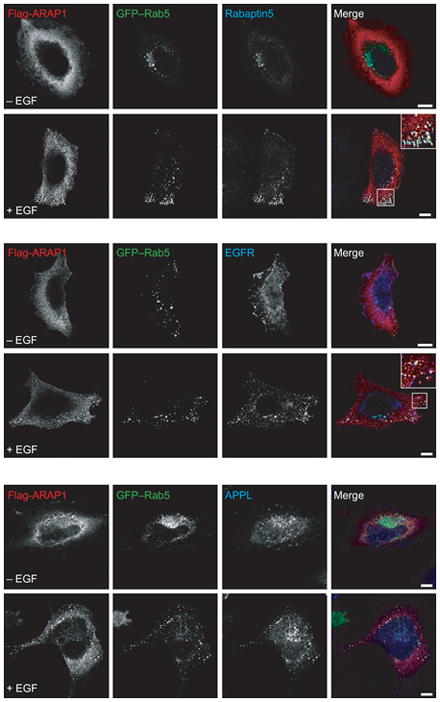
Figure 4.
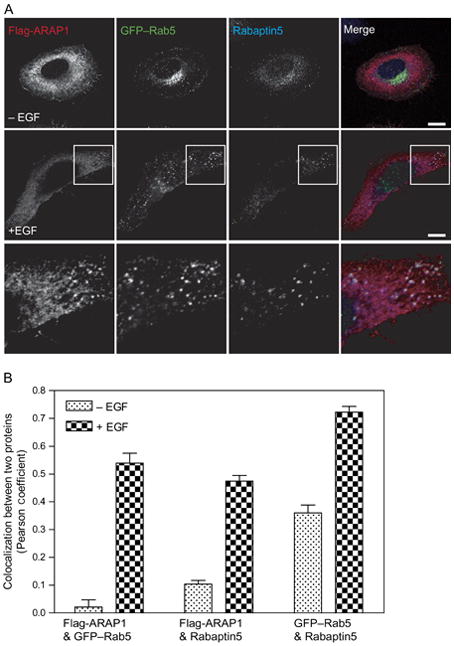
ARAP1 is recruited to a Rab5-positive compartment. A) Relative localization of ARAP1, Rab5 and Rabaptin 5. Serum-starved HeLa cells were treated with EGF for 5 min and stained for both ARAP1 and Rabaptin5, allowing visualization of ARAP1 (red), Rabaptin5 (blue) and Rab5 (green) in single cells. A higher magnification is shown in the inset. Scale bar, 10 μm. B) Colocalization of ARAP1 with Rab5 and Rabaptin5. Pearson coefficients were determined for the indicated combinations of proteins in 25 cells in which ARAP1, Rab5 and Rabaptin5 were simultaneously visualized. The errors bars represent the SEM.
To determine whether other Rab family proteins associated with the ARAP1-containing structures, recombinant ARAP1 was expressed with GFP–Rab4, GFP–Rab5, GFP–Rab7 and GFP–Rab11 (Figure 4 and Figure S3). Of these Rab proteins, only Rab5 colocalized with ARAP1. Flag-ARAP1, GFP–Rab5 and rabaptin 5 were together on punctate structures. On the basis of the association with Rab5, rabaptin5, EGFR and EGF, we conclude that the ARAP1-positive puncta represent endocytic intermediates.
We further characterized the EGF dependence of ARAP1 association with Rab5, determining whether we could identify a condition in which ARAP1 colocalized with the Rab5 effector EEA1. Cells expressing GFP–Rab5 and recombinant ARAP1 were treated with 1 to 100 ng/mL EGF for 5 min and stained with antibodies against the Flag tag of the recombinant ARAP1 and against endogenous EEA1 (Figure 5). Colocalization of ARAP1 with Rab5 was observed at every concentration of EGF examined. Colocalization was not detected in cells that were not treated with EGF. ARAP1 did not colocalize with EEA1 at any concentration of EGF that we examined.
Figure 5.
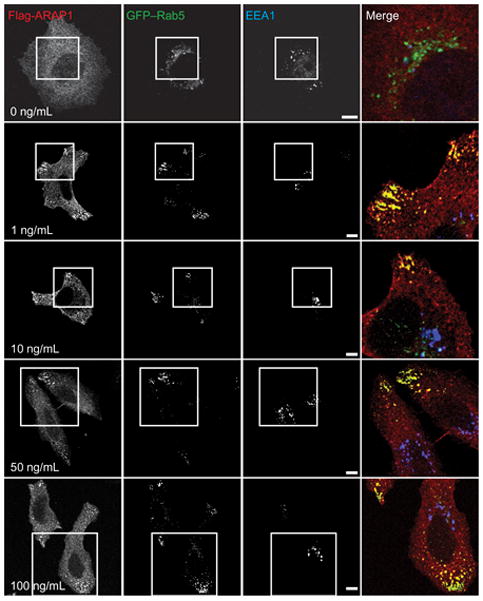
EGF dependence of ARAP1/Rab5 colocalization. Serum-starved HeLa cells transiently expressing Flag-ARAP1 and GFP–Rab5 were treated with the indicated concentrations of EGF for 5 min at 37°C. Fixed cells were immunostained with antibodies against Flag (red) and EEA1 (blue). The merged images are enlargements indicated by the boxed areas. Scale bars are 10 μm.
The time dependence of the ARAP1-containing puncta was determined in cells expressing Flag-ARAP1 and GFP–Rab5 (Figure 6A,B). Cells were treated with 100 ng/mL EGF. Rab5 localized with EEA1 prior to EGF treatment. Rab5 partly redistributed to ARAP1-containing puncta 3–5 min after EGF treatment. After 10 min of EGF treatment, colocalization of ARAP1 and Rab5 diminished and Rab5 associated primarily with EEA1-positive vesicles, which were distributed throughout the cell. The time dependence of ARAP1 association with Rab5 paralleled the time dependence of association with EGF (Figure 8A,D). ARAP1 did not colocalize with EEA1 during a 60 min period after addition of EGF (Figure 8A).
Figure 6.
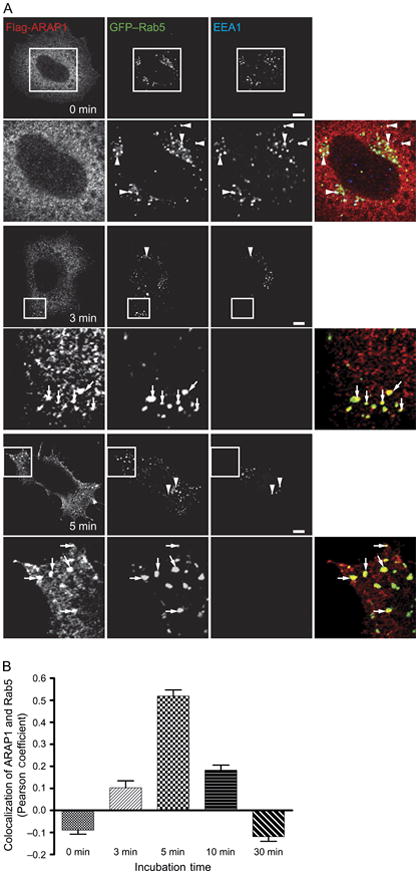
Time dependence of ARAP1/Rab5 colocalization. A) Images of ARAP1 colocalization. Serum-starved HeLa cells expressing Flag-ARAP1 and GFP–Rab5 were treated with 100 ng/mL EGF for the indicated period of time prior to fixing and staining. Inset is higher magnified area. Arrows indicate colocalization of Flag-ARAP1 and GFP–Rab5, and arrowheads are colocalization of GFP–Rab5 and EEA1. Scale bar, 10 μm. B) Colocalization of ARAP1 and Rab5 peaks at 5 min after EGF treatment. Pearson coefficients were determined for 25 cells at each time-point. The error bars represent the SEM.
Figure 8.
