Abstract
Background
Assays for assessing human islet cell quality which provide results prior to transplantation would be very beneficial to improving outcomes for islet transplantation therapy. Parameters such as percent beta cell apoptosis and cell composition are found to vary markedly between different islet preparations, and may serve as markers of islet quality. We have developed fluorescence-based assays using laser scanning cytometry (LSC) for assessing beta cell apoptosis and islet cell composition on serial sections of intact isolated islets.
Methods
Isolated human islets were fixed in formalin and embedded in paraffin. Serial sections were immunostained for the pancreatic hormones, acinar and ductal cell markers. DNA fragmentation was used to label apoptotic cells. Stained cells were quantified using an iCys laser scanning cytometer.
Results
Islet preparations from 102 human pancreatic islet isolations were analyzed. For the whole set of islet preparations we found a mean islet cell composition of 54.5±1.2% insulin positive; 33.9±1.2% glucagon; 12.1±0.7% somatostatin and 1.5±0.2% pancreatic polypeptide positive cells. The apoptotic beta cells were 2.85±0.4% with a range of 0.27% to 18.3%. The percentage of apoptotic beta cells correlated well (p<0.0001, n=59) with results obtained in vivo by transplantation of the corresponding islets in diabetic NODscid mice.
Conclusions
The analysis of whole, non-dissociated islets for cell composition and beta cell apoptosis using LSC is giving reliable and reproducible results and could be done both before islet transplantation, as well as on preserved cell blocks at any future time. Thus, they can be a powerful tool for islet quality assessment.
Keywords: Laser scanning cytometry, human pancreatic islets, islet cell composition, β cell apoptosis
Introduction
Islet transplantation continues to show promise as a treatment for type 1 diabetes (1). Several studies have evidenced the effectiveness of islet transplantation in “difficult-to-control” diabetic patients in terms of achieving insulin independence, reducing blood glucose lability, and eliminating hypoglycemia (2, 3); however, long-term success at maintaining insulin independence remains elusive (1, 4).
Transplantation outcomes are greatly influenced by the quantity and quality of isolated islets used for the procedure. Interestingly, the total islet numbers sufficient to achieve insulin independence range widely, indicating that islet quality likely has a significant impact on transplant success (1). To determine the quality of isolated islets, a number of new methods have been introduced (5-13); however, only a few of these can provide results prior to transplantation (14, 15).
Because cell composition and beta cell apoptosis vary markedly between different islet preparations, they may serve as markers for islet quality. The current standard methods of evaluating these parameters utilizes dissociated, single islet cells (10, 16, 17); however, this technique has a significant disadvantage, since islet dissociation process may lead to cell loss and increased cell apoptosis. An alternative method utilizes immunocytochemical analysis of undissociated islets analyzed by confocal microscopy. This approach has provided reliable results for islet architecture and cell composition (18), as well as apoptosis (19). Neither of the above two methods, however, allows for quantitation of apoptotic beta cells, an important parameter which could determine the outcome of the transplant procedure.
In this study we have developed a new fluorescence-based assay for assessing islet cell composition and beta cell apoptosis using serial sections of paraffin embedded, undissociated whole islet preparations. Islet sections were stained for specific markers and analyzed by laser scanning cytometry (LSC) (20, 21), which has been successfully utilized to evaluate apoptosis in tissue sections (22, 23). With our assay, we are able to complete islet sectioning, staining and analysis in “real time” (less than 24 hours) and provide quantitative results prior to the start of transplantation procedure. Since the clinical case numbers are too small at present, the analyzed results were compared with in vivo islet function by transplanting islets in diabetic NODscid mice. The apoptotic beta cell number in the islet preparations inversely correlated with success in reversing diabetes in mice, indicating that the number of healthy beta cells is critical to achieving success in clinical transplantation, and highlighting the potential value of LSC analysis of islet preparations.