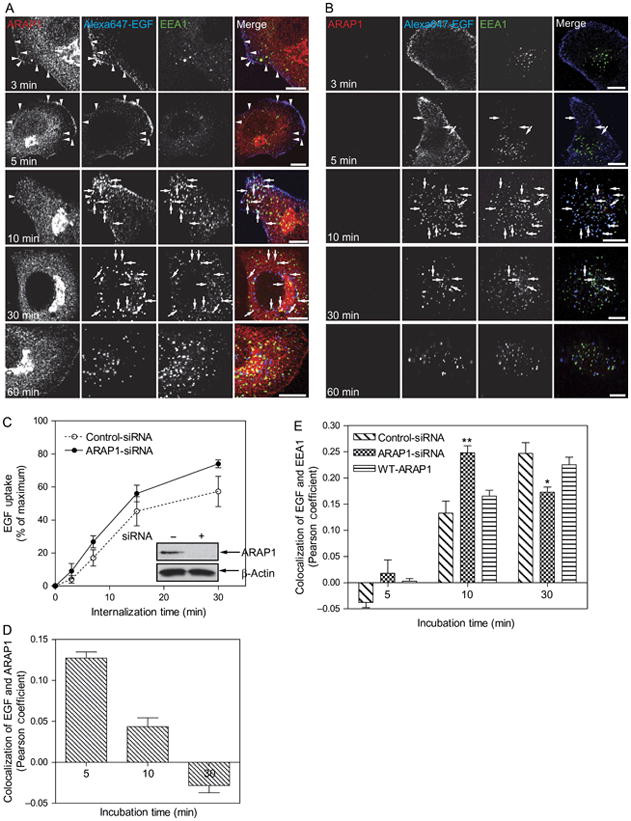
Effect of ARAP1 on EGF internalization. A) Time dependence of the relative localization of endogenous ARAP1, EGF and EEA1. HeLa Cells were treated with a nontargeting siRNA for 3 days prior to serum starvation and addition of 2 μg/mL of Alexa Fluor 647-labeled EGF. After the indicated length of time, cells were fixed and stained for endogenous ARAP1 and EEA1. EGF was visualized by Alexa Fluor 647 fluorescence. Arrowheads indicate colocalization of ARAP1 with Alexa Fluor 647-EGF, and arrows indicate colocalization of Alexa Fluor 647-EGF with EEA1. Scale bar, 10 μm. B) Effect of reduced ARAP1 expression on association with EEA1-positive compartment. HeLa cells treated with siRNA targeting ARAP1 were treated as described in (A). C) Time dependence of EGF internalization. Cells treated with nontargeting or ARAP1-siRNA were incubated with fluorescent EGF, and internalization was determined by FACS analysis as described in ‘Experimental Procedures’. Error bars are the SEM. D and E) Colocalization of EGF and ARAP1 (D) or EEA1 (E). Pearson coefficients were determined for 25 cells at each time-point. The error bars are the SEM. ** and * indicate significantly different from nontargeting-siRNA-treated cells, **p>0.001 and *p>0.01.
We determined if the association of ARAP1 with the punctate structures was affected by Rab5 (Figure 7). In these experiments, HeLa cells were transfected with plasmids directing the expression of Flag-ARAP1 and either wild-type Rab5, a dominant-negative form of Rab5 ([S34N]Rab5) or constitutively active Rab5 ([Q79L]Rab5) (60–64), fused to GFP. The cells were treated with EGF, and the localization of ARAP1 and GFP–Rab5 was determined. Without EGF, neither the wild type nor the Rab5 mutants colocalized with ARAP1 despite the presence of [Q79L]Rab5-positive punctate structures. Addition of EGF resulted in the colocalization of ARAP1 with wild type and [Q79L]Rab5. Puncta containing either ARAP1 or Rab5 did not form in cells expressing GFP–[S34N]Rab5.
Figure 7.
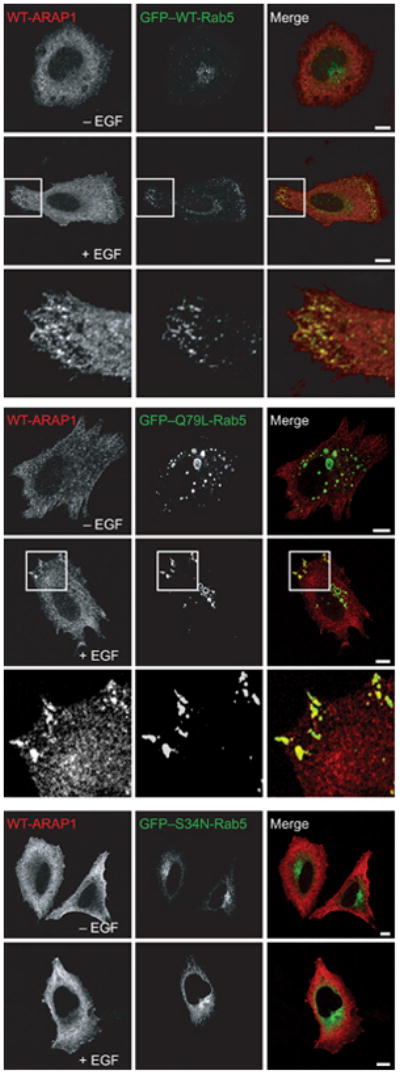
The effect of Rab5 on ARAP1 distribution. HeLa cells were transfected with plasmids encoding Flag-epitope-tagged ARAP1, GFP-tagged WT-, Q79L-and S34N-Rab5 for 24 h and were treated with 100 ng/mL EGF for 5 min. The cells were then fixed and stained with an antibody to the Flag epitope, and the cells were visualized by fluorescence microscopy. A higher magnification is shown in the inset. Scale bar, 10 μm.
The association of ARAP1 with Rab5-positive, EGF-containing vesicles led us to hypothesize that ARAP1 regulates internalization and endocytic trafficking of EGF. As a first test of the hypothesis, we used immunofluorescence to examine the intracellular trafficking of EGF. Cells transfected with nontargeting small interfering (siRNA), siRNA targeting the 3′-untranslated region (UTR) of ARAP1 or siRNA targeting ARAP1 with a plasmid directing expression of recombinant ARAP1 were treated with fluorescent EGF (Figure 8A). At the indicated times, cells were fixed and prepared for immunofluorescence to visualize ARAP1, EEA1 and EGF. EGF more rapidly associated with the EEA1-positive compartment in cells treated with siRNA targeting ARAP1 than in cells treated with nontargeting siRNA (Figure 8A,B with comparison of Pearson coefficients in Figure 8E). Expression of recombinant ARAP1 reversed the effect of reducing endogenous ARAP1. We did not detect an effect of reducing ARAP1 expression on transferrin trafficking to the EEA-positive compartment (Figure 9). The rate of EGF internalization was also determined. In these experiments, cells treated with either nontargeting or ARAP1-targeted siRNA were incubated with fluorescent EGF at 4°C. Unbound EGF was washed from the cells, and the cells were then warmed to 37°C. After the indicated incubation periods, extracellular EGF was stripped using an acidic buffer, and the amount of internalized fluorescent EGF was determined using a fluorescent-activated cell sorter (FACS). The rate of EGF internalization was approximately 20% greater in cells that were depleted of ARAP1 than in controls (Figure 8C). In contrast, the rate of transferrin internalization was the same in the control and ARAP1-depleted cells (Figure 9C).
Figure 9.
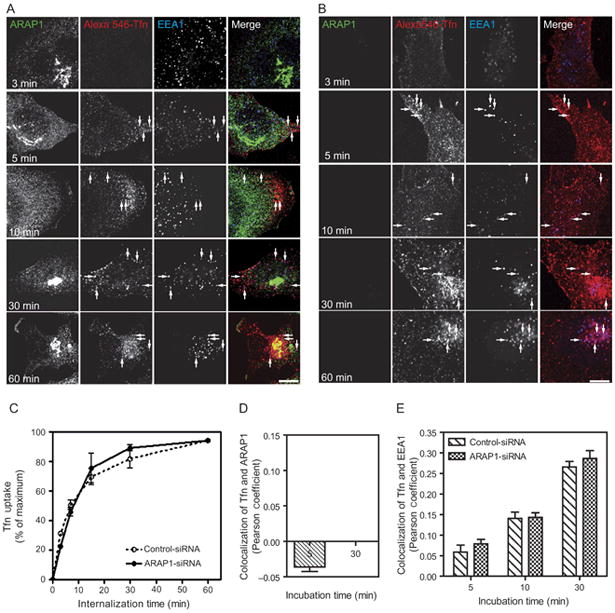
Figure 9. Effect of reduced ARAP1 expression on transferrin internalization. HeLa cells were treated with either (A) nontargeting or (B) ARAP1-targeted siRNA and serum-starved prior to the addition of Alexa Fluor 546–transferrin (Tfn). At the indicated times, cells were fixed and stained with antibodies to ARAP1 and EEA1. Arrows indicate colocalization of Alexa Fluor 546–Tfn and EEA1. Scale bar, 10 μm. C) Time dependence of transferrin internalization. HeLa cells treated with nontargeting or ARAP1-siRNA were incubated with fluorescent transferrin. Transferrin uptake was quantified by FACS analysis as described in ‘Experimental Procedures’. D) Colocalization of ARAP1 and transferrin. The colocalization of ARAP1 and fluorescent Tfn in HeLa cells was quantified by Zeiss lsm program. The error bars represent the SEM. E) Colocalization of EEA1 and transferrin. Pearson coefficients were determined for 25 cells at each time-point. The error bars are the SEM.
To corroborate the results of experiments in which fluorescent EGF was used to follow endocytosis, we examined the effect of reducing ARAP1 expression on internalization of EGFR (Figure 10). Cells were transfected with nontargeting siRNA or siRNA that targeted ARAP1. After treatment with EGF for the indicated periods, cells were chilled and EGFR on the cell surface was covalently modified with biotin using sulfo-NHS-LC-biotin. The cells were subsequently lysed, and biotinylated proteins were precipitated using streptavidin-conjugated beads. The precipitated proteins were fractionated by polyacrylamide gel electrophoresis, and EGFR was detected by immunoblotting. EGFR internalization was found to be accelerated in cells with reduced ARAP1 expression (Figure 10A). To exclude the possibility that the results were because of effects on the biosynthetic pathway, the experiment was repeated with cells pretreated with cycloheximide. As observed in cells that were not treated with cycloheximide, EGFR was more rapidly internalized in cells with reduced ARAP1 than in control cells (Figure 10B,C).
Figure 10.
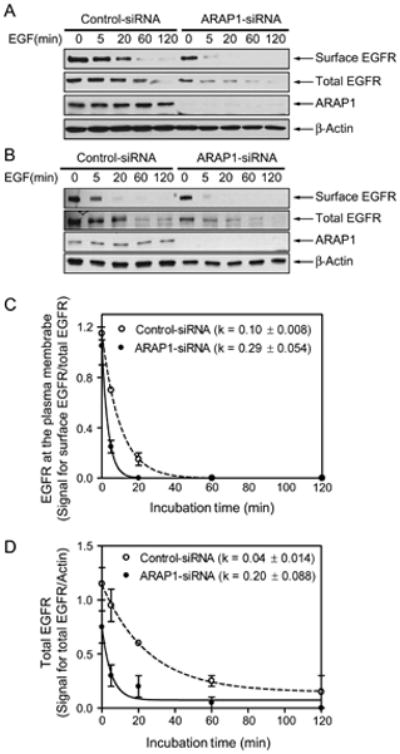
Effect of ARAP1 on EGFR. A and B) Internalization and degradation. HeLa cells treated with nontargeting siRNA or siRNA targeting ARAP1 were serum-starved and treated for the indicated duration with 25 ng/mL EGF in the absence (A) or presence (B) of cycloheximide (10 μg/mL). The cells were placed on ice and treated with sulfo-NHS-LC-biotin (0.1 mg/mL) prior to lysing the cells and precipitating proteins with streptavidin-conjugated beads. EGFR in the precipitate with streptavidin and in the total cell lysate was determined by immunoblotting. Data are representative of two separate experiments. Panel (B) shows data using cells preincubated with cycloheximide for 1 h and then treated EGF with cycloheximide. C and D) Quantification of surface (C) and total (D) EGFR. Western blots from two independent experiments were analyzed by densitometry. β-actin was used as a loading control and for normalizing the EGFR signal. The error bars are the SEM.
EGFR internalization is linked to its degradation. As another means of determining whether ARAP1 affected EGFR internalization and trafficking, total EGFR levels were measured in lysates of control cells and in cells with reduced expression of ARAP1 (Figure 10B,D). In these experiments, cells were treated with cycloheximide to block synthesis of EGFR. Cells with reduced ARAP1 degraded EGFR more rapidly than did control cells. We also performed the experiment with an siRNA targeting the open reading frame (ORF) in cells that were not treated with cycloheximide and obtained similar results (Figure S4).
Effects on receptor internalization are predicted to affect signaling and attenuation of signaling through the EGFR. Proteins phosphorylated in response to EGF include JNK, Akt and ERK. As indicators of signaling through EGFR, levels of phosphoEGFR (p-EGFR), phosphoJNK (p-JNK), phosphoAkt (p-Akt) and phosphoERK (p-ERK) (65–67)were measured in nontargeting and ARAP1-targeted siRNA-treated cells (Figure 11). In both controls and cells with reduced ARAP1 expression, a 5 min treatment with EGF increased the levels of p-EGFR, p-Akt, p-ERK and p-JNK. In control cells, p-ERK and p-JNK persisted for 2 h, whereas p-Akt was transient coming to prestimulation levels within 20 min. We did not detect an effect of reducing ARAP1 expression on the kinetics of EGFR or Akt phosphorylation and dephosphorylation. However, p-ERK and p-JNK levels were diminished and more transient in the cells with reduced ARAP1 expression than in control cells.
Figure 11.
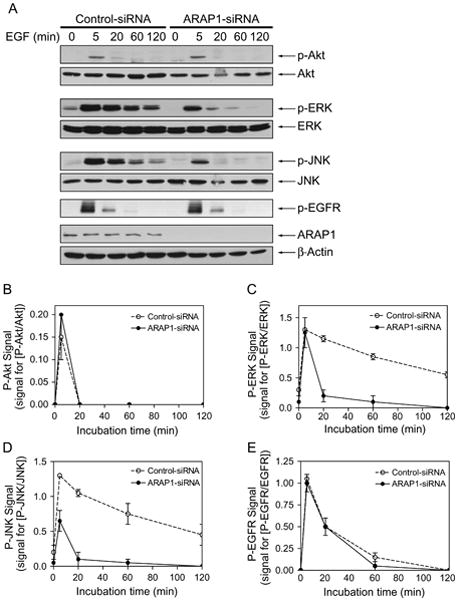
Effect of reduced ARAP1 expression on signaling through the EGF receptor. A) Immunoblots of p-Akt, p-ERK, p-JNK and p-EGFR. HeLa cells transfected with either nontargeting or ARAP1-siRNA were serum-starved and then treated with EGF (25 ng/mL) for the indicated times at 37°C. Whole cells lysates were probed with antibodies against p-Akt, Akt, p-ERK, ERK, p-JNK, JNK, p-EGFR (Y1173), ARAP1 and β-actin. The results are representative of two independent experiments. B–E) Quantification of immunoblots. Immunoblots from two experiments were digitally scanned, and signals were quantified using ImageJ program. Signals for the phosphorylated proteins were normalized to signals for the unphosphorylated proteins. The error bars are the range.
Discussion
EGF binds EGFR tightly and dissociates slowly. Regulation of the signal from the receptor depends on endocytosis. Several endocytic pathways that internalize EGFR have been described, and recently, Arf GTP-binding proteins have been found to be involved in at least one pathway. We have identified an Arf GAP called ARAP1 as a regulator of EGFR endocytosis. ARAP1 associated with an endosome compartment that temporally precedes the EEA1-positive early endosome. EGF and EGFR passed through the ARAP1-positive compartment prior to associating with EEA1-positive endosomes. Reduction of ARAP1 expression resulted in accelerated internalization and degradation of EGFR and reduced signaling through ERK and JNK. We propose a model in which ARAP1 is a feed forward regulator of EGFR signaling.