Results
Development and Evaluation of a Laser Scanning Cytometry Analysis of Isolated Human Islets
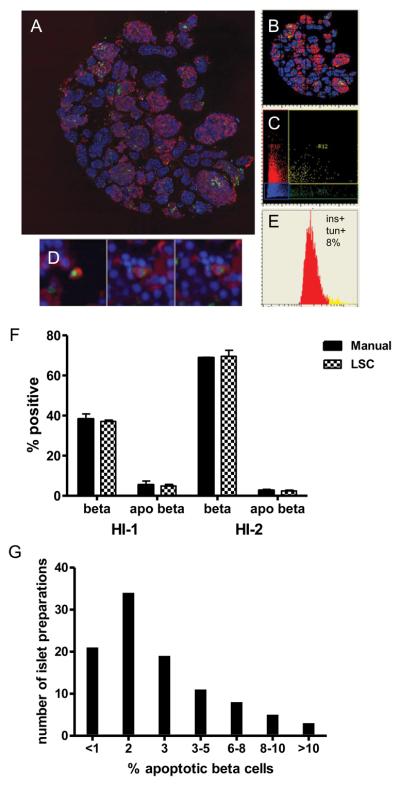
Human islet preparations of 1000 IEQ allowed the preparation of more than 50 serial sections, giving opportunity to quantitate beta cells, apoptotic beta cells, islet hormone-producing cells, and non-islet cells in duplicate. The remaining sections/blocks provided opportunities for additional future analyses. The stained preparations were scanned with an iCys laser scanning cytometer. For detecting apoptotic beta cells, slides were stained for insulin and TUNEL. TUNEL-positive nuclei were detected by green fluorescence using the 488-nm laser, while insulin cytoplasmic/peripheral red fluorescence was detected using the 633-nm laser. Figure 1A shows a merged mosaic image of a whole islet preparation stained for TUNEL and insulin. A corresponding XY scattergram is shown in Figure 1B. The TUNEL marker was gated based on green “Max pixel”, and insulin was gated on red “Peripheral max” parameters to construct scattergram (Figure 1C), where cells double-stained for both TUNEL and insulin are shown in yellow, indicating beta cells undergoing apoptosis. The staining of cells was confirmed using the iCys “Gallery” module which allows visualizing individual cells (Figure 1D). A percentage of apoptotic beta cells against the total beta cell number is shown by the histogram (Figure 1E) constructed from the scattergram (Figure 1C).
Figure 1. Evaluation of beta cell specific apoptosis.
Isolated human islets were fixed with formalin, embedded in paraffin, and processed for TUNEL staining to detect apoptotic nuclei followed by insulin staining to detect beta cells. The slides were scanned using the iCys LSC. (A) Merged image of a whole islet sample; (B) Corresponding X/Y axes scattergram; (C) Red (insulin) vs. green (TUNEL) scattergram; (D) “Gallery” images of cells with double-positive staining for TUNEL and insulin in the R12 area; (E) Histogram showing the distribution of insulin-positive cells into TUNEL-positive (yellow) and TUNEL-negative (red) areas; (F) Comparison of LSC analysis vs. manual count in two separate islet preparations (HI-1 and HI-2); (G) The distribution of apoptotic beta cell percentages in human islet preparations: the percentage of apoptotic beta cells in our islet preparations (n = 102) ranged from 0.27% to 18.3 %.
To compare results obtained by LSC with those obtained by conventional visual examination, images of samples analyzed by LSC were also captured by a conventional camera. TUNEL and insulin double-positive cells were counted visually, and the percentage of apoptotic beta cells calculated. Two human islet preparations that differed in beta cell and apoptotic beta cell numbers were selected in this study. Results were remarkably close: beta cell percentages obtained by visual vs. LSC (Figure 1F) were 38.28±2.55% vs. 37.08±1.94% in sample HI-1 and 68.22±0.26 vs. 69.58±2.96% in sample HI-2, respectively (p=0.997), and those of apoptotic beta cells were 4.88±0.63% vs. 5.44±0.51% in HI-1 and 2.71±0.50% vs. 2.41±0.23% in HI-2, respectively (p=0.837), demonstrating the reliability of the LSC data.
Results of the LSC apoptotic beta cell assessments for 102 islet preparations from the SC-ICRC are summarized in Figure 1G. The mean apoptotic beta cell percentage was 2.85±0.4%, with a range between 0.27% and 18.3%. Eighty-five out of 102 preparations contained less than 5% apoptotic beta cells.
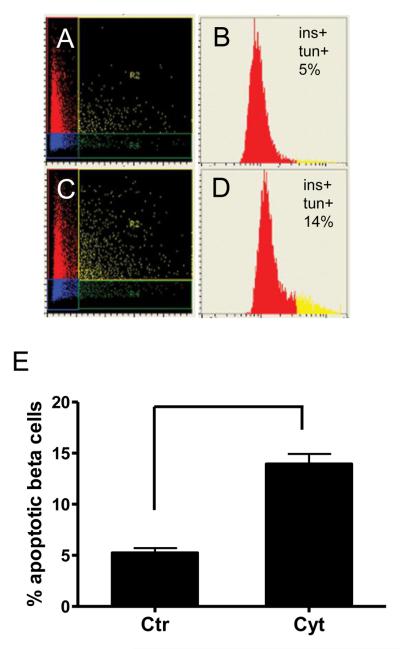
To test whether LSC analysis is capable of detecting cell damage of the same islet sample, apoptosis was induced by culturing human islets with a combination of inflammatory cytokines (TNFα, IFNγ, and IL1β) for 16 h (Figure 2A–2E). This treatment reproducibly increased beta cell apoptosis: the percentage of apoptotic beta cells increased to 13.22±0.49 % from 4.45±1.03 % in untreated islets (n=3, p<0.002; Figure 2E).