The substrates for Arf GAPs have been implicated in endocytosis of receptors in the PMs. Arf6 has been examined as a regulator of clathrin-independent and caveolin-independent endocytosis of transmembrane proteins in the PM, although EGFR has not been reported to be affected by Arf6. Arf1 has been implicated in endocytosis of EGFR (36). For both Arf1 and Arf6, the GTP-bound forms of the proteins accelerate endocytosis. Arf GAPs induce the hydrolysis of GTP on Arf. Therefore, reducing ARAP1 levels would reduce the conversion of the pool of Arf GTP to Arf GDP and, consequently, would be predicted to accelerate endocytosis of EGFR. We observed increased rates of both EGF uptake and EGFR internalization, consistent with the prediction. The decreased level of EGFR in cells with reduced levels of ARAP1 is also consistent with higher rates of constitutive EGFR degradation.
A role for ARAP1 in the regulation of EGFR internalization implies that a class 1 or class 2 Arf is activated consequent to EGFR activation. Although class 1 and 2 Arfs have not previously been found to be activated by extracellular signals, Arf1 has been found to be involved in EGFR endocytosis. Also, Arf6 has been found to be activated by extracellular signals. For instance, when neurotrophin 3 (NT3) binds to neurotrophin tyrosine kinase receptor C (TrkC), tamalin binds to the cytoplasmic tail of the receptor and recruits the exchange factor Arf nucleotide-binding site opener (ARNO) (68)resulting in the activation of Arf6. ARNO and Arf6 have been reported to function downstream of the insulin receptor (69,70). Gαq has also been found to bind to ARNO and activate Arf6 (71). ARNOs, exchange factors that function at the PM, have been found to be promiscuous in both in vivo and in vitro experiments using both Arf1 and Arf6 (72–80). An ARNO family protein may be regulated by EGFR, resulting in activation of a class 1 or class 2 Arf. ARAP1, recruited either by binding PIP3 or a Ras family protein or by being phosphorylated on tyrosine, could function as a negative regulator, limiting Arf GTP-driven endocytosis of EGFR.
Understanding the role of ARAP1 in modulating EGF signaling will require a description of the relationship of ARAP1 to other known regulatory mechanisms. Three major regulatory pathways of EGFR endocytosis and degradation have been identified. Rab5 is critical for both internalization and targeting to EEA1-positive endosomes (81). We found that formation of the ARAP1-containing endosomal compartment was blocked by dominant-negative Rab5, but ARAP1 was excluded from the puncta formed by expressing activated Rab5 in cells that were not treated with EGF. These results support the notion that there are at least two distinct Rab5 compartments. ARAP1 associates with an element specific to the Rab5 compartment that is an intermediate between the PM and the EEA1-positive compartment. We speculate that Arf functions upstream of or in parallel to Rab5 to drive the formation of the transport intermediate. ARAP1 would be incorporated into the forming vesicle, modulating the activity of Arf to regulate the rate of release of the vesicles from the PM. Once the transport intermediate is formed, ARAP1 may modulate the Rab5-driven fusion step by regulating RhoA, another regulator of endocytosis (31,30). The lifetime of the intermediate may affect sorting of the receptor or signaling from the endosome.
Cbl is another important regulator of EGFR internalization and degradation. Cbl binds to and ubiquitinates the receptor (11,15,16,82). It interacts with additional proteins that regulate internalization (83). One protein, CIN85, is an adaptor protein. ARAP3 binds to CIN85 (83), which may contribute to targeting ARAP3 to the PM after receptor activation.
ARAP1 functions downstream of EGFR in the model we are proposing. There are three signals from the EGFR that may impinge on ARAP1. One is through the PI 3-kinase pathway. ARAP1 has two PH domains that specifically bind PIP3. Thus, activation of PI 3-kinase by EGF could generate PIP3, which recruits ARAP1 to the PM or endosome. Second, EGFR activates Ras. ARAP1 has a Ras association domain. Ras could recruit or regulate ARAP1, which is similar to the mechanism described for activation of the Rab exchange factor Rin1 (29). Third, ARAP1 is phosphorylated by Src, also part of the EGFR pathway. The phosphorylation could directly affect ARAP1 catalytic activities or create binding sites for other regulatory proteins. Additional mechanisms may be discovered by identifying ARAP1-binding proteins.
Other Arf GAPs may have a role in EGFR trafficking. ASAP1 has been implicated in recycling (83,84), but a molecular mechanism has not been defined. We found that overexpressing several Arf GAPs can affect EGF internalization, but the effects have been distinct from those of ARAP1 (unpublished data). Furthermore, we have not observed an effect of reducing expression of other Arf GAPs on EGF internalization and, therefore, cannot exclude that possibility that the effects of these Arf GAPs are because of high expression levels, leading to mistargeting and/or unregulated GAP activity.
In summary, we propose that ARAP1 regulates EGFR by affecting a pre-early endosome. Given the domain structure of ARAP1, ARAP1 could function at more than one site in the endocytic pathway, be controlled by signals initiated by receptor occupancy and be tuned by several converging signals.
Materials and Methods
Monoclonal antibodies against γ-adaptin, GM130, EEA1, rabaptin5 and Rab5 were purchased from BD Biosciences. Mouse α-adaptin was from ABR. Mouse anti-M6PR was from Research Diagnostics, rabbit anti-APPL was from Abcam and mouse anti-EGFR Ab11 (clone 199.12) was obtained from LabVision. Mouse anti-Lamp 1 (H4A3) was from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development (NICHD) and maintained by the Department of Biological Sciences, University of Iowa, Iowa City. Polyclonal antibodies against p-Akt, phospho-p42/44 mitogen-activated protein kinase (MAPK), p-JNK, EGFR, Akt and JNK were purchased from Cell Signaling, and anti-p-EGFR(Y1173) and anti-MAPK 1/2 were from Upstate. Rabbit anti-β-actin was from Rockland, and monoclonal anti-Flag (clone M5) and polyclonal anti-Flag were from Sigma. Antisera to ARAP1 have been previously described (44). Alexa 633-conjugated goat anti-mouse or goat anti-rabbit secondary antibodies were from Invitrogen. Fluorescence-or Texas Red-conjugated secondary antibodies were purchased from Jackson Immunoresearch. Horse-peroxidase-conjugated anti-rabbit immunoglobulin G was from Bio-Rad Laboratories.
Plasmids
We cloned the complementary DNA (cDNA) for a splice variant of human ARAP1. The encoded protein has a SAM domain at the N-terminus and is otherwise identical to the previously described ARAP1 protein (44). To obtain the amino terminal sequence of ARAP1-containing SAM domain, we utilized the 5′-rapid amplification of cDNA ends technique with human skeletal muscle poly A+ RNA (Clontech) as a template. According to the manufacturer’s instructions, we used an antisense, gene-specific primer that corresponded to part of the ARAP1 5′-UTR of the message (5′-CTCAAAACAGCTCCCGCCTCAGGC-3′) and a sense primer (5′-AAGCTCAGGCTGCTGGTATG-3′). For polymerase chain reaction (PCR) amplification, the Advantage Polymerase GC2 PCR kit (Clontech) was used under the following cycling conditions: 95°C for 30 seconds, followed by 10 cycles, each consisting of 95°C for 30 seconds, 53°C for 1 min and 68°C for 2 min and subsequently 25 cycles with 95°C for 30 seconds, 55°C for 1 min and 68°C for 2 min. The reaction was completed at an elongation by 72°C for 5 min and analyzed on a 1% agarose gel. Fragments corresponding to positive bands were subcloned into pCR4-Topo vector (Invitrogen) and sequenced. The coding region for ARAP1 was amplified by PCRs and subcloned into the XhoI and NotI sites of pCI (Promega) with a Flag tag (DYKDDDDK) for mammalian expression. We have submitted the human ARAP1 full-length sequence to the GenBank (accession number AY553630) with the name ARAP1b. We refer to the protein as ARAP1 in this paper. GFP-tagged Rab4, 5, 7 and 11 were generous gifts from Juan Bonifacino [National Institute of Child Health and Human Development, National Institutes of Health (NIH), Bethesda, MD, USA]. GFP–[S34N]Rab5 and GFP–[Q79L]Rab5 were generous gift from Julie Donaldson (National Heart, Lung and Blood Institute, NIH).