Figure 2. Detection of increased beta cell apoptosis by LSC following treatment with inflammatory cytokines.
Isolated islets were treated with cytokines for 16 h (C and D), fixed, embedded and processed as in Figure 1. Control islets (A and B) were cultured in medium alone. Scattergrams (A) and (C) with staining for insulin (red) and TUNEL (green). Histograms (B) and (D) of insulin-positive cells showing apoptotic beta cells in yellow. Treatment with inflammatory cytokines (E) results in a significant increase (p < 0.002) in the number of apoptotic beta cells, as assessed by LSC (n = 3).
Analysis of cell composition in isolated human islets by LSC
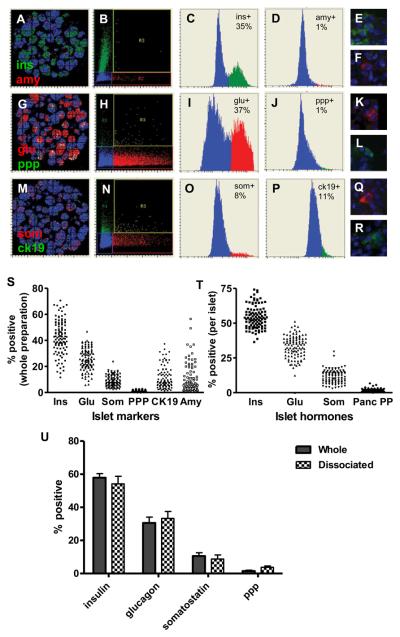
To evaluate cells contained in islet preparations, sets of serial histology sections of each islet preparation were stained for the following sets of markers: insulin and amylase, glucagon and pancreatic polypeptide, and somatostatin and cytokeratin 19. Fluorescein(FITC/Cy2)-conjugated secondary antibodies were used for insulin, pancreatic polypeptide, and cytokeratin 19, while conjugates with Cy5/Alexa647 was used for amylase, glucagon, and somatostatin. Slides were scanned as described (Figure 3). XY scattergrams of the corresponding scans are shown on the far left panels in Figure 3 (3A, 3G, and 3M). The markers were gated on red vs. green “Peripheral max” scattergrams in the second column (Fig.3B, 3H, and 3N), and the corresponding histograms for each channel are shown in the third and fourth columns (Fig.3C, 3D, 3I, 3J, 3O, and 3P). Individual positive cells were visualized using the “Gallery” module in the last column (Fig.3E, 3F, 3K, 3L, 3Q, and 3R).
Figure 3. Evaluation of cell compositions in islet cell preparations.
Parallel sections of human islet preparations were stained for insulin (green) and amylase (red) (A-F); glucagon (red) and pancreatic polypeptide (green) (G-L) and for somatostatin (red) and cytokeratin 19 (green) (M-R). Left to right on the figure: X/Y axes scattergrams (A, G and M); scattergrams of insulin vs. amylase (B); glucagon vs. pancreatic polypeptide (H), and somatostatin vs. cytokeratin 19 (N); histograms for insulin (C); amylase (D); glucagon (I); pancreatic polypeptide (J); somatostatin (O); and cytokeratin 19 (P); Corresponding “Gallery” images for insulin (E), amylase (F), glucagon (K), pancreatic polypeptide (L), somatostatin (Q), and cytokeratin 19 (R).
Figure 3(S and T) cell compositions in different islet preparations: distribution of cell compositions of 102 islet cell preparations are shown: (S) cell compositions examined in whole islet preparations: (T) islet hormone-positive cell compositions examined in islets only.
Figure 3(U): Islet preparations (n = 9) were processed by two methods and immunostained for LSC analysis: one used single cells prepared by dissociation of islets (30) and the other used undisrupted islet preparations. Results of both analyses were compared by calculating for “specific cell composition per islet.”
Cell composition in the whole islet preparations, or calculated “per islet” was analyzed in a total of 102 islet preparations and the percentages of each cell type are shown in Figures 3S (whole islet preparations) and 3T (islets alone). In islets, the percentage of insulin-positive cells ranged 36.4%-72.4% with a mean value of 54.5±1.2%; glucagon-positive cells: 12.4%-48.2 % with a mean of 33.9±1.2%; somatostatin-positive cells: 2.9%-29.5% with a mean of 12.1±0.7%; and pancreatic polypeptide-positive cells: 0.5-4.2% with a mean of 1.5±0.2%.
These results were compared with the data for the islet cell composition obtained using LSC analysis of dissociated islets (Figure 3U). In this set of experiments, islets were dissociated, fixed on slides, immunostained for islet hormones and evaluated by LSC. The comparison of data between dissociated and undissociated islets of the same preparations showed a very close correlation (p=0.9936) for all islet hormones, with the only exception being pancreatic polypeptide (3.75±0.79% in dissociated islets vs. 1.52±0.53% in undissociated islets). A possible explanation for this difference could be the low number of pancreatic polypeptide-positive cells in the islet preparations.
Validation of reproducibility and reliability of LSC analysis
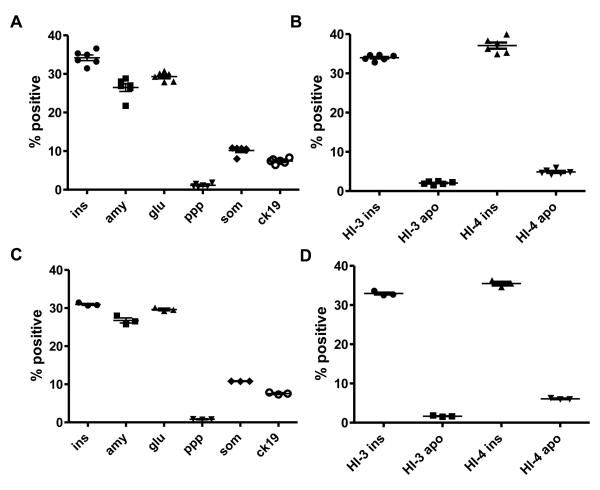
Reproducibility and reliability of LSC results were evaluated further using scanning and analysis of multiple sections of the same islet preparation, and analysis on the same section multiple times. Both tests were performed on islet sections stained for islet cell markers, as well as for insulin and TUNEL. Figure 4A/B shows LSC analysis results obtained from six randomly selected sections of one human islet sample (HI-3) stained for islet cell markers (4A), and from two islet samples (HI-3 and HI-4) stained for insulin and TUNEL (4B). There were no marked differences in results between slides, as indicated by the small coefficients of variation (CV<39% with ranges of 2.2% to 38.5%) of six readings for each staining , demonstrating that the LSC results reliably represent the islet cell composition and beta cell apoptosis in the islet preparation.
Figure 4. Reproducibility of the results obtained by LSC analysis.
A/B. Analysis of islet cell composition and beta cell apoptosis of multiple random sections of individual islet preparations. LSC analysis of islet cell composition (A) of one islet cell preparation (HI-3) and beta cell apoptosis (B) of two islet cell preparations (HI-3 and HI-4) was performed using 6 randomly selected sections prepared from each block.
C/D. Analysis of multiple scans of the same slides. Individual samples for islet cell composition (C) and beta cell apoptosis (D) were scanned and analyzed by LSC three consecutive times.
One potential problem with fluorescent staining is the decay of intensity, caused by long and multiple-excitation light exposures. As shown on Figure 4C/D the fluorescence intensity of all markers was found to remain stable throughout three separate scans of the same slide with low variability between the individual scans (CV<10% with ranges of 0.30% to 9.6%). Thus, our results indicate that multiple scans of the same slide are possible for repeated analysis.
Correlation between islet quality assessed by LSC and in vivo islet functionality
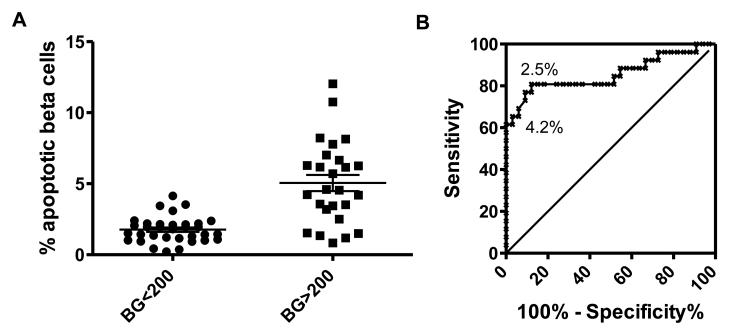
To determine whether there is a correlation between islet quality (as assessed by LSC) and islet functionality, LSC analysis results were compared with in vivo islet function following transplantation of 1200-1600 IEQ in diabetic NODscid mice. In vivo data were divided into two groups depending on average blood glucose levels measured between weeks 3-5: islet preparations that reversed diabetes (blood glucose<200) and islet preparations that did not reverse diabetes (blood glucose>200). Comparison of beta cell apoptosis and diabetes reversal vs. non-reversal showed highly significant differences (p<0.0001; n=59; Figure 5A). In Figure 5A all of the islet preparations that achieved diabetes reversal in NODscid mice had less than 5% apoptotic beta cells. The predictive power of the beta cell apoptosis for transplant efficiency in mice was assessed by Receiver Operating Curves (ROC) analysis (Figure 5B), and was found to be highly significant. The area under the curve (AUC) was 0.8561 (sensitivity 100% and specificity 75%; 95% confidence interval: 0.7474-0.9647), where 1 is equivalent full predictability and 0.5 indicates no predictive relationship. None of the preparations with >4.2% apoptotic beta cells (16 out of 59) reversed diabetes in mice (negative predictive value 1.00); whereas over 80% of the preparations with <2.5% apoptotic beta cells (26 out of 59) reversed diabetes (positive predictive value 0.85). Analyses of other sets of LSC results, however, including beta cell content, did show much weaker correlations with the mouse transplant data (not shown).