Cell culture and transfection
HeLa cells were cultured in DMEM with 10%FBS, 100 U/mL penicillin and 100 μg/mL streptomycin (Invitrogen) at 37°C with 5% CO2. Cell transfection was performed using FuGENE6 (Roche) reagent following the manufacturer’s instructions for plasmid DNA. For RNA interference (RNAi) assays, 19-nucleotide siRNA duplexes with 3′-UU overhangs specific for the 3′-UTR (5′-GUUCAGACCUCUUGGCCCAUU-3′) and the ORF (ON-TARGETplus SMARTpool) of Human ARAP1 Genebank accession number (NM_139181) were purchased from Dharmacon. siCONTROL non-targeting siRNA no. 2 (Dharmacon) was used as a negative control. The siRNA targeting the 3′-UTR was used for all experiments shown except where indicated. RNAi transfection has been described previously (45).
Confocal microscopy
HeLa cells were plated onto fibronectin (15 μg/mL)-coated coverslips for 6 h in OPTi-MEM media and were treated with EGF at the indicated concentration for the indicated periods of time. The cells were fixed in 2% formaldehyde in PBS for 10 min at room temperature. For uptake assays, HeLa cells were plated on glass coverslips in DMEM media containing 10% FBS for overnight and were serum-starved for 1 h at 37°C in prewarmed uptake media DMEM containing 20 mm HEPES, pH 7.4, and 1% BSA. The cells were incubated with Alexa Fluor 647–EGF complex biotinylated EGF complexed to AlexaFluor 647-conjugated streptavidin obtained from Molecular Probes and referred to as Alexa Fluor 647–EGF in text (2 μg/mL) or Alexa Fluor 548–transferrin (Molecular probes, 50 μg/mL) on ice for 30 min, washed once in ice-cold PBS and then placed in prewarmed uptake media for indicated time at 37°C. After incubation, cells were placed on ice and incubated in acidic PBS (pH 4) for 6 min on ice to remove fluorescent EGF and transferrin. The cells were washed two times with ice-cold PBS, fixed and processed for immunofluorescence. Images were captured with a confocal microscope Zeiss 510 LSCM (Carl Zeiss Inc.) equipped with a differential interference contrast (DIC) 63× Plan-Apochromat objective (NA 1.4) (Carl Zeiss Inc.). The acquired images were processed with Adobe Photoshop.
Receptor internalization assay
HeLa cells grown on 100-mm plates were transfected with RNAi using Oligofectamine. After 36 h, the cells were serum-starved for overnight and treated with 25 ng/mL of EGF at 37°C with or without 10 μg/mL cycloheximide (Sigma). After the indicated incubation, cells were placed on ice, washed three times quickly with ice-cold PBS (pH 8.0) to remove amine-containing culture media and biotinylated with 0.1 mg/mL of Sulfo-NHS-LC-Biotin (Pierce) for 30 min on ice. Unreacted biotin was quenched and removed by washing two times with ice-cold PBS (pH 8.0) containing 0.1 m glycine. The cells were washed two more times with ice-cold PBS. The cells were then lysed in 50 mm Tris–HCl, pH 7.4, 150 mm NaCl, 0.25% deoxycholate, 1% Nonidet P-40, 1 mm ethylenediaminetetraacetic acid (EDTA), 1 mm sodium orthovanadate and one mini complete EDTA-free protease inhibitor (Roche) (one tablet per 10 mL buffer). The lysates were centrifuged at 10000×g at 4°C for 10 min. The cleared lysates were incubated with streptavidin-conjugated beads (Pierce) for 1 h at 4°C, and precipitated proteins were analyzed by western blot with specific antibodies and detected by SuperSignal® West Dura Extended Duration Substrate (Pierce). In assays for p-Akt, p-JNK and p-ERK and p-EGFR, whole cell lysates were directly analyzed by immunoblotting without immunoprecipitation.
Flow cytometry
EGFR and transferrin internalization were determined using flow cytometry with an assay that was modified from (85). For EGFR internalization, cells at 90% confluence were serum-starved for 1 h in serum-free uptake media (DMEM containing 20 mm HEPES, pH 7.4, and 1% BSA). The cells were then incubated with 1 μg/mL of Alexa Fluor 647-labelled EGF on ice water for 30 min. After washing with ice-cold PBS, cells were incubated at 37°C for the indicated times to allow internalization. The cells were placed on ice to stop internalization and then subjected to an acidic wash (0.2 m acetic acid and 0.5 m NaCl, pH 3.5) for 2 min to remove non-internalized fluorescent-conjugated EGF. Cells were collected and suspended in FACS resuspension buffer (PBS containing 0.1% BSA and 0.1% sodium azide) before fixation by adding an equal volume of 4% formaldehyde. The fluorescence emission of internalized EGF was detected by flow cytometry, and the percentage of EGF that was internalized at each time-point was calculated by subtracting background (fluorescence of cells subjected to acid wash without allowing internalization) and then divided by the starting fluorescence intensity. For transferrin internalization, cells were serum-starved for 2 h and then cells were incubated with 50 μg/mL Alexa Fluor 546-labelled transferrin on ice water for 1 h. The cells were then processed in the same way that cells were processed to determine EGF internalization. Each experiment was done in duplicate.
Quantitation of fluorescence intensity at the cell edge
IPLab Ver3.9 software (Scanalytics, Inc.) was used for the quantification of fluorescence intensity at the cell edge. Image files were imported in tagged-information file (TIFF) format. The color channels were separated and converted to gray scale images. The edge of the cell was selected as the region of interest using the brush tool. Twenty-five images for each condition were analyzed.
Supplementary Material
Figure S1. Characterization of antibody for ARAP1. HeLa cells transfected with or without plasmids encoding Flag-ARAP1 and total cell lysates were analyzed by immunoblotting using the rabbit sera raised against ARAP1, a monoclonal antibody (M5) for the Flag epitope or a polyclonal antibody for the Flag epitope. The arrows indicate ARAP1. Lane 1: HeLa and Lane 2: HeLa cells transfected with Flag-ARAP1.
Figure S2. Effect of EGF on the distribution of recombinant ARAP1 and GFP–Rab5. Serum-starved HeLa cells expressing Flag-ARAP1 and GFP–Rab5 were treated with 100 ng/mL EGF for 5 min where indicated. The cells were fixed and prepared for immunofluorescence staining to visualize the indicated proteins. Scale bar, 10 μm.
Figure S3. ARAP1 and Rab family members. HeLa cells transfected with plasmids encoding Flag-ARAP1 and GFP–Rab4, 5, 7 or 11 were treated with EGF for 5 min at 37°C where indicated. Fixed cells were immunostained against the Flag epitope. A higher magnification is shown in the inset. Scale bar, 10 μm.
Figure S4. Effect of siRNA targeting the ORF of ARAP1 on EGFR degradation. HeLa cells treated with siRNA targeting the ORF of ARAP1 were serum-starved and treated for the indicated period of time with 25 ng/mL EGF. EGFR in the cell lysates was determined by immunoblotting.
Acknowledgments
We thank Drs Stanley Lipkowitz, Carole Parent and Lawrence Samelson and the members of the Randazzo laboratory group for insightful discussions. This study was supported by the Intramural Research Program of the National Cancer Institute, Department of Health and Human Services.