Figure 5.
Correlation of percentages of beta cell apoptosis and in vivo functionality of transplanted human islets into streptozotocin-diabetic NODscid mice (n=59): Laser scanning cytometry data for beta-cell-specific apoptosis were plotted against blood glucose data of diabetic NODscid mice obtained 3-5 weeks post-transplantation and analyzed (A) by a two tailed t-test and (B) ROC curve analysis, giving a negative predictive value of 1.00 for 4.2% apoptotic beta cells and a positive predictive value of 0.85 for 2.4% apoptotic beta cells .
Discussion
We have developed a new method to evaluate islet cell composition and beta-cell-specific apoptosis in pancreatic islet preparations without dissociating islets into single cells. Our method allows for the examination of both islet cell composition and morphological islet integrity in large samples (3000 to 30000 cells). In terms of islet cell composition, our results, obtained from a large number (n=102) of human islet preparations, were similar to the data previously reported by several other investigators using different methods, showing a very similar composition of various hormone-positive cells (e.g., 54.6% for beta cells, 33.6% for alpha cells, 12% for delta cells, and 1.6% for pancreatic polypeptide-positive cells) (9, 10, 18, 24).
Our method additionally allows for the evaluation of beta-cell-specific apoptosis without the need for islet cell dissociation, which is known to induce cell damage and loss, including apoptosis. Indeed, our data show significantly lower apoptotic beta cell values than results obtained using the islet dissociation method (10). This discrepancy may also be related to the apoptosis determination method. Because we used TUNEL to measure DNA damage, our results reflect late events in the apoptosis cascade, compared to the mitochondrial membrane damage method in (10). It is interesting to note that our data in human islets are comparable to those reported in isolated rat islets (25), which also showed low TUNEL-positive beta cell apoptosis examined in paraffin sections of fresh or short-cultured islets.
Within a large set of data (n=59), the beta-cell-specific apoptosis correlated well (p<0.0001) with in vivo islet function following transplantation of the corresponding islets into diabetic immunodeficient animals, demonstrating the potential value of the LSC assay in evaluating islet quality. The in vivo assessment of human islet preparations in immunodeficient mice is the only currently known method to assess islet function following transplantation, and is considered the gold standard to evaluate islet quality. However since there are only limited data available to correlate mouse transplant results with clinical islet transplantation outcomes (15), it remains to be elucidated whether our beta-cell-specific apoptosis data assessed by LSC will be capable of predicting the outcome of human islet cell transplantation.
In conclusion, the results of this study demonstrate the quantitative evaluation of beta-cell-specific apoptosis and cell composition in human islet preparations without disturbing islet structure. The method also provides data in “real time,” allowing for results to be obtained prior to clinical islet transplantation and could represent a powerful new tool for quantitation of islet quality.
Materials and Methods
Human pancreatic islets
Islets were isolated from human pancreata from healthy adult donors with a proper consent for research use and approval by the Southern California Islet Cell Resources Center (SC-ICRC) and the Institutional Review Board of the City of Hope. Islet isolations were performed using standard collagenase method for pancreas digestion and islet purification (26) following SC-ICRC operating procedures. All islet samples used in this study were cultured for 24-72 h in the standard media, serum-free CMRL1066-based islet culture medium (Mediatech, Manassas, VA), used for human islets for transplantation. In apoptosis induction experiments, islets were cultured in media containing 50 ng/mL TNFα, 750 U/mL IFNγ, and 75 U/mL IL1β (all from R&D Systems, Minneapolis, MN) for 16 h (25, 27).
Antibodies
Primary antibodies used were: guinea pig anti-human insulin (DAKO, Carpinteria, CA); mouse anti-human glucagon (Sigma, St Louis, MO); rabbit anti-human somatostatin (DAKO); rabbit anti-human pancreatic polypeptide (Millipore/LINCO, St Charles, MO); rabbit anti-human α-amylase (Sigma); mouse anti-human cytokeratin 19 (DAKO). Corresponding secondary antibodies were conjugated with FITC, Cy2, Cy5 (Jackson ImmunoResearch, West Grove, PA), or with Alexa Fluor 647 (Invitrogen, Carlsbad, CA).
Immunohistochemistry
Islets (1000 IEQ) aliquots were fixed with 10% formalin (Fisher Scientific, Pittsburg, PA), embedded in 3% agarose Type VII (Sigma), dehydrated and processed for paraffin embedding. Parallel 5 μm sections were prepared, and immunohistochemical staining was performed as described previously (28). The sections were deparaffinized, hydrated and treated with antigen unmasking solution (Vector Laboratories, Burlingame, CA) following manufacturer's protocol. The slides were washed in PBSM (Dulbecco's PBS containing 2 mM MgCl2), blocked with 1% human serum albumin (HSA) and incubated for 1 h at 37°C with first antibodies appropriately diluted with 1% HSA. The slides were then washed extensively in PBSM containing 0.02% Triton X100 (Sigma), blocked with 1% HSA, and incubated for 1 h at 37°C with appropriate dilutions of secondary antibodies. Finally, the slides were stained for 10 min with 0.2 μg/ml 4,6-diamidino-phenylindole (DAPI) (Sigma) in PBSM/TX100, washed and mounted in Vectashield (Vector Laboratories).
Cells undergoing apoptosis were detected by DNA fragmentation using terminal-deoxynucleotidyl-transferase-mediated dUTP-nick-end labeling (TUNEL) assay (29) utilizing the ApopTag Plus Fluorescein In Situ Apoptosis Detection Kit (Millipore/Chemicon, Temecula, CA) following supplier's instructions. Insulin staining followed after apoptosis staining using an anti-insulin antibody and a Cy5 conjugated secondary antibody as described above. For visual examination, we used an Olympus BX51 fluorescent microscope, equipped with a Pixera CL600 camera.
Laser scanning cytometry
The slides were scanned using an iCys laser scanning cytometer (Compucyte, Westwood, MA) for quantitation of cells expressing desired markers. LSC was performed using 405, 488, and 633 nm lasers and the iCys 3.2.5-3.4 software. The areas of interest were scanned through a 40x objective across sequential 0.5 μm increments. Nuclei stained with DAPI were contoured using the 405 nm laser. Cells stained for islet markers were scanned and recorded using 488 nm or 633 nm lasers. The scanning was performed using the “Watershed” module to distinguish overlapping cells and nuclei. The acquired data during scanning included: area, X/Y position, fluorescence maximal intensity (“Max Pixel”), and peripheral maximal intensity (“Peripheral Max”) for all channels. Fluorescence intensities were recorded on scattergrams and histograms and corresponding areas were designated/gated. Cell morphology and staining were confirmed by visual examination of a selected region in a scattergram or histogram using the “Gallery” module. Scanning and analysis of each sample were performed on at least two randomly selected sections, covering the entire sections containing 3000 to 30000 cells. Composition of a specific islet cell type was expressed as a percentage in a whole islet preparation, as well as “per islet” (10) calculated by the formula: the percentage of a specific islet hormone cell type (β, α, δ, or ppp) divided by the total islet hormone cell (β + α + δ + ppp) percentage × 100. The whole procedure from islet fixation to evaluation could be completed in less than 24 h, and thus, results made available prior to clinical islet transplantation.
Evaluation of cell composition of dissociated islets
Isolated human islets were dissociated into single cells, attached to glass slides, fixed, immunostained and scanned on an iCys laser scanning cytometer (Compucyte) as described previously (30).
Assessment of in vivo islet function in diabetic NODscid mice
Male NODscid mice, ages 10-12 weeks, were obtained from the Animal Resources Center of the City of Hope and used as islet recipients. Mice were rendered diabetic by intraperitoneal injections of 50 mg/kg streptozotocin (Sigma) on three consecutive days. Those that exhibited hyperglycemia (>350 mg/dL) for two consecutive days were used as recipients. Islets (1200 and/or 1600 IEQ) were transplanted under the left kidney capsule of diabetic mice and blood glucose levels were measured 2-3 times weekly. Recipient mice that maintained a blood glucose <200 mg/dL after the third week post-transplantation were considered to have reversed diabetes. Glucose levels between weeks 3 and 5 (days 15-35) were averaged and used as “mouse blood glucose” for correlation analysis studies. At the end of each experiment, the graft was removed by nephrectomy to confirm graft dependence. A total of 137 mice transplanted with 1200-1600 IEQ from 59 islet preparations were used for correlation. Animal procedures followed protocols approved by the Institutional Animal Care and Use Committee of the City of Hope.