References
- 1.Barbieri MA, Ramkumar TP, Fernadez-Pol S, Chen PI, Stahl PD. Receptor tyrosine kinase signaling and trafficking. Curr Top Microbiol Immunol. 2004;286:1–20. [PubMed] [Google Scholar]
- 2.Riese DJ, Gallo RM, Settleman J. Mutational activation of ErbB family receptor tyrosine kinases: insights into mechanisms of signal transduction and tumorigenesis. Bioessays. 2007;29:558–565. doi: 10.1002/bies.20582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roskoski R. The ErbB/HER receptor protein-tyrosine kinases and cancer. Biochem Biophys Res Comm. 2004;319:1–11. doi: 10.1016/j.bbrc.2004.04.150. [DOI] [PubMed] [Google Scholar]
- 4.Shawver LK, Slamon D, Ullrich A. Smart drugs: tyrosine kinase inhibitors in cancer therapy. Cancer Cell. 2002;1:117–123. doi: 10.1016/s1535-6108(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 5.Marmor MD, Yarden Y. Role of protein ubiquitylation in regulating endocytosis of receptor tyrosine kinases. Oncogene. 2004;23:2057–2070. doi: 10.1038/sj.onc.1207390. [DOI] [PubMed] [Google Scholar]
- 6.Sebastian S, Settleman J, Reshkin SJ, Azzariti A, Bellizzi A, Paradiso A. The complexity of targeting EGFR signalling in cancer: from expression to turnover. Biochim Biophys Acta. 2006;1766:120–139. doi: 10.1016/j.bbcan.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter G. The EGF receptor: a nexus for trafficking and signaling. Bioessays. 2000;22:697–707. doi: 10.1002/1521-1878(200008)22:8<697::AID-BIES3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 8.Lichtner RB. Estrogen/EGF receptor interactions in breast cancer: rationale for new therapeutic combination strategies. Biomed Pharmacother. 2003;57:447–451. doi: 10.1016/j.biopha.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550–6565. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- 10.Huang FT, Khvorova A, Marshall W, Sorkin A. Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference. J Biol Chem. 2004;279:16657–16661. doi: 10.1074/jbc.C400046200. [DOI] [PubMed] [Google Scholar]
- 11.Jiang XJ, Sorkin A. Epidermal growth factor receptor internalization through clathrin-coated pits requires Cbl RING finger and proline-rich domains but not receptor polyubiquitylation. Traffic. 2003;4:529–543. doi: 10.1034/j.1600-0854.2003.t01-1-00109.x. [DOI] [PubMed] [Google Scholar]
- 12.Sorkin A, Mckinsey T, Shih W, Kirchhausen T, Carpenter G. Stoichiometric interaction of the epidermal growth-factor receptor with the clathrin-associated protein complex Ap-2. J Biol Chem. 1995;270:619–625. doi: 10.1074/jbc.270.2.619. [DOI] [PubMed] [Google Scholar]
- 13.Sorkina T, Bild A, Tebar F, Sorkin A. Clathrin, adaptors and eps15 in endosomes containing activated epidermal growth factor receptors. J Cell Sci. 1999;112:317–327. doi: 10.1242/jcs.112.3.317. [DOI] [PubMed] [Google Scholar]
- 14.Nesterov A, Carter RE, Sorkina T, Gill GN, Sorkin A. Inhibition of the receptor-binding function of clathrin adaptor protein AP-2 by dominant-negative mutant mu 2 subunit and its effects on endocytosis. EMBO J. 1999;18:2489–2499. doi: 10.1093/emboj/18.9.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grovdal LM, Stang E, Sorkin A, Madshus IH. Direct interaction of Cbl with pTyr 1045 of the EGF receptor (EGFR) is required to sort the EGFR to lysosomes for degradation. Exp Cell Res. 2004;300:388–395. doi: 10.1016/j.yexcr.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Huang FT, Sorkin A. Growth factor receptor binding protein 2-mediated recruitment of the RING domain of Cbl to the epidermal growth factor receptor is essential and sufficient to support receptor endocytosis. Mol Bio Cell. 2005;16:1268–1281. doi: 10.1091/mbc.E04-09-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang FT, Jiang XJ, Sorkin A. Tyrosine phosphorylation of the beta 2 subunit of clathrin adaptor complex AP-2 reveals the role of a di-leucine motif in the epidermal growth factor receptor trafficking. J Biol Chem. 2003;278:43411–43417. doi: 10.1074/jbc.M306072200. [DOI] [PubMed] [Google Scholar]
- 18.Jiang XJ, Huang FT, Marusyk A, Sorkin A. Grb2 regulates internalization of EGF receptors through clathrin-coated pits. Mol Bio Cell. 2003;14:858–870. doi: 10.1091/mbc.E02-08-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polo S, Pece S, Di Fiore PP. Endocytosis and cancer. Curr Opin Cell Biol. 2004;16:156–161. doi: 10.1016/j.ceb.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Buccione R, Orth JD, McNiven MA. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nature Rev Mol Cell Biol. 2004;5:647–657. doi: 10.1038/nrm1436. [DOI] [PubMed] [Google Scholar]
- 21.McNiven MA. Big gulps: specialized membrane domains for rapid receptor-mediated endocytosis. Trends Cell Biol. 2006;16:487–492. doi: 10.1016/j.tcb.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Orth JD, Krueger EW, Weller SG, McNiven MA. A novel endocytic mechanism of epidermal growth factor receptor sequestration and internalization. Cancer Res. 2006;66:3603–3610. doi: 10.1158/0008-5472.CAN-05-2916. [DOI] [PubMed] [Google Scholar]
- 23.Cavalli V, Corti M, Gruenberg J. Endocytosis and signaling cascades: a close encounter. FEBS Lett. 2001;498:190–196. doi: 10.1016/s0014-5793(01)02484-x. [DOI] [PubMed] [Google Scholar]
- 24.Miaczynska M, Pelkmans L, Zerial M. Not just a sink: endosomes in control of signal transduction. Curr Opin Cell Biol. 2004;16:400–406. doi: 10.1016/j.ceb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Schnatwinkel C, Christoforidis S, Lindsay MR, Uttenweiler-Joseph S, Wilm M, Parton RG, Zerial M. The Rab5 effector rabankyrin-5 regulates and coordinates different endocytic mechanisms. Plos Biol. 2004;2:1363–1380. doi: 10.1371/journal.pbio.0020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lanzetti L, Palamidessi A, Areces L, Scita G, Di Fiore PP. Rab5 is a signalling GTPase involved in actin remodelling by receptor tyrosine kinases. Nature. 2004;429:309–314. doi: 10.1038/nature02542. [DOI] [PubMed] [Google Scholar]
- 27.De Renzis S, Sonnichsen B, Zerial M. Divalent Rab effectors regulate the sub-compartmental organization and sorting of early endosomes. Nature Cell Biol. 2002;4:124–133. doi: 10.1038/ncb744. [DOI] [PubMed] [Google Scholar]
- 28.Zerial M, McBride H. Rab proteins as membrane organizers. Nature Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 29.Tall GG, Barbieri MA, Stahl PD, Horazdovsky BF. Ras-activated endocytosis is mediated by the Rab5 guanine nucleotide exchange activity of RIN1. Dev Cell. 2001;1:73–82. doi: 10.1016/s1534-5807(01)00008-9. [DOI] [PubMed] [Google Scholar]
- 30.Lamaze C, Chuang TH, Terlecky LJ, Bokoch GM, Schmid SL. Regulation of receptor-mediated endocytosis by Rho and Rac. Nature. 1996;382:177–179. doi: 10.1038/382177a0. [DOI] [PubMed] [Google Scholar]
- 31.Kaneko T, Maeda A, Takefuji M, Aoyama H, Nakayama M, Kawabata S, Kawano Y, Iwamatsu A, Amano M, Kaibuchi K. Rho mediates endocytosis of epidermal growth factor receptor through phosphorylation of endophilin A1 by Rho-kinase. Genes Cells. 2005;10:973–987. doi: 10.1111/j.1365-2443.2005.00895.x. [DOI] [PubMed] [Google Scholar]
- 32.Lin Q, Lo CG, Cerione RA, Yang W. The Cdc42 target ACK2 interacts with sorting nexin 9 (SH3PX1) to regulate epidermal growth factor receptor degradation. J Biol Chem. 2002;277:10134–10138. doi: 10.1074/jbc.M110329200. [DOI] [PubMed] [Google Scholar]
- 33.Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nature Rev Mol Cell Biol. 2006;7:347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- 34.Nie ZZ, Hirsch DS, Randazzo PA. Arf and its many interactors. Curr Opin Cell Biol. 2003;15:396–404. doi: 10.1016/s0955-0674(03)00071-1. [DOI] [PubMed] [Google Scholar]
- 35.Moss J, Vaughan M. Molecules in the ARF orbit. J Biol Chem. 1998;273:21431–21434. doi: 10.1074/jbc.273.34.21431. [DOI] [PubMed] [Google Scholar]
- 36.Kumari S, Mayor S. ARF1 is directly involved in dynamin-independent endocytosis. Nature Cell Biol. 2008;10:30–41. doi: 10.1038/ncb1666. [DOI] [PubMed] [Google Scholar]
- 37.Donaldson JG, Jackson CL. Regulators and effectors of the ARF GTPases. Curr Opin Cell Biol. 2000;12:475–482. doi: 10.1016/s0955-0674(00)00119-8. [DOI] [PubMed] [Google Scholar]
- 38.Randazzo PA, Hirsch DS. Arf GAPs: multifunctional proteins that regulate membrane traffic and actin remodelling. Cell Signal. 2004;16:401–413. doi: 10.1016/j.cellsig.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Gillingham AK, Munro S. The small G proteins of the Arf family and their regulators. Ann Rev Cell Dev Biol. 2007;23:579–611. doi: 10.1146/annurev.cellbio.23.090506.123209. [DOI] [PubMed] [Google Scholar]
- 40.Inoue H, Randazzo PA. Arf GAPs and their interacting proteins. Traffic. 2007;8:1465–1475. doi: 10.1111/j.1600-0854.2007.00624.x. [DOI] [PubMed] [Google Scholar]
- 41.Nie ZZ, Randazzo PA. Arf GAPs and membrane traffic. J Cell Sci. 2006;119:1203–1211. doi: 10.1242/jcs.02924. [DOI] [PubMed] [Google Scholar]
- 42.Kahn RA, Buford E, Inoue H, Logsdon JM, Nie Z, Premont RT, Randazzo PA, Satke M, Theibert AB, Zapp ML, Cassel D. Consensus nomenclature for the human Arf GAP domain-containing proteins. J Cell Biol. 2008;182:1039–1044. doi: 10.1083/jcb.200806041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Randazzo PA, Inoue H, Bharti S. Arf GAPs as regulators of the actin cytoskeleton. Biol Cell. 2007;99:583–600. doi: 10.1042/bc20070034. [DOI] [PubMed] [Google Scholar]
- 44.Miura K, Jacques KM, Stauffer S, Kubosaki A, Zhu KJ, Hirsch DS, Resau J, Zheng Y, Randazzo PA. ARAP1: a point of convergence for Arf and Rho signaling. Mol Cell. 2002;9:109–119. doi: 10.1016/s1097-2765(02)00428-8. [DOI] [PubMed] [Google Scholar]
- 45.Yoon H-Y, Miura K, Cuthbert EJ, Davis KK, Ahvazi B, Casanova JE, Randazzo PA. ARAP2 effects on the actin cytoskeleton are dependent on Arf6-specific GTPase-activating protein activity and binding to RhoA. J Cell Sci. 2006;119:4650–4666. doi: 10.1242/jcs.03237. [DOI] [PubMed] [Google Scholar]
- 46.Stacey TTI, Nie ZZ, Stewart A, Najdovska M, Hall NE, He H, Randazzo PA, Lock P. ARAP3 is transiently tyrosine phosphorylated in cells attaching to fibronectin and inhibits cell spreading in a RhoGAP-dependent manner. J Cell Sci. 2004;117:6071–6084. doi: 10.1242/jcs.01526. [DOI] [PubMed] [Google Scholar]
- 47.Krugmann S, Anderson KE, Ridley SH, Risso N, McGregor A, Coadwell J, Davidson K, Eguinoa A, Ellson CD, Lipp P, Manifava M, Ktistakis N, Painter G, Thuring JW, Cooper MA, et al. Identification of ARAP3, a novel PI3K effector regulating both Arf and Rho GTPases, by selective capture on phosphoinositide affinity matrices. Mol Cell. 2002;9:95–108. doi: 10.1016/s1097-2765(02)00434-3. [DOI] [PubMed] [Google Scholar]
- 48.Krugmann S, Andrews S, Stephens L, Hawkins PT. ARAP3 is essential for formation of lamellipodia after growth factor stimulation. J Cell Sci. 2006;119:425–432. doi: 10.1242/jcs.02755. [DOI] [PubMed] [Google Scholar]
- 49.Krugmann S, Williams R, Stephens L, Hawkins PT. ARAP3 is a P13K-and rap-regulated GAP for RhoA. Curr Biol. 2004;14:1380–1384. doi: 10.1016/j.cub.2004.07.058. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura N, Rabouille C, Watson R, Nilsson T, Hui N, Slusarewicz P, Kreis TE, Warren G. Characterization of a cis-Golgi matrix protein, GM130. J Cell Biol. 1995;131:1715–1726. doi: 10.1083/jcb.131.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakamura N, Lowe M, Levine TP, Rabouille C, Warren G. The vesicle docking protein p115 binds GM130, a cis-Golgi matrix protein, in a mitotically regulated manner. Cell. 1997;89:445–455. doi: 10.1016/s0092-8674(00)80225-1. [DOI] [PubMed] [Google Scholar]
- 52.Robinson MS, Bonifacino JS. Adaptor-related proteins. Curr Opin Cell Biol. 2001;13:444–453. doi: 10.1016/s0955-0674(00)00235-0. [DOI] [PubMed] [Google Scholar]
- 53.Robinson MS. Cloning and expression of gamma-adaptin, a component of clathrin-coated vesicles associated with the Golgi-apparatus. J Cell Biol. 1990;111:2319–2326. doi: 10.1083/jcb.111.6.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mu FT, Callaghan JM, Steelemortimer O, Stenmark H, Parton RG, Campbell PL, Mccluskey J, Yeo JP, Tock EPC, Toh BH. EEA1, an early endosome-associated protein – EEA1 is a conserved alpha-helical peripheral membrane-protein flanked by cysteine fingers and contains a calmodulin-binding IQ motif. J Biol Chem. 1995;270:13503–13511. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- 55.Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nature Rev Mol Cell Biol. 2007;8:622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- 56.Ni X, Canuel M, Morales CR. The sorting and trafficking of lysosomal proteins. Histol Histopath. 2006;21:899–913. doi: 10.14670/HH-21.899. [DOI] [PubMed] [Google Scholar]
- 57.Dintzis SM, Pfeffer SR. The mannose 6-phosphate receptor cytoplasmic domain is not sufficient to alter the cellular-distribution of a chimeric Egf receptor. EMBO J. 1990;9:77–84. doi: 10.1002/j.1460-2075.1990.tb08082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stenmark H, Vitale G, Ullrich O, Zerial M. Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell. 1995;83:423–432. doi: 10.1016/0092-8674(95)90120-5. [DOI] [PubMed] [Google Scholar]
- 59.Miaczynska M, Christoforidis S, Giner A, Shevchenko A, Uttenweiler-Joseph S, Habermann B, Wilm M, Parton RG, Zerial M. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell. 2004;116:445–456. doi: 10.1016/s0092-8674(04)00117-5. [DOI] [PubMed] [Google Scholar]
- 60.Li G, Liang Z. Phosphate-binding loop and Rab GTPase function: mutations at Ser(29) and Ala(30) of Rab5 lead to loss-of-function as well as gain-of-function phenotype. Biochem J. 2001;355:681–689. doi: 10.1042/bj3550681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li G, Barbieri MA, Colombo MI, Stahl PD. Structural features of the Gtp-binding defective Rab5 mutants required for their inhibitory activity on endocytosis. J Biol Chem. 1994;269:14631–14635. [PubMed] [Google Scholar]
- 62.Li G, Stahl PD. Structure-function relationship of the small GTPase Rab5. J Biol Chem. 1993;268:24475–24480. [PubMed] [Google Scholar]