Data analysis
Data are presented as a mean±standard error. Paired two-tailed Student's t-test and Receiver Operating Curves (ROC) analyses (GraphPad Prizm) were used to compare differences between islet batches to investigate the association between LSC analysis results and with in vivo islet transplant outcome in mice (6, 7). A p value of less than 0.05 was considered significant. The data analysis was performed by statistical SAS 9.1 for Windows software package (SAS Institute Inc., Cary, NC) or by GraphPad Prizm (GraphPad Software, La Jolla, CA).
Acknowledgments
We would like to thank the islet isolation team of the SC-ICR Center, the Anatomic Pathology and Analytical Cytometry Core facilities at City of Hope for their help and support, and the team at Compucyte Corporation for their assistance and advice in the development of our LSC technology, as well as Koi Anunta for her help in the preparation of the manuscript.
Grant Support: U42 RR16607 (NIH) (F.K); 8-2006-1014 (JDRF) (F.K.)
Abbreviations
- Cy
cyanine
- DAPI
4,6-diamidino-phenylindole
- IFNγ
interferon γ
- IL1β
interleukin 1β
- LSC
laser scanning cytometry
- SC-ICRC
Southern California Islet Cell Resources Center
- TNFα
tumor necrosis factor α
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
Footnotes
Authors' Contribution: I.T, F.K., and Y.M. designed research; I.T., I.N., A.A-M., J.R., K.O., T.I., L.V, I.I-M and I.H.A performed experiments; I.T., I.N., C.O., K.D.S, and K.F. analyzed data; K.D.S., C.O. and K.F. performed statistical analysis; I.T. and Y.M. prepared, and F.K., K.O., K.F. and I.H.A. critically reviewed, the manuscript; F.K. supported the project.
Conflict of interest: All authors declare no conflict of interest for the study
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alejandro R, Barton FB, Hering BJ, Wease S. 2008 Update from the Collaborative Islet Transplant Registry. Transplantation. 2008;86(12):1783. doi: 10.1097/TP.0b013e3181913f6a. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 3.Fiorina P, Shapiro AM, Ricordi C, Secchi A. The clinical impact of islet transplantation. Am J Transplant. 2008;8(10):1990. doi: 10.1111/j.1600-6143.2008.02353.x. [DOI] [PubMed] [Google Scholar]
- 4.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54(7):2060. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 5.Wang W, Upshaw L, Strong DM, Robertson RP, Reems J. Increased oxygen consumption rates in response to high glucose detected by a novel oxygen biosensor system in non-human primate and human islets. J Endocrinol. 2005;185(3):445. doi: 10.1677/joe.1.06092. [DOI] [PubMed] [Google Scholar]
- 6.Sweet IR, Gilbert M, Jensen R, et al. Glucose stimulation of cytochrome C reduction and oxygen consumption as assessment of human islet quality. Transplantation. 2005;80(8):1003. doi: 10.1097/01.tp.0000178381.35014.37. [DOI] [PubMed] [Google Scholar]
- 7.Sweet IR, Gilbert M, Scott S, et al. Glucose-stimulated increment in oxygen consumption rate as a standardized test of human islet quality. Am J Transplant. 2008;8(1):183. doi: 10.1111/j.1600-6143.2007.02041.x. [DOI] [PubMed] [Google Scholar]
- 8.Papas KK, Colton CK, Nelson RA, et al. Human islet oxygen consumption rate and DNA measurements predict diabetes reversal in nude mice. Am J Transplant. 2007;7(3):707. doi: 10.1111/j.1600-6143.2006.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabrera O, Jacques-Silva MC, Berman DM, et al. Automated, high-throughput assays for evaluation of human pancreatic islet function. Cell Transplant. 2008;16(10):1039. [PMC free article] [PubMed] [Google Scholar]
- 10.Ichii H, Inverardi L, Pileggi A, et al. A novel method for the assessment of cellular composition and beta-cell viability in human islet preparations. Am J Transplant. 2005;5(7):1635. doi: 10.1111/j.1600-6143.2005.00913.x. [DOI] [PubMed] [Google Scholar]
- 11.Goto M, Holgersson J, Kumagai-Braesch M, Korsgren O. The ADP/ATP ratio: A novel predictive assay for quality assessment of isolated pancreatic islets. Am J Transplant. 2006;6(10):2483. doi: 10.1111/j.1600-6143.2006.01474.x. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto T, Horiguchi A, Ito M, et al. Quality control for clinical islet transplantation: organ procurement and preservation, the islet processing facility, isolation, and potency tests. J Hepatobiliary Pancreat Surg. 2009;16(2):131. doi: 10.1007/s00534-009-0064-z. [DOI] [PubMed] [Google Scholar]