- 63.Stenmark H, Parton RG, Steelemortimer O, Lutcke A, Gruenberg J, Zerial M. Inhibition of Rab5 Gtpase activity stimulates membrane-fusion in endocytosis. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoffenberg S, Sanford JC, Liu SB, Daniel DS, Tuvin M, Knoll BJ, Wesslingresnick M, Dickey BF. Biochemical and functional-characterization of a recombinant Gtpase, Rab5, and two of its mutants. J Biol Chem. 1995;270:5048–5056. doi: 10.1074/jbc.270.10.5048. [DOI] [PubMed] [Google Scholar]
- 65.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 66.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 67.Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN. The protein-kinase encoded by the Akt protooncogene is a target of the Pdgf-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 68.Esteban PF, Yoon HY, Becker J, Dorsey SG, Caprari P, Palko ME, Coppola V, Saragovi HU, Randazzo PA, Tessarollo L. A kinase-deficient TrkC receptor isoform activates Arf6-Rac1 signaling through the scaffold protein tamalin. J Cell Biol. 2006;173:291–299. doi: 10.1083/jcb.200512013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li HS, Shome K, Rojas R, Rizzo MA, Vasudevan C, Fluharty E, Santy LC, Casanova JE, Romero G. The guanine nucleotide exchange factor ARNO mediates the activation of ARF and phospholipase D by insulin. BMC Cell Biol. 2003;4:1–10. doi: 10.1186/1471-2121-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hafner M, Schmitz A, Grune I, Srivatsan SG, Paul B, Kolanus W, Quast T, Kremmer E, Bauer I, Famulok M. Inhibition of cytohesins by SecinH3 leads to hepatic insulin resistance. Nature. 2006;444:941–944. doi: 10.1038/nature05415. [DOI] [PubMed] [Google Scholar]
- 71.Giguere P, Rochdi MD, Laroche G, Dupre E, Whorton MR, Sunahara RK, Claing A, Dupuis G, Parent JL. ARF6 activation by G(alpha q) signaling: G(alpha q) forms molecular complexes with ARNO and ARF6. Cell Signal. 2006;18:1988–1994. doi: 10.1016/j.cellsig.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 72.Cohen LA, Honda A, Varnai P, Brown FD, Balla T, Donaldson JG. Active Arf6 recruits ARNO/cytohesin GEFs to the PM by binding their PH domain. Mol Biol Cell. 2007;18:2244–2253. doi: 10.1091/mbc.E06-11-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Franco M, Boretto J, Robineau S, Monier S, Goud B, Chardin P, Chavrier P. ARNO3, a Sec7-domain guanine nucleotide exchange factor for ADP ribosylation factor 1, is involved in the control of Golgi structure and function. Proc Natl Acad Sci U S A. 1998;95:9926–9931. doi: 10.1073/pnas.95.17.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frank S, Upender S, Hansen SH, Casanova JE. ARNO is a guanine nucleotide exchange factor for ADP-ribosylation factor 6. J Biol Chem. 1998;273:23–27. doi: 10.1074/jbc.273.1.23. [DOI] [PubMed] [Google Scholar]
- 75.Kremer W, Steiner G, Beraud-Dufour S, Kalbitzer HR. Conformational states of the small G protein Arf-1 in complex with the guanine nucleotide exchange factor ARNO-Sec7. J Biol Chem. 2004;279:17004–17012. doi: 10.1074/jbc.M312780200. [DOI] [PubMed] [Google Scholar]
- 76.Monier S, Chardin P, Robineau S, Goud B. Overexpression of the ARF1 exchange factor ARNO inhibits the early secretory pathway and causes the disassembly of the Golgi complex. J Cell Sci. 1998;111:3427–3436. doi: 10.1242/jcs.111.22.3427. [DOI] [PubMed] [Google Scholar]
- 77.Paris S, BeraudDufour S, Robineau S, Bigay J, Antonny B, Chabre M, Chardin P. Role of protein-phospholipid interactions in the activation of ARF1 by the guanine nucleotide exchange factor Arno. J Biol Chem. 1997;272:22221–22226. doi: 10.1074/jbc.272.35.22221. [DOI] [PubMed] [Google Scholar]
- 78.Santy LC, Casanova JE. Activation of ARF6 by ARNO stimulates epithelial cell migration through downstream activation of both Rac1 and phospholipase D. J Cell Biol. 2001;154:599–610. doi: 10.1083/jcb.200104019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Macia E, Chabre M, Franco M. Specificities for the small G proteins ARF1 and ARF6 of the guanine nucleotide exchange factors ARNO and EFA6. J Biol Chem. 2001;276:24925–24930. doi: 10.1074/jbc.M103284200. [DOI] [PubMed] [Google Scholar]
- 80.Casanova JE. Regulation of Arf activation: the Sec7 family of guanine nucleotide exchange factors. Traffic. 2007;8:1476–1485. doi: 10.1111/j.1600-0854.2007.00634.x. [DOI] [PubMed] [Google Scholar]
- 81.Ceresa BP. Regulation of EGFR endocytic trafficking by rab proteins. Histol Histopath. 2006;21:987–993. doi: 10.14670/HH-21.987. [DOI] [PubMed] [Google Scholar]
- 82.Levkowitz G, Waterman H, Ettenberg SA, Katz M, Tsygankov AY, Alroy I, Lavi S, Iwai K, Reiss Y, Ciechanover A, Lipkowitz S, Yarden Y. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol Cell. 1999;4:1029–1040. doi: 10.1016/s1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]
- 83.Kowanetz K, Husnjak K, Holler D, Kowanetz M, Soubeyran P, Hirsch D, Schmidt MHH, Pavelic K, De Camilli P, Randazzo PA, Dikic I. CIN85 associates with multiple effectors controlling intracellular trafficking of epidermal growth factor receptors. Mol Biol Cell. 2004;15:3155–3166. doi: 10.1091/mbc.E03-09-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nie Z, Hirsch DS, Luo R, Jian X, Stauffer S, Cremesti A, Andrade J, Lebowitz J, Marino M, Ahvazi B, Hinshaw JE, Randazzo PA. A BAR domain in the N terminus of the Arf GAP ASAP1 affects membrane structure and trafficking of epidermal growth factor receptor. Curr Biol. 2006;16:130–139. doi: 10.1016/j.cub.2005.11.069. [DOI] [PubMed] [Google Scholar]
- 85.Wang Q, Villeneuve G, Wang ZX. Control of epidermal growth factor receptor endocytosis by receptor dimerization, rather than receptor kinase activation. EMBO Rep. 2005;6:942–948. doi: 10.1038/sj.embor.7400491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Characterization of antibody for ARAP1. HeLa cells transfected with or without plasmids encoding Flag-ARAP1 and total cell lysates were analyzed by immunoblotting using the rabbit sera raised against ARAP1, a monoclonal antibody (M5) for the Flag epitope or a polyclonal antibody for the Flag epitope. The arrows indicate ARAP1. Lane 1: HeLa and Lane 2: HeLa cells transfected with Flag-ARAP1.
Figure S2. Effect of EGF on the distribution of recombinant ARAP1 and GFP–Rab5. Serum-starved HeLa cells expressing Flag-ARAP1 and GFP–Rab5 were treated with 100 ng/mL EGF for 5 min where indicated. The cells were fixed and prepared for immunofluorescence staining to visualize the indicated proteins. Scale bar, 10 μm.
Figure S3. ARAP1 and Rab family members. HeLa cells transfected with plasmids encoding Flag-ARAP1 and GFP–Rab4, 5, 7 or 11 were treated with EGF for 5 min at 37°C where indicated. Fixed cells were immunostained against the Flag epitope. A higher magnification is shown in the inset. Scale bar, 10 μm.
Figure S4. Effect of siRNA targeting the ORF of ARAP1 on EGFR degradation. HeLa cells treated with siRNA targeting the ORF of ARAP1 were serum-starved and treated for the indicated period of time with 25 ng/mL EGF. EGFR in the cell lysates was determined by immunoblotting.