- 13.Omori K, Mitsuhashi M, Todorov I, et al. Microassay for glucose-induced preproinsulin mRNA expression to assess islet functional potency for islet transplantation. Transplantation. 2010;89(2):146. doi: 10.1097/TP.0b013e3181c4218d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertuzzi F, Ricordi C. Prediction of clinical outcome in islet allotransplantation. Diabetes Care. 2007;30(2):410. doi: 10.2337/dc06-1233. [DOI] [PubMed] [Google Scholar]
- 15.Caiazzo R, Gmyr V, Kremer B, et al. Quantitative in vivo islet potency assay in normoglycemic nude mice correlates with primary graft function after clinical transplantation. Transplantation. 2008;86(2):360. doi: 10.1097/TP.0b013e31817ef846. [DOI] [PubMed] [Google Scholar]
- 16.Ichii H, Miki A, Yamamoto T, et al. Characterization of pancreatic ductal cells in human islet preparations. Lab Invest. 2008;88(11):1167. doi: 10.1038/labinvest.2008.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armann B, Hanson MS, Hatch E, Steffen A, Fernandez LA. Quantification of basal and stimulated ROS levels as predictors of islet potency and function. Am J Transplant. 2007;7(1):38. doi: 10.1111/j.1600-6143.2006.01577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brissova M, Fowler MJ, Nicholson WE, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53(9):1087. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- 19.Boffa DJ, Waka J, Thomas D, et al. Measurement of apoptosis of intact human islets by confocal optical sectioning and stereologic analysis of YO-PRO-1-stained islets. Transplantation. 2005;79(7):842. doi: 10.1097/01.tp.0000155175.24802.73. [DOI] [PubMed] [Google Scholar]
- 20.Kamentsky LA, Kamentsky LD. Microscope-based multiparameter laser scanning cytometer yielding data comparable to flow cytometry data. Cytometry. 1991;12(5):381. doi: 10.1002/cyto.990120502. [DOI] [PubMed] [Google Scholar]
- 21.Pozarowski P, Holden E, Darzynkiewicz Z. Laser scanning cytometry: principles and applications. Methods Mol Biol. 2006;319:165. doi: 10.1007/978-1-59259-993-6_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grace MJ, Xie L, Musco ML, et al. The use of laser scanning cytometry to assess depth of penetration of adenovirus p53 gene therapy in human xenograft biopsies. Am J Pathol. 1999;155(6):1869. doi: 10.1016/S0002-9440(10)65506-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darzynkiewicz Z, Li X, Bedner E. Detection of DNA strand breakage in the analysis of apoptosis and cell proliferation by flow and laser scanning cytometry. Methods Mol Biol. 1999;113:607. doi: 10.1385/1-59259-675-4:607. [DOI] [PubMed] [Google Scholar]
- 24.Street CN, Lakey JR, Shapiro AM, et al. Islet graft assessment in the Edmonton Protocol: implications for predicting long-term clinical outcome. Diabetes. 2004;53(12):3107. doi: 10.2337/diabetes.53.12.3107. [DOI] [PubMed] [Google Scholar]
- 25.Cattan P, Berney T, Schena S, et al. Early assessment of apoptosis in isolated islets of Langerhans. Transplant Proc. 2001;33(1-2):264. doi: 10.1016/s0041-1345(00)02006-6. [DOI] [PubMed] [Google Scholar]
- 26.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37(4):413. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 27.Matsuda T, Omori K, Vuong T, et al. Inhibition of p38 pathway suppresses human islet production of pro-inflammatory cytokines and improves islet graft function. Am J Transplant. 2005;5(3):484. doi: 10.1046/j.1600-6143.2004.00716.x. [DOI] [PubMed] [Google Scholar]
- 28.Todorov I, Omori K, Pascual M, et al. Generation of human islets through expansion and differentiation of non-islet pancreatic cells discarded (pancreatic discard) after islet isolation. Pancreas. 2006;32(2):130. doi: 10.1097/01.mpa.0000202945.78331.93. [DOI] [PubMed] [Google Scholar]
- 29.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119(3):493. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iglesias I, Bentsi-Barnes K, Umeadi C, Brown L, Kandeel F, Al-Abdullah IH. Comprehensive analysis of human pancreatic islets using flow and laser scanning cytometry. Transplant Proc. 2008;40(2):351. doi: 10.1016/j.transproceed.2008.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]